Abstract
The successful development and implementation of any healthcare technology requires input from multiple stakeholders including clinical leads, trust information technology directorates as well as project management and procurement. In this process however, a key stakeholder that is often overlooked is the patient.
This paper illustrates the crucial importance of patient involvement to avoid poor design and poor uptake of technology and subsequently poor health outcomes.
To highlight this, we share a case example evidencing involvement of people with lived experience of foot ulcers resulting from Diabetic foot neuropathy throughout identification of unmet technology needs, design requirements for the device and iterative device development and evaluation.
1. Introduction
In the UK, 4.9 million people have type 2 diabetes, of which are 85,000 undiagnosed, and 13.6 million people are at increased risk of developing the condition, persistently increasing the burden of the disease [Citation1]. The NHS spends £10 billion, or approximately 10% of its annual budget on diabetes, as people with diabetes are twice as likely to be admitted to hospital as the general population [Citation1].
Diabetes-related amputations have now reached an all-time high, with diabetic foot disease costing the NHS an estimated £580 million every year [Citation2]. Most amputations are preceded by foot ulceration which occurs in one in four people with diabetes over the course of their lifetime. Almost all people with foot ulcers have sensory loss secondary to diabetic peripheral neuropathy (nerve damage) [Citation2].
In England, diabetes leads to approximately 9600 major lower limb amputations every year or 26 every single day. People with diabetes related amputations also have an elevated risk of mortality (with a life expectancy of less than 3 years) [Citation3]. Most amputations can be prevented by simple foot care advice, regular podiatry, and prompt referral to multidisciplinary diabetic foot clinics when an ulcer develops.
According to NICE diabetes foot care guidelines, commissioners and service providers should ensure the following is in place [Citation4]:
A foot protection service for preventing diabetic foot problems, and for treating and managing diabetic foot problems in the community.
A multidisciplinary foot care service for managing diabetic foot problems in hospital and in the community that cannot be managed by the foot protection service. This may also be known as an interdisciplinary foot care service.
Robust protocols and clear local pathways for the continued and integrated care of people across all settings including emergency care and general practice. The protocols should set out the relationship between the foot protection service and the multidisciplinary foot care service.
Regular reviews of treatment and patient outcomes, in line with the National Diabetes Foot Care Audit [2015] [Citation4].
The challenges in achieving such standards and service delivery are multifaceted and include:
Employing a robust process to ensure all service providers are integrated as well as utilising technology to enable this.
Service bottleneck, e.g., secondary care at capacity whereas community services not being utilised to full potential.
Underutilisation of capacity building solutions: For example, telehealth models as well as poor patient adherence to treatments, or willingness of patients to use the service.
2. Current innovation management approaches
Technology solutions to support the diabetes foot care pathway are both vast and varied in their development (innovation cycle) as well as uptake (). The current COVID-19 pandemic has resulted in expansion of digital solutions and differentiating value propositions of each of the solutions can be a challenge for healthcare professionals. A widely accessible NHS platform could aid in providing clarity around available solutions however, this currently only exists in fragmented forms such as individual procurement hubs and NHS framework Agreements.
Figure 1. The innovation cycle. https://medium.com/@tonyokoro/the-innovation-cycle-vs-the-innovation-funnel-6d291ffa84c3 [2021 Oct 21].
![Figure 1. The innovation cycle. https://medium.com/@tonyokoro/the-innovation-cycle-vs-the-innovation-funnel-6d291ffa84c3 [2021 Oct 21].](/cms/asset/0f8371e9-f619-4ab8-8452-e45afb4fa5f9/ijmt_a_2089259_f0001_b.jpg)
Innovators can experience high levels of resistance when it comes to the initial uptake of their ideas and solutions (). There also exists a necessary approval and sign off process from identifying an unmet need through to imbedding the technology in any given care pathway. The role of an internal Project Manager is crucial in acting as a catalyst in such instances, ensuring the overall initiative runs on course. Lack of project management and ownership is one of the reasons why projects can risk not progressing [Citation5].
Figure 2. Diffusion of innovation theory. https://sphweb.bumc.bu.edu/otlt/mph-modules/sb/behavioralchangetheories/behavioralchangetheories4.html [2021 Oct 21].
![Figure 2. Diffusion of innovation theory. https://sphweb.bumc.bu.edu/otlt/mph-modules/sb/behavioralchangetheories/behavioralchangetheories4.html [2021 Oct 21].](/cms/asset/7a39c409-6340-4fe4-a998-5a74482a3543/ijmt_a_2089259_f0002_b.jpg)
Additional challenges in technology uptake also include cost of a solution, economies of scale and lack of funding. This, however, is being addressed to a greater extent than previously due to the push for telehealth models during COVID-19.
Stakeholder approval and sign off is imperative at each stage (), ensuring all parties are in agreement of their roles, ensuring that the project is complete and solution is delivered, ultimately addressing a potentially critical, unmet need.
Figure 3. Project cycle: innovation to implementation. A Dea. Opportunities and Challenges in Digital Healthcare for Devices for Dignity. Team presentation; 2021 Feb 22; England, UK.
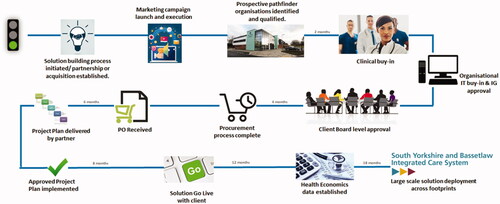
Looking at from a critical standpoint, a key challenge that has been reported is the lack of health technology uptake in patients; the technology is embedded into the care pathway, but patients are reluctant to take on. A fundamental reason for this is that many technologies are being developed in silos and without consulting patients, essentially reverse engineering them into patient care. Examples of this are seen by the NIHR Devices for Dignity MedTech Co-operative through collaboration requests from innovators in diabetic foot care. Often the support requested is geared around route to market with little to no patient and public involvement work done previously (PPI).
A key stakeholder missing in is the patient themself who would be able to articulate the unmet need from first-hand experience and optimise usability by being involved in iterative development from the earliest stages, throughout evaluation and into commercialisation and adoption. however is an example of the project cycle as in other cases patients are involved.
3. Methodology: embedding user involvement throughout technology innovation – illustration of the model used by devices for dignity
NIHR Devices for Dignity Med Tech Cooperative (D4D) embed engagement and involvement of people with lived experience throughout each of their projects. This approach is illustrated in and we will now work through his model illustrating the range of patient and public involvement activities at each stage.
Figure 4. Devices for Dignity Six Stages of Innovation Model, illustrating PPI activities and Technology Readiness Levels (TRL) www.devicesfordignity.org.uk.
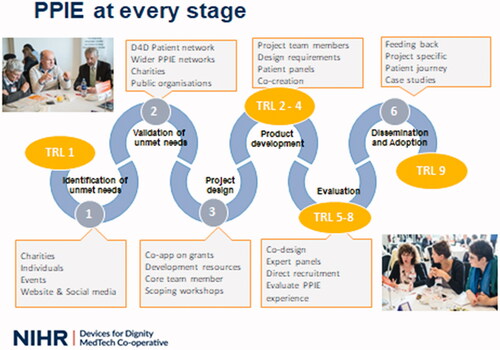
3.1. Stages 1 and 2: unmet need identification and validation
In contrast to prior approaches of designing technology within research or industry settings and then taking prototypes or completed products to people with lived experience for feedback, D4D commence involvement and engagement of patients and public (PPIE) at the earliest possible stage – by identifying what devices may be needed and what the user requirements for these should be in order to validate and prioritise project plans and to help strengthen bids for research funding. Validation and exploration of unmet needs can be undertaken in many ways – focus groups surveys or by large scale Unmet Needs events.
We share here an example of an Unmet Needs event held in June 2019, in Sheffield. NIHR Devices for Dignity MedTech Cooperative (D4D), Lab4Living (a design institute with Sheffield Hallam University and the Yorkshire and Humber Academic Health Sciences Network (AHSN) worked in partnership to run a Technology Unmet Needs Event to identify and explore unmet needs for people living with Type 1 and Type 2 Diabetes and to create project teams to develop solutions to address those needs. We will then illustrate how this foundation was progressed through project design and device evaluation stages of the development of the technology.
Attendees at the event included people living with and/or caring for people with diabetes, researchers, leading health care professionals, designers, digital technology experts and commissioners from localities across the UK. shows one of the tables of participants at the event, which was attended by 36 people, from all over the UK, including professionals from a wide range of disciplines and 8 people with lived experience. All attendees were adults, as this was a full day, with a busy and interactive agenda; therefore children’s needs were addressed by their parents acting as advocates.
During the first half of the workshop, via a series of small group co-creation activities (with bespoke tools), attendees built a shared holistic understanding of needs/impacts related to the lived experience of diabetes.
These and other activities were used to develop a holistic understanding of challenges experienced and shortfalls in the assistance currently available via currently available support systems, health education and health technology.
The James Lind Diabetes Unmet Needs were also considered which had been previously reported: Type 1 (May 2011) [Citation6] and Type 2 (Oct 2017) [Citation7]. These were combined with the newly developed challenges and refined into “Statements of Need” which attendees then ranked for priority. This Unmet Needs Event is described in detail by Langley et al. [Citation8] and in paper [McCarthy AD, Moody L, Reeves M, et al. Usability engineering in practice: developing an intervention for post-stroke therapy during a global pandemic. JMET. 2022.} of this Special Edition.
One of the most popular Unmet Need in technology for diabetes was: Maintaining motivation for self-management. Comments from attendees at the event included:
“Some people can be “complacent” or demotivated at times.”
“Life doesn’t move in straight lines, it has peaks and troughs', there is a need for tools to help sustain and/or help re-engagement with self-management.”
The second half of the workshop was used to identify ideas for potential solutions and/or discuss potential developing technology solutions. One of the priorities selected by voting was a device to support people to comply with advice about their foot health – in particular adhering to “foot offloading advice” – i.e. balancing time standing and walking with rest periods in order to avoid development of foot ulcers due to pressure on skin which has reduced sensation resulting from diabetic peripheral neuropathy.
Development of smart insoles was one of the potential solutions discussed and attendees were positive about this and selected this as a potential solution which may help them to maintain engagement with foot offloading advice from their clinicians and to embed this via adaptations within their daily routines as they would be able to receive reminders in real time. A participant mentioned that “we know what we have been advised to do but it can be difficult to put that into practice in everyday situations”.
3.2. User involvement throughout technology innovation: stages 3 and 4: project design and product development
Attendees at the workshop (people with lived experience of diabetes, clinicians, designers, engineers, public sector representatives) then worked in partnership to identify design requirements for development of the smart insoles. These included:
Use of memory foam for comfort
A discreet insole, which would be more acceptable than some of the currently available orthotic footwear, which are often considered unappealing and therefore not used
Sensors to measure temperature, pressure, shear, dampness, and activity
Potential to alert the wearer to potential problems and help with foot offloading
It would link with a smart phone or watch to give the user prompts in real time to support motivation to adhere to foot care advice
It may even include artificial intelligence, to learn individual’s activity and foot offloading behaviours, to give personalised prompts and support
D4D and the Diabetes Clinical Specialists at Sheffield Teaching Hospitals NHS FT (STH) and at the University of Sheffield then researched what technologies were being developed which met most of these requirements or whether it would be necessary to secure funding to develop a device.
Two published studies were identified which were using in-shoe pressure feedback technology; a validation study [Citation9] and a pilot prospective cohort study with people with history of diabetic foot ulcer [Citation10] however we found no research using devices in the management of active diabetic foot ulcers.
The clinical diabetes team at STH and D4D identified work byFeetMe®, a digital health company based in Paris, France who were developing a smart technology platform for in-clinic and real-world disease gait diagnostics, monitoring and rehabilitation for subjects with mobility impairments. They were developing a smart shoe insole which met the majority of the design requirements identified by the Unmet Needs Technology Event which would connect to a smartphone or smart watch to offer real time feedback to patients about their foot offloading behaviours and provide connectivity to healthcare professionals in their treating team. A decision was therefore made to use this prototype as a starting point with a view to exploring clinician and patient experience of its use and to base further iterative design improvements upon user evaluation. We therefore formed a partnership to undertake a Proof-of-Concept trial. This is currently work in progress and therefore, once the results of this trial are complete, we can build the user evaluation feedback into the future iterative development of the device and move forward into co-designing the user interface of the smartphone app. This work reflects the need to revisit parts of the innovation pipeline to develop other component parts supported by user engagement, prior to the whole insole system being evaluated.
3.3. User involvement throughout technology innovation: stage 5: evaluation
The trial of the FeetMe® Monitor Smart Insole system is the STRIDER project (Study of Pressure Sensing Insoles in Diabetic Foot Ulcer Recovery, STH20449).
The Lay ADvice for Diabetes and Endocrine Research (LADDER) Public Involvement Group at Sheffield Teaching Hospitals NHS FT then provided essential feedback on the STRIDER trial design and on the patient information resources.
The aims for this Proof-of-Concept clinical trial are:
To provide proof of concept that a new smart insole system (FeetMe®, SAS) which monitors plantar pressures and gait spatiotemporal parameters can be used in patients with diabetic foot ulcers in multidisciplinary diabetic foot clinics.
To evaluate the performance and reliability of the FeetMe® Smart Insole plantar pressure alert generating thresholds to confirm these are appropriate to promote ulcer healing.
Twenty patients will be assessed over a 12-week period in an open labelled study. We will also pilot key components and processes which will inform the design and implementation of a future main study. All patients will receive the FEETME Smart Insoles system fitted in orthotic shoes. Smartphone applications will provide a visual depiction of foot pressures in real-time. This information will be used to provide personalised education on effective foot pressure offloading for each patient and all components of usual wound care will be maintained throughout the study. This trial is currently underway, and results will be evaluated and published.
3.4. User involvement throughout technology innovation: stages 6: adoption and dissemination – future steps
As the Proof-of-Concept trial is still underway it is not yet possible to describe completed adoption and dissemination activity. Instead we shall therefore outline work being undertaken in order to prepare for these stages. The next important phase of this work will involve co-design of the user interface for the smart device.
This work reflects the need to revisit parts of the innovation pipeline to develop other component parts supported by user engagement, prior to the whole insole system being evaluated. This iterative development is common in the NIHR Devices for Dignity Innovation pathway, especially in cases such as this where there are a number of components parts to the overall device and helps ensure that the final product is as fit for purpose as possible. A range of examples of this approach can be found throughout this special edition.
After the proof of concept work on the smart insoles is completed, work will be undertaken to link the insoles with a digital phone application. In preparation for this, user consultation work has already been undertaken to identify the design requirements for this user interface. This will be crucial to achieve the overall aim of supporting adherence with foot offloading advice, by providing real time prompts in an accessible, easy to use way.
A group of eight public contributors with lived experience of foot ulcers were recruited by the study Principal Investigator, and D4D then conducted a series of semi-structured interviews with them – either face to face or by telephone according to individual preference. The group included six males and two females with ages ranging from 42–70 years. All public contributors had experience of foot ulcers and five of the group had current foot ulcers; three patients had no current skin concerns. The treating physician confirmed the group to be a representative sample.
3.3.1. Iterative development of the foot insoles and co-design of the patient interface on the app
Despite having had significant foot health issues, including amputation experience in 6 of the 8 cases, compliance with foot health advice was very variable. This further validated the need for tools to enable patients to self-manage foot offloading more effectively and consistently.
Reasons given for difficulty in being able to foot offload consistently included:
Not wearing prescribed orthotic boots/shoes due to negative impact on self- confidence due to the unacceptable appearance of the boots/shoes
Having job roles which entailed prolonged periods of standing/driving
Having leisure interests (e.g., hill walking) or other commitments (e.g. dog walking)
Lack of information on how much rest is required, and lack of feedback sensation in their feet
Not inspecting their feet regularly (prior to having their first ulcer) due to reduced awareness of health risks.
We gathered information on each public contributor’s foot health history, the advice they reported being given on foot care and foot offloading, the facilitators and challenges they experienced in complying with this advice and their perceptions and experience of using technology in other areas of their everyday lives.
We discussed options for:
The next iteration of the form of the shoe insoles,
The visual appearance of information to be shared on the app interface
The types of information they would like to receive and data display preferences (by sharing options of potential images to select from)
The frequency and type of reminder alert stimuli (for example, vibration, auditor tone, combined, snooze option etc.)
Whether they preferred feedback only to alert them when foot pressures approached critical thresholds, so that adaptive measures could be taken – or also positive feedback when positive behaviour change had been achieved
Data storage and sharing preferences
Technical support requirements for set up of the app and how this support could most helpfully be offered.
Based on the interviews with the public contributors, suggested improvements to the app can be summarised as follows:
Option to personalise the app at set up. Patients would like to be able to personalise some aspects, including:
Whether they receive positive feedback
Whether alerts are silent or have a tone
The option to snooze alerts if undertaking an activity preventing them from immediately taking appropriate foot offloading behaviour
Technical support. One patient has previously abandoned apps due to difficulties in downloading and setting it up. He felt it would make a significant difference to good adoption/use of the app
Navigating/ease of use of the app. Requirements were for a menu on opening the app so that patients can then select (with minimal button clicks) the type of information they were interested in at that time (but still be reminded of other aspects via the menu). This was preferred to the current system of scrolling down a series of pages
Simplicity of information. It was suggested that the app is kept “streamlined” – so not including pages which some felt might be extraneous – for example space to note questions to ask at the next appointment, or foot offloading advice, “I know what I should be doing – I just need reminders to actually do it”
Accessibility of information. Some of the example visual images were described as readily understandable – for example, the images relating to battery life of the insoles, others were felt to be ambiguous and so will be discarded/adapted.
All collaborators were enthusiastic about having the opportunity to offer this feedback and about receiving further information about the project. They all expressed interest in being involved with further project activity – for example prototype testing.
The information from this user consultation was shared with FeetMe® to inform the next stage of development of the technology.
4. Discussion and conclusions
We have described the D4D model we have developed to guide our patient and public involvement and engagement, illustrating this approach by working through a case illustration of validation and prioritisation of unmet needs, design requirement identification and prototype evaluation.
We hope that this evidences the need to commence user involvement at the very earliest technology readiness level, to achieve the necessary balance between technology development driven by what is possible and needs led development, driven by what public contributors find would add most value and impact. Given that health technology development is to a considerable extent publicly funded, it is an ethical imperative to consult future device users about its potential value.
The pandemic has highlighted concerns about the risks of digital exclusion [Citation11–13] of those who may not be able to afford technology and those who may have physical and/or cognitive challenges or economic restrictions which make it harder for them to access it without additional support. We are therefore collaborating with a broad range of ethnic community groups and charity organisations such as Ability Net to address these issues and facilitate access to laptops and tablets to help ensure that our efforts to develop health technology do not widen these inequities. This is an area which we acknowledge we have much further work still to do.
The case example shared here illustrates how it is possible to work with potential future users of technology in order to learn from them what they would require of a device in order to make it acceptable, useful and usable. The learning from the Unmet Needs Events helped identify and validate support for self management of their health as a priority concern and to validate selection of smart insoles as one of the potential solutions which they felt merited further investigation and development work.
Next steps will be to undertake further stakeholder consultation, both people with lived experience of diabetes and also health professionals in the development of the digital smart phone interface and in the usability for both groups as potential future users of the technology. We will also explore how best to fit the technology within existing clinical pathways and processes in order to facilitate adoption and uptake.
We have put forward a case illustration of the D4D model for clinician, designer, commercial and patient and public involvement. We feel that this stakeholder collaboration is crucial to development of technology which will optimise fitness for purpose and user acceptability and by doing so, support optimal health outcomes, by supporting better engagement with self-management, and better communication between patients and healthcare clinicians [Citation14,Citation15]. Finally, it also has potential to deliver cost savings for the NHS and for commercial partners, by reducing spending on unadopted technology and additional health and social care requirements [Citation16].
Acknowledgements
The authors wish to acknowledge the public contributors, staff and students, colleagues and the stakeholders who shared their valuable time, views, ideas, and experiences in the project described. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or FeetMe®.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- https://www.diabetes.org.uk/professionals/position-statements-reports/statisticshttps://pubmed.ncbi.nlm.nih.gov/28865844/. [cited 2021 Oct 10].
- https://www.kingsfund.org.uk/publications/what-are-health-inequalities. [cited 2021 Oct 21].
- Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the national health service in England. Diabet Med. 2014;31(12):1498–1504.
- https://www.nice.org.uk/guidance/ng19/resources/diabetic-foot-problems-prevention-and-management-pdf-1837279828933. [cited 2021 Oct 21].
- https://www.england.nhs.uk/improvement-hub/wp-content/uploads/sites/44/2017/11/Tips-for-Successful-Improvement-Projects.pdf. [cited 2022 Feb 2].
- James Lind Alliance Priority Setting Partnerships. [cited 2021 Nov 17]. https://www.jla.nihr.ac.uk/priority-setting-partnerships/diabetes-type-1/
- James Lind Alliance Priority Setting Partnerships. [cited 2021 Nov 17]. https://www.jla.nihr.ac.uk/priority-setting-partnerships/diabetes-type-2/
- Langley J, Wheeler G, Partridge R, Bec R, Wolstenholme D, Sproson L, editors. Designing with and for older people; intelligent systems reference library. vol 167. Cham (CH): Springer; 2020. p. 3–19.
- Razak AH, Zayegh A, Begg RK, et al. Foot plantar pressure measurement system: a review. Sensors (Basel). 2012;12(7):9884–9912.
- Najafi B, Ron E, Enriquez A, et al. Smarter sole survival: will neuropathic patients at high risk for ulceration use a smart insole-based foot protection system? J Diabet Sci Technol. 2017;11(4):702–713.
- Watts G. COVID-19 and the digital divide in the UK. Lancet Digit Health. 2020;2(8):e395–E396.
- https://www.england.nhs.uk/ltphimenu/digital-inclusion/digital-inclusion-in-health-and-care/ [2021 Nov 17].
- https://www.goodthingsfoundation.org/what-we-do/news/health-inequalities-and-digital-exclusion/ [2021 Nov 17].
- Clavelle JT. Leveraging technology to increase patient and family engagement and improve outcomes. Nurs Adm Q. 2018;42(3):246–253.
- Sawesi S, Rashrash M, Phalakornkule K, et al. The impact of information technology on patient engagement and health behavior change: a systematic review of the literature. JMIR Med Inform. 2016;4(1):e1. URL: https://medinform.jmir.org/2016/1/e: DOI:10.2196/medinform.4514
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5810684/. [cited 2022 Mar 3].

