Abstract
NIHR (National Institute for Health Research) Devices for Dignity MedTech Cooperative (D4D) and NIHR Children and Young People MedTech Cooperative (CYPMedTech) have established track records in keeping patient and public involvement (PPI) at the core of medical technology development, evaluation and implementation. The 2020 global COVID-19 pandemic presented significant challenges to maintaining this crucial focus. In this paper we describe prior successful methodologies and share examples of the adaptations made in order to continue to engage with patients and the public throughout the pandemic and beyond. We reflect on learning gained from these experiences, and new areas of scope and focus relating to broadening the reach of engagement and representation, along with associated resource requirements and impact metrics.
Introduction
NIHR Devices for Dignity (D4D) and NIHR Children and Young People MedTech Cooperative (CYPMedTech) are two of eleven national MedTech and In-vitro diagnostic Co-operatives (MICs). D4D’s focus is across the life course and CYPMedTech is the only MIC dedicated to children and young people. Effective partnerships with patients (including children and young people), carers and the public as equal partners alongside academics, engineers, designers and healthcare professionals are embedded throughout our work.
NIHR Centre for Engagement and Dissemination (NIHR CED) defines patient and public involvement (PPI) as “research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’ ‘about’ or ‘for’ them.” NIHR CED has replaced NIHR Involve, the previous group that presented PPI within the NIHR [Citation1].
We use the NIHR definitions of Involvement, Engagement and Participation. By patients and public, we refer to:
Patients in specific condition groups (and/or multiple conditions)
Family/friends/unpaid carers
General public
Expert patient consultants
Patient organisations and charities
Patient and public involvement brings a breadth of insight, experience, knowledge and skills, and ensures that technology development is shaped by the patients and the public who will be using the technology, in order to ensure it is:
Needed
Usable
Acceptable
Fit for purpose
We aim to engage with a representative range of patient and public voices. To do this, we have our own established collaborators with whom we have built meaningful relationships over time. We also work with many other patient organisations and charities to extend our reach. It is worth noting that there is no “one-size-fits-all” approach. Flexibility is required to tailor patient and public involvement to the topic, research question, methods and resources available [Citation2], this is where the flexibility of the combined MIC approach adds extra value.
Historically, the National Health Service (NHS) and academic PPI approach has struggled to receive the appropriate funding or importance within the research and innovation community with patients and members of the public simply being asked to approve and rubber stamp nearly completed projects without appropriate consultation on the work undertaken [Citation3]. In contrast, our approach to PPI means that the user/patient/member of the public is considered and involved where applicable at every stage of the project, we also undertake significant work with funders to recognise that PPI is key to a project being successful or not. Both MIC’s have worked alongside the Small Business Research Initiatives (SBRI) competition, NIHR Innovation for Invention (I4I) and Innovate UK to increase PPI involvement in a meaningful way. Significant funding bids have been secured, PPI representation on funding panels facilitated and invitations to coordinate and create learning resources on PPI for medical technology development for EUPATI (European Patient Training Academy), MDMC (Medical Device Manufacturing Centre). D4D have also collaborated with the EPSRC (Engineering & Physical Sciences Research Council, https://epsrc.ukri.org/) in their review of PPI support for their membership and embedding a greater focus on PPI within their future funding calls.
Work to better understand the health challenges of people with long term and multiple conditions is fundamental to our approach of identifying priorities for research and building collaborative project teams to develop innovative technology to meet these needs.
This collaboration principle (co-production) is included throughout the development, evaluation and adoption stages of each project. Both MIC’s share a similar approach, this is illustrated in . The Six Stages of Innovation Model, incorporating examples of PPI activities appropriate for each stage of innovation and also mapping onto this model the Technology Readiness Levels (TRLs) from the scale originally developed by NASA (National Aeronautics and Space Association) which is widely used to describe the stage of development and maturity of different technologies [Citation4].
Figure 1. Devices for dignity six stages of innovation model, illustrating PPI activities and Technology Readiness Levels (TRL). www.devicesfordignity.org.uk.
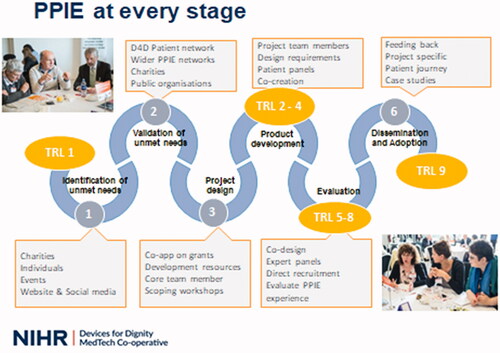
We include individuals or groups of patients and carers, or patient-representing organisations such as charities, in several different roles and activities. This allows them to add their individual personal lived experiences and also a collective voice of issues affecting the majority of patients within a specific group.
Roles:
Membership of our Steering Committees
Expert Advisors in Project Steering Groups
Co-applicants on funding bids and project teams
Participants of focus groups, surveys, interviews, webinars and design workshops
Co-authors on dissemination outputs, including journal publications
Activities:
Identification, validation and prioritisation of unmet needs
Idea generation and incubation
Identification of design, acceptability and usability requirements for new devices and digital applications
Iterative prototype development and evaluation
Collaboration in bid development and review
Collaboration in strategy development and review
Feedback on experiences of health and research involvement underrepresentation from minority groups and advice on cultural sensitivity in order to address this going forward
Development and review of trial design, participant information materials, outcome measures and device instruction leaflets
Collaboration on interpretation and dissemination of study findings
Impact of COVID-19 on PPI practice
The COVID-19 pandemic has had a devastating impact on healthcare systems [Citation5] and care delivery, changing the context for patient and public involvement with research and innovation. It has certainly highlighted the fragility of patient and public involvement and engagement in research and innovation developments.
Challenges relating to language, digital literacy and socio-economic status have all been well documented for a number of years and became reinforced barriers during the COVID-19 pandemic as alternative ways of undertaking patient involvement and engagement activities were looked at.
PPI in research depends traditionally on personal relationships [Citation6], on face-to-face meetings, on gradually building PPI capacity among both researchers and PPI partners. It is widely acknowledged that establishing these relationships takes time and commitment, from both researchers and PPI partners. In some research teams with an existing PPI ethos, re-assignment of key researchers to other roles and prioritising support for front-line activities, meant that PPI skills may not be readily available. So, in many cases, it has been easier to discount PPI in research during the pandemic, rather than find alternative ways to maintain existing, or build new, PPI relationships.
The pandemic affects everyone in society, but it does not affect everyone in the same way. The public at large, and those from minority or marginalised groups in particular, (who are disproportionately affected), can play an important role in shaping research that explores the impact of the pandemic on our working lives, our home life and how we are coping with our “new normal”. It is important to recognise and harness the different types of knowledge and experiences brought by diverse communities and individuals: this input can help reveal the true natures of the varying experiences of the pandemic [Citation7].
In April 2020, the National Institute for Health Research published its position around PPIE in health research and innovation [Citation8]; “NIHR re-affirms its support for patient and public involvement, engagement, and participation during the COVID-19 pandemic.” As NIHR organisations this was something that both D4D and CYPMedTech fully supported and ensured our messaging was aligned, that we have a continued commitment to ensuring patients, carers and the public have a say in and help to shape health and care research during the COVID-19 pandemic, in line with the long standing commitment to patient and public involvement, engagement and participation (PIE) in health and care research.
Given the critical contribution of patient and public involvement, engagement and participation activities in medical technology innovation, it was imperative that we were able to find solutions for these activities to continue. In the case studies that follow multiple methodologies were adopted to allow patient engagement and involvement to continue. These case studies demonstrate the breadth of activities across the age spectrum to include participation of children and their families, adults and older adults as well as carers and wider members of the public.
During the pandemic, networking between organisations became even more valuable than usual and new networks were set up specifically to support professionals whose remit was to continue patient and public engagement and to advance projects – despite unprecedented circumstances. One of these new networks, the Co-Production collective, who were instrumental in the Co-Pro Covid and Beyond meetings [Citation9]. These were a huge help to NHS organisations who were speedily moving face to face consultations to an online medium.
The opportunity to share experiences online across the UK and also to try out new online tools for co-production (for example Google Jam, online polling and Miro tools) is immensely valuable for making changes in a way agile enough to enable continued progress for many projects.
Case examples
Dementia and co-design of devices and assistive technology (D4D)
This group is hugely underrepresented in partnership working in user evaluation – and particularly in engineering and technology development.
This is in spite of this population representing a large and growing proportion of society; currently there are around 55 million people living with dementia worldwide and this increases by 10 million cases each year [Citation10].
Most of these people and their families will require assistive equipment and technology in order to maintain independence and quality of life, to stay in their own homes and to access their local community for as long as possible. The physical, psychological, economic and social challenges experienced by people with dementia also affect their families, health and social care systems and society as a whole. It is perhaps as a result of the wide range of physical, cognitive and communicative challenges faced by this population that researchers, healthcare professionals, designers and engineers may be hesitant to involve people with lived experience as partners in technology development.
The World Health Organisation (WHO) Global Action Plan on the Public Health Response to Dementia (2017 − 2025) calls for development of innovative technologies which will “respond to the physical, psychological and social needs of people with dementia, their carers or people at risk of developing dementia” [Citation11]. Innovative design and engineering responses are required for aspects including earlier diagnosis, monitoring and assistive technology. WHO also calls for creation of more equitable opportunities for people with lived experience to be part of this development and for this development work to be increased significantly in settings of economic deprivation.
Co-designing digital technology equitably: PPI with ethnic minority groups (D4D)
Researchers from D4D, the University of Sheffield and commercial partners Therapy Box Ltd (https://therapy-box.co.uk/) are collaborating to further develop a digital doctor, CognoSpeak™ [Citation12], which will use artificial intelligence (AI), validated cognitive assessment stimulus questions and speech analysis to detect cognitive impairment. In order for novel treatments to be tested on populations at risk, technology is required to diagnose people in the prodromal or even preclinical stage of diseases that cause dementia. For example Mild Cognitive Impairment (MCI) is a diagnosis of memory complaints not sufficient to impair activities of daily living. People with MCI have a risk of approximately 50% of developing dementia within 5 years.
An advanced prototype has been developed with extensive involvement of people with dementia, their families and healthcare professionals. It will enable access to assessment online; at GP practices, community centres or in their own homes. This will help reduce waiting times and improve earlier access for families to appropriate medical and social support.
Earlier trials established that CognoSpeak™ is at least as reliable as the pen and paper assessments currently used by GPs. In 2020, the project team were ready to progress to working to ensure that the AI was as reliable with people who speak English with a regional accent, or as a result of speaking English as an additional language. A further schedule of face to face meetings in order to test out the device would have been the usual approach, however in the light of the pandemic, it became necessary to rethink how best to proceed.
PPI activity for other projects had been successfully moved to online methods, however achieving this task for groups with memory problems and possible dementia and reaching out to build new collaboration partnerships in ethnic communities across the city presented a particular area of challenge.
Using a small PPI grant award from the Sheffield Biomedical Research Centre, the project team were able to provide a laptop and internet connectivity for the Israac Community Centre based in Sheffield (israac.org.uk). We were then able to run test online meetings with collaborators at the centre and used these, plus phone calls and WhatsApp messaging to undertake collaboration around involvement from key stakeholders at Israac. Relationships built pre-pandemic enabled this transfer to online communications with existing partners, but also to incorporate new PPI collaborators and to work on a funding application which employs three of the Israac team on a part time basis to act as research champions within Somali and South Asian communities in Sheffield.
Consultation with the PPI partners at Israac has led to new network connections (again via social media) to other ethnic community action groups in Sheffield; Asiana (a South Asian women’s group), South Yorkshire Home Education Community and Deep End Yorkshire and Humber (a group of GPs working to reduce health inequalities in economically deprived areas of the city).
Now that new connections have been established in this way, further work will be undertaken in order to understand:
The experiences ethnic communities and people from economically deprived areas have had of the current assessment process for dementia
Issues of sensitivity and taboo around dementia in different ethnic groups
Perspectives on technology development, AI, data ownership and data sharing in these groups
Preferences for the visual appearance and voice of the digital doctor so that future users of the system will be able to choose who they would feel most comfortable interacting with.
Any culturally sensitive adjustments required to the phrasing of the questions
As we emerge from the pandemic, we will retain the use of social media as we discovered that this is the preferred channel of communication for many of these groups. We will continue to complement the online and social media channels we have established during lock down with a return to face to face meetings, in order to combine the reach and benefits of all approaches.
We have built into the technology trial design the processes necessary for recruitment and participation in user testing of CognoSpeak™ by email links, so that older adults with dementia who may still feel vulnerable post pandemic will be able to participate in the trial in their own homes, with family at hand for support of they would feel more comfortable to do so. Participants will be recruited to train the AI in interacting with people who have accented English, to ensure that the previously demonstrated reliability and sensitivity can be maintained in a wider range of the population.
Co-designing a new approach to evaluate fitness to drive: online PPI with people with dementia (D4D)
With life expectancy constantly increasing, the number of elderly drivers will increase proportionally. There has been a 30% overall increase in drivers over 70 years in the last 14 years [Citation13]. Increasing age and the challenges this can bring can impact negatively on safety to drive. This is particularly an issue for people who have neurodegenerative conditions.
Inability to drive contributes to loneliness, as it becomes difficult to maintain hobbies/activities (e.g., religious meetings, visiting friends and relatives, walking groups, library visits and many others) and the burden on carers to provide transport is increased. It has been linked also to health problems, institutionalisation and increased depression and death rates [Citation14].
Currently fitness to drive is assessed by a number of different methods; self-reports, medical reports that take into account factors such as short-term memory, orientation, attention control and decision making, which may or may not accurately reflect on road driving ability, and referral from a specialist or GP for on road driving assessment. There is a long waiting list for these assessments however and many families are confused and frustrated by the differing routes to assessment of fitness to drive.
A team of researchers from D4D, the University of Sheffield and commercial partner, The Floow (https://www.thefloow.com/) have secured funding for two complementary projects investigating driving behaviour in patients with Mild Cognitive Impairments and fitness to drive. The projects are funded respectively by UKRI Digital Health Catalyst, Economic and Social Research Council (ESRC) and by the Road Safety Trust.
We have mentioned the array of physical and cognitive challenges that people with dementia can experience. As we moved online during the pandemic in order to maintain progress on technology development and to keep patients, families and public contributors as partners in this, we developed additional support mechanisms to help older adults and those with a diagnosis of MCI or mild dementia to remain active partners in this process.
In order to reach out to contact older adults who might be interested in new approaches for driving assessment, we collaborated with Sheffield Carers Trust, Alzheimer’s Society, Age UK, Driving Mobility. We also published invitations to participate on websites such as NIHR People In Researchand VOICE Global in order to reach a broad and representative range of participants.
We contacted each older adult individually, by phone or email as each preferred, in order to explain what would be involved in an online focus group, which platform they may have access to, and what individual additional support they may require.
Two people opted to share their views outside of online meetings but of the remaining interested people, Zoom™ was identified as the most commonly used platform. We decided to limit the group size to a maximum of 8 per online group meeting, in order to avoid crowded screens or lack of visibility of some participants.
We developed publicity posters and two tailored pre-meeting information booklets: one for the Healthy Older Adults Meeting and one for the People with Lived Experience of Dementia Meeting which were posted out prior to the meeting.
The booklets explained how to use Zoom™, what the purpose of the online meetings would be and also shared the questions ahead of the call, in order to allow time to think and discuss with loved ones. Space was provided for notes as an aide memoire for the meeting. This was designed to avoid people feeling under pressure to “think on the spot” and also meant that anyone who could not take part on the day also had the option of sending in their views by post if they wished to.
We wanted to understand each of their perspectives and experiences on the current methods of fitness to drive assessment and their perceived benefits & concerns. We wanted to seek feedback on the trial study design and recruitment approaches, the study information materials, preferred formats for sharing study findings and also feedback on iterative design for user facing interfaces for the device platform and on barriers and facilitators to adoption and roll out.
The focus groups were very successful, with participants engaging actively and reporting a positive experience of the meetings and a wish to remain engaged as participants in future project activities.
The Stakeholder consultation strongly validated the need for, and potential benefits of the research study. Useful feedback was gained on the research design – many of these points can be incorporated into the design for the user interfaces that allow visualising data about driving behaviour.
We found that online participation did not hamper engagement, particularly with the extra measures taken ahead of the meetings in order to facilitate this. It also opened up the opportunity to participate across a wider geographical area than face to face meetings would have permitted. Thank you vouchers were provided as a gesture of thanks for participants’ valuable time and input and also to offset costs of broadband connectivity time.
As we move out of lock down, we aim to remain in contact with participants by both face to face and online methods, as preferred by individuals.
Listening to children and young people (CypMedTech)
CypMedTech are supported by GenerationR Liverpool Young Person’s Advisory Group (YPAG) through our PPI Theme Lead, Jenny Preston. GenerationR YPAG was established in 2006 at Alder Hey Children’s Hospital in Liverpool, as part of the then NIHR Medicines for Children Research Network (MCRN). Since its inception the group has supported, advised and guided multiple grants and applications in research and innovation. Prior to the COVID-19 pandemic, the group met face to face every six weeks. The direct impact of COVID-19 on children and young people (CYP) seems to be less severe than on adults, but indirect and hidden consequences are having a lasting effect. Many of the decisions taken over the pandemic have excluded the voices of CYP [Citation15]. While pubs, restaurants and non-essential shops opened, the majority of children were not able to attend school. CYP felt their voices and opinions had not been heard during the pandemic and indeed that major decisions had been taken about them, without them [Citation15]. The YoungMinds (2020) survey findings highlights the positive suggestions from CYP about what they feel would be best for them, confirming the vital importance of hearing and listening to them and acting on the solutions that they propose [Citation16].
GenerationR and CYPMedTech recognised this in the early stages of the pandemic and quickly and efficiently supported ways of listening and learning from CYP to resume involvement in the YPAG sessions and with other innovations related PPI groups within CYPMedTech. CYP were empowered to get involved in shaping research and innovation once more and were able to apply this expertise to the numerous COVID-19 studies that had requested support and involvement from the CYP.
Having identified suitable dates/times for meetings and assessing CYP support and accessibility the meetings were held through the Zoom™ platform. Where required training was also offered on installing and using Zoom™. Safeguarding considerations were applied throughout the online sessions and plans were tailored around CYP to ensure that they were supported in order to get the most out of the virtual meeting. Many CYP had adopted this approach having had their school lessons moved from face to face to online learning.
The first online meeting was a great success, the CYP were confident in using the Zoom™ platform and felt listened to and understood, the researchers presenting also felt that the outcomes were very positive and that they had chance to discuss their project and elicit robust feedback and input from the CYP. Attendance was high and the CYP were enthusiastic to attend future virtual meetings, the group were soon asked to rapidly review COVID-19 studies often with only 24 h notice. The CYP rose to the challenge and felt that their ideas and thoughts were valued, they helped to shape studies that would impact the lives of CYP globally during the pandemic.
Throughout the entirety of the situation, we overcame the online barriers of zoom™ and were still able to successfully help ongoing clinical studies regarding COVID-19 as well as many others. I loved how able we were to change and although there were a few complications, we were still able to make great progress for helping others. Anshul (YPAG member)
Different PPI approaches taken with children and young people during COVID-19
Sleep survey for CYP (CypMedTech)
CYP with Special Educational Needs (SEN) are more likely to experience disturbed sleep and poorer mental wellbeing [Citation17]. A study undertaken by CYPMedTech, Sheffield Children’s NHS Foundation Trust, and The Sleep Charity during the COVID-19 pandemic explored the differential impact of the pandemic on the sleep and mental wellbeing of CYP with and without SEN. The online survey was shared via social media platforms between June 2020 and August 2020. Online surveys became an important tool for COVID-19 based research when conventional study survey methods were not feasible [Citation18].
A total of 585 parents/carers completed the survey, this will have taken a fraction of the time and cost required to deliver a similar face to face research study. However, scientific rigour might be questioned with online health research studies, Andrade [Citation19] argues that “online surveys commonly suffer from two serious methodological limitations: the population to which they are distributed cannot be described, and respondents with biases may select themselves into the sample. Research is of value only when the findings from a sample can be generalised to a meaningful population”. While it is not in the scope of this paper to discuss the findings, the study suggests that, while the majority of CYP in both groups reported sleep changes due to the pandemic, CYP with SEN experienced more sleep disturbance, these findings are being used by The Sleep Charity and clinicians at Sheffield Children's NHS Foundation Trust to tailor care and support further for SEN CYP. Online surveys were increasing before COVID-19, and COVID-19 measures are only accelerating this trend—the invitations to complete an online survey will probably continue [Citation18]. This important study highlights how, by using the correct tools and approach, we can quickly and effectively gather data to help support health care delivery and policy. Clearly with the large numbers of respondents, people were keen to contribute and support research efforts in the early days of the COVID-19 pandemic.
Ostique PPI project (CypMedTech)
Ostique are a UK-based small/medium enterprise (SME) who are developing innovative, affordable stoma/ostomy appliances that outperform current products, overcoming their shortcomings by combining improved functionality with customisable aesthetics to improve quality of life. Inflammatory bowel disease, cancer and/or trauma can result in surgery where an artificial opening (stoma) is made in the abdomen. An ostomy bag is placed over the stoma, collecting stool, which people must constantly wear. Leakages, odours and skin reactions impairs peoples’ physical and psychosocial wellbeing. Many suffer loss of dignity and self-confidence with at least 25% experiencing anxiety, depression and suicidal thoughts [Citation20].
Ostique, CYPMedTech and clinicians from Sheffield Children's NHS Foundation Trust worked together to undertake PPI sessions with CYP and families to elicit their thoughts and feelings about their current stoma supplies, and their hopes for new ostomy products. Traditionally, this session would have taken place in person within a clinical space in the hospital, however an online video approach was taken due to the limits on social gatherings at the time, and to ensure that unnecessary visits to the hospital were reduced. The added benefits of this approach became apparent as the session planning and subsequent delivery unfolded:
Enabling families’ participation in their own homes may provide a ‘safe space’ for them to discuss potentially sensitive issues, with the knowledge that they can leave the session quickly and easily at any time if they wish. This was particularly useful for CYP, with the added benefit of being surrounded by their own toys or activities to support them in expressing themselves, and keeping entertained if they chose to sit out any of the activities.
Several barriers to participation were removed for families, for example logistical challenges (the time/expense of travelling, finding parking, locating the venue), time pressures (fitting in these events in between school, evening meals, bedtimes of children of various ages, and so on) and concurrent commitments (i.e., childcare for siblings).
The online approach significantly reduced costs for the project team (i.e., venue hire, refreshments, potentially covering participant’s travel costs), meaning that families could be involved in informing the scope of future research projects from the earliest point, prior to funding applications.
Whilst the above points could be applied to many projects, it is important to note that for this group, sub-optimal ostomy product performance may impair and/or reduce confidence in visiting unfamiliar places. As such, participating in the workshop from home removed this worry, as participants had access to their own bathroom, and all of their ostomy supplies, at all times.
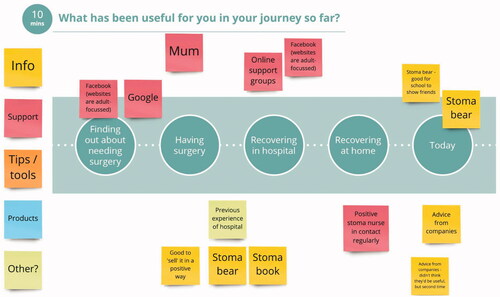
The families were asked to undertake some reflective activities at home prior to the session, including an online survey for parents, and a paper-based activity postal pack for the child (the latter were scanned and emailed back to the team prior to the group workshop). This approach was particularly important given the sensitive nature of the topic, and it allowed families to have more control as it allowed young children to share their thoughts and ideas without being put ‘on the spot’ in front of other families in the workshop, parents were able to share thoughts they may not wish to raise in front of their children. The approach was to harness the best elements of a face-to-face session with the best elements of an online session. Through our experience and expertise, we know that with young children, keeping the energy and engagement going is key ().
This approach also allowed those who couldn't attend or have the equipment to join the online session to complete the surveys and send their thoughts back to the research team.
In order to undertake this important PPI work, online was the only option available due to the COVID-19 pandemic, however it became apparent that this approach added value to the overall findings and gave the research team more information compared with undertaking the traditional face to face approach. The children and families were able to express themselves and share information about their lives to share vital information to support the development of new ostomy products ().
Discussion
As set out in the case studies it is evident that the increased use of digital platforms and online surveys has removed some of the long-standing barriers to patient and public involvement, engagement and participation. Significantly, these have been noted against costs and time. With the reduction in travel for participants the barriers around geography were removed, whether that be travel by car or public transport, which also had a positive effect on costs associated with travel to participate. In addition it also meant that involvement was less disruptive to people’s daily lives as they were able to engage from the comfort of their home environment. It is reassuring to see amongst our own work as well as others within the field of healthcare technology development that work has continued and research and innovation have still moved forward throughout the pandemic. Whilst the increased use of digital platforms has reduced many barriers, it has also had its challenges for those that are not digitally literate, nor have access to such digital platforms [Citation21] and this needs to be addressed now, in the post-COVID world.
It is clear from the significant amount of published research that during the COVID-19 pandemic, research and innovation in healthcare carried on and methods of interaction were constantly reviewed and changed in order to maximise participation for all. For both D4D and CYPMedTech, work on early MedTech development and identification of unmet needs never stopped and in fact increased. We adapted quickly and efficiently to continue with the same level of PPI across our portfolio of projects and activities.
As we look to a post-COVID world, it will be important to retain many of the practices across PPIE that have been successful and not simply return to ‘Business as usual’.
Conclusion
Perhaps most important of all, increased use of digital platforms has removed some of the long-standing barriers to patient and public engagement, such as geography, inaccessible environments and cost, and has enabled global conversations in a way that was not possible before. Enforced it may have been, but virtual working and digital connection have powerfully enhanced patient engagement, although there is a continued need to work in complementary ways with those who are digitally excluded, including those groups dealing with poverty and/or cognitive and communication challenges, without provision of additional support.
We believe that PPI is fundamental to the successful development and delivery of technologies for all. The involvement of children, young people, and their families as well as adults together with their carers ensures that technology is developed for and with the user, adopted more rapidly, and better accepted into clinical practice.
The events of 2020 and 2021 have allowed both the MICs to review the standard model of working with patients and the public. The research and innovation community, including patients and the public, must build on the lessons learned during COVID-19 to strengthen the foundation for patient and public involvement, engagement and participation in research and policy.
In the post-COVID world a blended model of virtual and face to face meetings will increase inclusion in research and innovation, which will have a positive impact on the PPI activities within both D4D and CYPMedTech. Our focus will be to take the positives of what we have learnt over the last 2 years and build this into our PPI model of the future.
Acknowledgments
The authors wish to acknowledge the project team members, users and stakeholders who have shared their time, views and experiences during the evolution and delivery of these projects. The research reported in this publication was supported by the National Institute for Health Research Children and Young People MedTech Co-operative and National Institute for Health Research Devices for Dignity MedTech Co-operative and Sheffield Biomedical Research Centre and The University of Sheffield. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- NIHR Centre for Engagement and Dissemination: UK standards for Public Involvement in Research: Better public involvement for better health and social care research. [Internet]. [cited 2021 Nov 13]. Available from: https://www.nihr.ac.uk/news/nihr-launches-new-centre-for-engagement-and-dissemination/24576.
- Hoddinott P, Pollock A, O'Cathain A, et al. How to incorporate patient and public perspectives into the design and conduct of research. F1000Res. 2018;7:752.
- Gray-Burrows KA, Willis TA, Foy R, et al. Role of patient and public involvement in implementation research: a consensus study. BMJ Qual Saf. 2018;27(10):858–864.
- Mihaly H. From NASA to EU: the evolution of the TRL scale in public sector innovation. Innov J. 2017;22:1–23.
- Cadel L, Marcinow M, Sandercock J, et al. A scoping review of patient engagement activities during COVID-19: more consultation, less partnership. PLoS One. 2021;16(9):e0257880.
- Murphy E, Tierney E, Ní Shé É, et al. COVID-19: Public and patient involvement, now more than ever. HRB Open Res. 2020;3:35.
- Marston C, Renedo A, Miles S. Community participation is crucial in a pandemic. Lancet. 2020;395(10238):1676–1678.
- NIHR: Reaffirms its support for patient and public involvement, engagement and participation during the COVID-19 pandemic. [Internet]. [cited 2021 Nov 13]. Available from: https://www.nihr.ac.uk/news/nihr-reaffirms-its-support-for-patient-and-public-involvement-engagement-and-participation-during-the-covid-19-pandemic/24641.
- UCL Co-Production Collective : co-producing change together. [Internet]. [cited 2021 Nov 3]. Available from: https://www.ucl.ac.uk/culture/projects/co-production-collective.
- World Health Organisation, Dementia Fact Sheet, 2021. [Internet]. [cited 2021 Nov 3]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/dementia.
- Global action plan on the public health response to dementia 2017–2025. World Health Organization, 2017. ISBN 978-92-4-151348-7
- CognoSpeak [Internet]. [cited 2021 Oct 29]. Available from: https://cognospeak.github.io/website/.
- UK Government, Older Car Drivers Fact Sheet [Internet]. [cited 2021 Oct 29]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/706517/older-car-drivers-factsheet.pdf.
- Chihuri S, Mielenz TJ, DiMaggio CJ, et al. Driving cessation and health outcomes in older adults. J Am Geriatr Soc. 2016;64(2):332–341.
- Association for Young People’s Health,Summarising what we know so far about the impact of Covid-19 on young people [Internet]. [cited 2021 Nov 4]. Available from: https://www.youngpeopleshealth.org.uk/wp-content/uploads/2021/02/Impact-of-Covid-19-on-young-people-briefing.pdf.
- YoungMinds. Impact of COVID-19 on children’s and young people’s mental health: Results of survey with parents and carers. [Internet]. [cited 2021 Nov 4]. Available from: https://youngminds.org.uk/.
- Elphick H, Howsley P, Mills N, et al. The impact of the COVID-19 pandemic on the sleep and mental wellbeing of children and young people with and without special educational needs. BMJ Open Respiratory Research. 2021;8(Suppl 1):A1–A31.
- Hlatshwako TG, Shah SJ, Kosana P, et al. Online health survey research during COVID-19. Lancet Digit Health. 2021;3(2):E76–e77.
- Andrade C. The limitations of online surveys. Indian J Psychol Med. 2020;42(6):575–576.
- Nicholas DB, Swan SR, Gerstle TJ, et al. Struggles, strengths, and strategies: an ethnographic study exploring the experiences of adolescents living with an ostomy. Health Quality Life Outcomes. 2008;6:114. 10.1186/1477-7525-6-114
- Watts G. COVID-19 and the digital divide in the UK. Lancet Digital Health. 2020;2(8):e395–396.