Abstract
The purpose of this study was to experimentally assess the effect of a one touch clamp and rack smart pump on the time needed for patient mobilisation and the clinical staff`s perceived burden associated with these procedures when compared with the traditional type infusion system. Participants were randomly assigned to one of three groups: Multi-layer rack group (MULTI), One-touch pole clamp group (ONE), and a Control group (CON). In CON, traditional type pumps were replaced from pole stands to pole stands. In MULTI, the smart pumps were replaced from multi-layer rack to multi-layer rack, and in ONE the smart pumps were replaced from pole stands to multi-layer rack. In each group, the subject round-transported the simulated patient and re-replaced the pumps. The time of both pump replacements and the transport time were significantly shorter in MULTI and ONE compared with CON (p < 0.0001). The perceived burden of the replacement and transport were all significantly lower in MULTI and ONE than CON (p < 0.0001). The one touch clamp and rack smart pump reduced the time needed and perceived burden of mobilisation. These findings are anticipated to translate into progression in the delivery of early mobilisation and ultimately rehabilitation outcomes.
1. Introduction
In recent years, in various fields of medicine, a body of evidence has emerged on the beneficial effects of early mobilisation after the onset of disease, injury, or surgery on mental and physical outcomes. Among them, Schweickert et al. [Citation1] reported that early mobilisation in a group of critically ill patients under mechanical ventilation increased the number of patients who could independently perform activities of daily living (ADL) following rehabilitation compared with a control group that did not receive early mobilisation. Engel et al. [Citation2] also report that an early mobilisation program in the intensive care unit (ICU) may improve outcomes and reduce medical costs. As a result, early mobilisation is recommended in many clinical guidelines as well as postoperative protocols [Citation3–6].
However, acute phase patients often have unstable respiratory and circulatory dynamics, and are connected to various devices and infusion lines necessary for life support. Therefore, in addition to barriers associated with patients` disease and injury, the management and handling of equipment is an additional factor that may limit the use of early mobilisation during acute phase rehabilitation. Indeed, several reports have indicated that the handling of medical devices and intravenous infusion lines form a major barrier to early mobilisation [Citation7–9]. Therefore, Fuest et al. [Citation10] state that while early mobilisation improves the outcome of postoperative patients in the ICU, early mobilisation can be difficult to implement and strategies to overcome this barrier need to be explored.
To this aim, a one touch clamp and rack smart pump (), consisting of a smart Internet of Things (IoT) pump, a one-touch pole clamp and a multi-layer rack, has been available. In addition to enhanced infusion accuracy, the drug library and the Information Technology (IT) connection function, the smart pump itself can be attached to and detached from the rack with one touch. Further, when the one-touch pole clamp is attached to the smart pump, it can be easily attached and detach to the drip pole stand, while the multi-layer rack can install multiple pumps. Together, the one touch clamp and rack smart pump may form a device with an improved mobility and functionality when compared with currently used devices, and partly alleviate the barrier to perform early mobilisation associated with the management of medical devices.
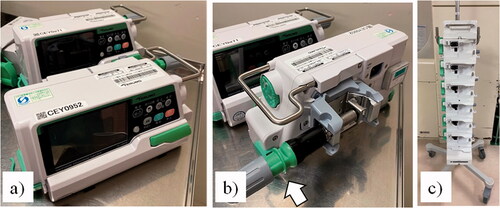
Figure 1. A one touch clamp and rack smart pump. (a) smart Internet of Things (IoT) pump; (b) one-touch pole cramp attached with smart pump; (c) multi-layer rack. The one-touch pole clamp allows the pump to be easily attached to or removed from the pole using the clamp indicated by the white arrow in Figure 1(b). In addition, the pump can be mounted in the multi-layer rack with the one-touch clamp attached.
Despite the promise of the one touch clamp and rack smart pump, there are limited studies on its use in acute phase rehabilitation, including its handling and mobility. It is also unclear whether the one touch clamp and rack smart pump can reduce the perceived burden of staff members engaged in acute phase rehabilitation medicine.
Therefore, the purpose of this study was to experimentally assess the time needed and the perceived burden of the replacement and transport of one touch clamp and rack smart pump compared with traditional type pumps. It was hypothesised that the one touch clamp and rack smart pump leads to a reduction in time needed and perceived burden among staff members involved in early mobilisation in acute phase rehabilitation when compared to the traditional type pumps.
2. Methods
2.1. Participants
Participants were the staff members in our department. The inclusion criterium was: engaging in acute phase rehabilitation including early mobilisation at ICU, while the exclusion criterium was having an illness or physical disability that interfered with study participation. This study was approved by the Research Ethics Review Committee of our Medical University (#2934) and each participant provided signed consent before enrolment into the study.
Following the provision of written informed consent, participants were randomly assigned using the allocation table kept by the registration office in the department. The allocation table was created under the block size 3 by the permuted block method.
2.2. Study design
The study consisted of three people, one in the role of a subject, one in the role of a simulated patient, and one in the role of a wheelchair assistant. In addition, these three people took on the role of the subject by changing roles on different days within the same group. Those who did not participate in this study were the time measurers. Six infusion lines (three lines to the left side of the neck with central venous catheter, one line to the left forearm, two lines to the right forearm) from two infusion pumps and four syringe pumps were attached to the body surface of the simulated patient with tape, and the simulated patient was lying on the bed. In each group, the subject moved the simulated patient attached with the infusion lines from the bed to the wheelchair after the pumps were replaced. The subject handled two mobile drip-pole stands or one mobile multi-layer rack while managing the infusion lines, and made a round transport from the laboratory to the rehabilitation room. After returning to the laboratory, the subject moved the simulated patient from the wheelchair to the bed, and the subject detached the pumps from the mobile drip-pole stand or mobile multi-layer rack to the original drip pole stand or multi-layer rack ().
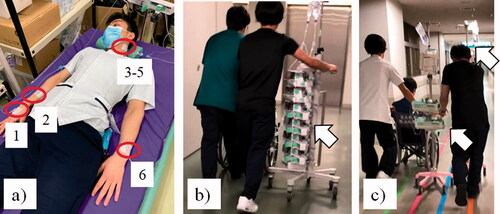
Figure 2. Study Design. (a) Simulated patient attached through six infusion lines with two infusion pumps and four syringe?pumps; (b) Round-transport of MULTI and ONE; (c) Round-transport of CON Multi-layer rack group (MULTI), One-touch pole clamp group (ONE), control group (CON) Red circle in Figure 2(a) shows the six lines attached to the simulated patient with tape, two lines to the right forearm (1,2), three lines to the left part of the neck with a central venous catheter (3–5), and one line to the left forearm (6). White arrow in Figure 2(b) shows two smart infusion pumps and four smart syringe pumps mounted in the multi-layer rack in MULTI and ONE groups. White arrows in Figure 2(c) show old traditional type two infusion pumps and four syringe pumps attached with two drip-poles in CON group (One pole is behind the subject).
2.3. Groups
Subjects were randomly assigned to one of three groups: Multi-layer rack group (MULTI), One-touch pole clamp group (ONE), or a control group (CON) ().
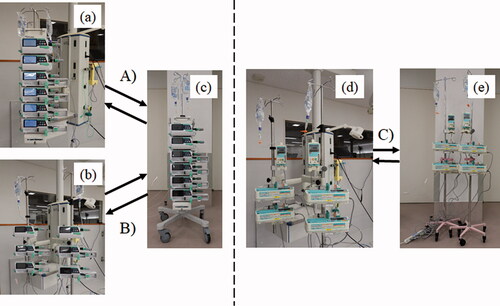
Figure 3. The experimental groups. (A) Experimental group (1) (MULTI); (B) Experimental group (2) (ONE); (C) Control group (CON) (A) In MULTI group, the subject detached the four smart syringe pumps and two smart infusion pumps from the one multi-layer rack (a) and attached to the one mobile multi-layer rack for transportation (c). (B) In ONE group, the subject detached the four smart syringe pumps and two smart infusion pumps, that were attached one-touch pole clamp from the two-drip pole stands (b) and attached to the one mobile multi-layer rack for transportation (c). (C) In CON group, the subject detached the four traditional type syringe pumps and two traditional type infusion pumps from the two drip-pole stands (d) and attached to the two-mobile drip-pole stands for transportation (e). (A–C) After returning to the laboratory, the subject detached the pumps from the mobile drip-pole stand or mobile multi-layer rack to the original drip pole stand or multi-layer rack.
MULTI: The subject detached the four smart syringe pumps (Terumo FusionⓇ SS type; Terumo Co., Ltd., Tokyo, Japan) and two smart infusion pumps (Terumo FusionⓇ LM type; Terumo Co., Ltd., Tokyo, Japan) from the one multi-layer rack (Terumo FusionⓇ IoT rack system; Terumo Co., Ltd., Tokyo, Japan) and attached to the one mobile multi-layer rack for transportation. After returning to the laboratory from the rehabilitation room, the subject detached the smart syringe pumps and infusion pumps from the one mobile multi-layer rack and attached to the original one multi-layer rack.
ONE: The subject detached the four smart syringe pumps and two smart infusion pumps, that were attached one-touch pole clamp (Terumo FusionⓇ one-touch pole clamp; Terumo Co., Ltd., Tokyo, Japan), from the two drip pole stands and attached to the one mobile multi-layer rack for transportation. After returning to the laboratory from the rehabilitation room, the subject detached the smart syringe pumps and infusion pumps with one-touch pole clamp from the one mobile multi-layer rack and attached to the original two drip-pole stands.
CON: The subject detached the four traditional type syringe pumps (Terumo Fusion syringe pump TE-331S type; Terumo Co., Ltd., Tokyo, Japan) and two traditional type infusion pumps (Terumo Fusion infusion pump TE-161Stype; Terumo Co., Ltd., Tokyo, Japan) from the two drip-pole stands and attached to the two mobile drip-pole stands for transportation. After returning to the laboratory, the subject detached the traditional type syringe pumps and infusion pumps from the two mobile drip-pole stands and attached to the original two drip-pole stands.
2.4. Outcome measures
(1) Replacement time (initial replacement time and second replacement time): We used a stopwatch to measure the replacement time and round transport time. For initial replacement time, the stopwatch was started when the subject started detaching the four syringe pumps and two infusion pumps from the two original drip-pole stands or one original multi-layer rack and stopped the stopwatch when the subject finished attaching the pumps to the mobile two drip-pole stands or one mobile multi-layer rack. For second replacement time, the stopwatch was started when the subject started detaching the pumps from the two mobile drip-pole stands or one mobile multi-layer rack and stopped the stopwatch when the subject finished attaching the pumps to the original two drip-pole stands or one original multi-layer rack.
(2) Round transport time (between the laboratory and the rehabilitation room (250 m)): The stopwatch was started when the subject started transferring the simulated patient from the bed to wheelchair and kept measuring round transport time and stopped when the subject finished transferring the simulated patient from the wheelchair to the bed.
(3) Number of incidents.: We counted the number of the mechanical and clinical adverse events when the subject replaced the pumps and transported the simulated patient.
(4) Perceived burden, as assessed by a 1–100 mm Visual Analogue Scale (VAS) immediately following the end of the experiment: burden for the initial and second replacement of the pumps, and management of the infusion lines during replacement. Lesage et al. [Citation11] reported that VAS showed a good correlation with the general burden and stress scale of hospital staff.
(5) Perceived burden, as assessed by a 1–100 mm VAS immediately following the end of the experiment: feeling of stability, complexity of line handling, and the feeling of insecurity about the handling of the device.
2.5. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD). Normality of the data was checked with the Shapiro–Wilk test. One-way ANOVAs were used to test for a difference one of the outcome measures between groups. Tukey HSD post-hoc tests were used when a significant main effect was detected. Data analysis was performed using JMP® pro version 14.1 (SAS Institute Inc.) and p < 0.05 was considered statistically significant.
3. Results
3.1. Participants
Of the 62 staff members in our department, 45 met the criteria. The enrolled participants were randomly assigned to three groups, 15 participants in each group (). The professional background of the participants is shown in . All trials were successfully completed; however, in CON, one mounting screw failure, two loose mounting of the pump, and one spontaneous line removal occurred.
Figure 4. Flow Diagram. Multi-layer rack group (MULTI), One-touch pole clamp group (ONE), control group (CON).
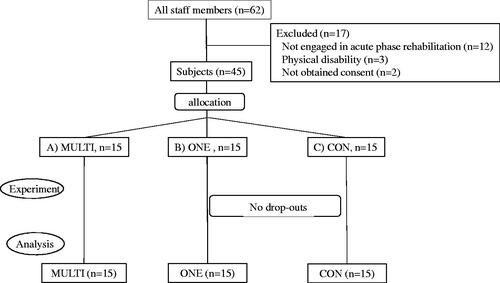
Table 1. Demographic characteristics of the subjects.
3.2. Pump replacement time
The initial replacement time was significantly shorter in MULTI and ONE when compared with CON (p < 0.0001). The round-transport time was also significantly shortened in MULTI and ONE compared with CON (p < 0.0001). Similarly, the second replacement time was significantly shorter in MULTI and ONE compared with CON (p < 0.0001). Further, the second replacement time was significantly shorter in MULTI compared with ONE (p = 0.0007) ().
Table 2. Time needed for pump replacement and the round-transport.
3.3. Perceived burden of pump replacement
The perceived burden of initial and second replacement, and line arrangement were significantly lower in MULTI and ONE compared with CON (p < 0.0001). In addition, the perceived burden of the second replacement was lower in MULTI compared with ONE (p = 0.0223) ().
Table 3. Perceived burden of the pump replacement.
3.4. Perceived burden of round-transport
The perception of instability, the complexity of line handling, and the feeling of insecurity about the handling of the device were all significantly lower in MULTI and ONE compared with CON (p < 0.0001) ().
Table 4. Perceived burden of the round-transport.
4. Discussion
This study showed that a one touch clamp and rack smart pump may reduce the time needed for early mobilisation as well as the perceived burden on staff in charge of acute phase rehabilitation. However, this was not the study assessing the utility of a particular brand of pump.
There are several potential reasons why the time needed for mobilisation and perceived burden were lowered with the one touch clamp and rack smart pump. The traditional type pump used as a comparator in the present needs to be fixed by a screw when it is attached to or detached from the drip pole stand. On the other hand, the smart pump can be detached from the rack and attached to the rack with one touch. In addition, the smart pump can be detached from and attached the drip pole stand with one touch by connecting the one-touch pole clamp. Even if the one-touch pole clamp is attached to the smart pump, it can be detached from and attached to the rack with one touch. As for the multi-layer rack, multiple smart pumps can be installed in one multi-layer rack, improving the stability of the device. On the other hand, the traditional drip-pole stand is generally unstable, and it is difficult to install four or more smart pumps in one drip-pole stand.
In this study, six infusion lines were attached to the subjects with tape, and a total of two infusion pumps and four syringe pumps were used; representative of the number of lines and pumps used when mobilising a patient at the ICU. Therefore, in this study, two drip pole stands were required when moving six pumps. In the ONE condition, it was necessary to remove 6 pumps from one rack and attach them to 2 poles at the time of second replacement after the round-transport. This may be the reason that ONE took more time and that the perceived burden was increased as compared with MULTI, in which the smart pumps attached to one rack were replaced to one rack. Moreover, it was considered that the stable moving of one rack in MULTI shortened the time compared to the unstable moving of two drip pole stands, and the feeling of stability, security, and line handling were improved.
The smart pump is a smart IoT infusion system with features such as a drug library function and an in-hospital IT connection function. This has been shown to reduce errors associated with continuous drug administration [Citation12], while the IT connection also allows it to be used remotely. The latter makes mobility and stability during transportation increasingly important. However, most of previous studies on smart pumps have reported on accurate drug administration, drug library function, and risk management of the infusion pump itself [Citation13–16]. In addition, there are some reports on the health economic effects of these functions [Citation17], however they were not focussed on the mobility and stability of the one touch clamp and rack smart pump.
In the other hand, additional devices that could enhance mobility have emerged. For example, Skryabina and Dunn [Citation18] have reviewed disposable infusion pumps and reported that these can be used in a variety of clinical settings. However, the fact that they are non-electric limits their usefulness in a large-scale medical setting. Seo et al. [Citation19] have developed a wearable intravenous infusion device, which may greatly enhance the mobility of the patient. Finally, while hospital staff members use smart pump technology with drug libraries and medication safety software, most home fluid providers continue to use traditional infusion pumps that do not provide those features [Citation20]. While this may be sufficient for most situations, the findings of the present study provide tentative support for further research into its use in settings other than acute rehabilitation settings.
As with all studies, the present study had limitations that need addressing. Firstly, the present study was an acute experimental study using simulated patient mobilisation, rather than a clinical study conducted with patients. In our hospital, all infusion systems in the ICU have been replaced with one touch clamp and rack smart pump, and it was therefore difficult to create a control group in the clinical setting. Secondly, the staff members rotated the role of subject, simulated patient, and wheelchair assistant. We divided 15 people into five groups, three people each in the same group, and avoided them participating in other groups. However, any bias due to the order of roles in the same group cannot be excluded. Fourthly, in this experimental study, we focussed on the one touch clamp and rack smart pump only, and did not use patient monitoring or ventilation equipment. As patient monitoring and ventilation equipment may influence the handling of the pumps, this may be considered a limitation of the study. However, patient monitoring equipment and a ventilator are additional barriers of early mobilisation [Citation7–9] and by reducing the required time and perceived burden on staff members with the one touch clamp and rack pump, time is freed to focus on patient monitoring and ventilation. Finally, the studied sample did not include nurses. Nurses may also be involved in early mobilisation for mildly ill patients. However, as early mobilisation for critically ill patients in the ICU is routinely performed by a rehabilitation specialist and therapist, this study was limited to only these members of staff.
Intravenous infusion management is one of the barriers for early mobilisation of critically ill patients [Citation7–10]. Therefore, it is expected that the one touch clamp and rack smart pump will lead to improvements in clinical practice by reducing the required time for pump replacement and the burden on staff. This may in turn reduce the health risks associated with early mobilisation result in meaningful progress in the early mobilisation for critically ill patients. The one touch clamp and rack smart pump is equipped with a drug library function and connected to electronic medical records via the Internet. Therefore, in the future, in addition to the reduction effect of clinical burden and risk, we plan to test the accuracy of infusion and risk management when patients are transported outside the acute phase ward. This would open up the opportunity to conduct and monitor walking training, aerobic training and muscle strengthening training in the early phases of acute rehabilitation; potentially further contributing to improved rehabilitation outcomes.
5. Conclusion
The one touch clamp and rack smart pump experimentally reduced the time needed for pump replacement and transportation as well as the perceived burden associated with these procedures. A reduction in the required time and perceived burden of the handling of medical devices in clinical practice is expected to further promote the use of early mobilisation in acute phase rehabilitation; a strategy that has previously been shown to lead to better mental and physical function outcomes.
Acknowledgements
We thank our staff for their participation in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Schweickert WD, Pohlman MC, Kress JP, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients. Lancet. 2009;373(9678):1874–1882.
- Engel HJ, Needham DM, Gropper MA, et al. ICU early mobilization: from recommendation to implementation at three medical centers. Crit Care Med. 2013;41(9 Suppl 1):S69–S80.
- Lang JK, Paykel MS, Hodgson CL, et al. Clinical practice guidelines for early mobilization in the ICU: a systematic review. Crit Care Med. 2020;48(11):e1121–e1128.
- Batchelor TJP, Rasburn NJ, Naidu B, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the enhanced recovery after surgery (ERAS®) society and the european society of thoracic surgeons (ESTS). Eur J Cardiothorac Surg. 2019;55(1):91–115.
- Gould MK, Garcia DA, Samama CM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians Evidence-Based clinical practice guidelines. Chest. 2012;141(2):e227S–e277S.
- Aquim EE, Bernardo WM, Verona C, et al. Brazilian guidelines for early mobilization in intensive care unit. Rev Bras Ter Intensiva. 2019;31(4):434–443.
- Dubb R, Nydahl P, Needham DM, et al. Barriers and strategies for early mobilization of patients in intensive care units. Ann Am Thorac Soc. 2016;13(5):724–730.
- Needham DM, Korupolu R. Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil. 2010;17(4):271–281.
- Koenders N, Weenk M, Bredie SJH, et al. Exploring barriers to physical activity of patients at the internal medicine and surgical wards: a retrospective analysis of continuously collected data. Disabil Rehabil. 2019;6:1–7.
- Fuest K, Schaller SJ. Recent evidence on early mobilization in critical-Ill patients. Curr Opin Anaesthesiol. 2018;31(2):144–150.
- Lesage FX, Berjot S, Deschamps F. Clinical stress assessment using a visual analogue scale. Occup Med. 2012;62(8):600–605.
- Larsen GY, Parker HB, Grant MC, et al. Standard drug concentrations and smart-pump technology reduce continuous-medication-infusion errors in pediatric patients. Pediatrics. 2005;116(1):e21–e25.
- Wilson K, Sullivan M. Preventing medication errors with smart infusion technology. Am J Health Syst Pharm. 2004;61(2):177–183.
- Skledar SJ, Niccolai CS, Urban A, et al. Quality-improvement analytics for intravenous infusion pumps. Am J Health Syst Pharm. 2013;70(8):680–686.
- Rothschild JM, Keohane CA, Bates DW, et al. A controlled trial of smart infusion pumps to improve medication safety in critically ill patients. Crit Care Med. 2005;33(3):533–540.
- Jani YH, Chumbley GM, Franklin B, et al. The potential role of smart infusion devices in preventing or contributing to medication administration errors: a descriptive study of 2 data sets. J Patient Saf. 2020;2020:751.
- Biltoft J, Finneman L. Clinical and financial effects of smart pump-electronic medical record interoperability at a hospital in a regional health system. Am J Health Syst Pharm. 2018;75(14):1064–1068.
- Skryabina EA, Dunn T. Disposable infusion pumps. Am J Health Syst Pharm. 2006;63(13):1260–1268.
- Seo WH, Cho KH, Loh BG. A wearable intravenous infusion device facilitating ambulation: a pre-clinical report. J Med Eng Technol. 2014;38(1):42–48.
- Brown TD, Michael M, Grady DS. Implementation of smart pump technology with home infusion providers: an assessment of clinician workflow and patient Satisfaction. J Infus Nurs. 2018;41(6):344–349.
