Abstract
Obsolete organochlorine pesticides (OSPs) are currently prohibited as persistent organic pollutants that contaminate the environment. If undisposed, they continue to pollute soil and water, to accumulate in the food chain and to harm plants, animals and the human body. The aim of the study was to assess water and soil pollution around the storehouses of undisposed, banned OSPs and their possible genotoxic effect. The storehouses in four villages near Almaty, Kazakhstan were investigated. Chemical analysis confirmed contamination of water and soil around storehouses with OSPs. The genotoxic effect of water and soil samples was evaluated using model objects: S.typhymurium, D.melanogaster, sheep lymphocytes cultures and human lymphocytes cultures. It was found that water and soil samples caused mutagenic effect in all model systems. They increased the frequency of revertants in Salmonella, the frequency of lethal mutations in Drosophila chromosomes, and the frequency of chromosome aberrations in cultures of human and sheep lymphocytes. Although a genotoxic effect was demonstrated for each of these models, various models showed different sensitivity to the effects of pesticides and they varied degree of response. The association between the total content of OCPs in soil and the level of mutations for different model systems was discovered.
Introduction
It is known that many pesticides used in agriculture are toxic to humans and warmblooded animals. Among them, organochlorine pesticides (OCPs) are the most resistant to chemical and biological degradation. Persistent organic pollutants (POPs) are considered to be the most toxic among OCPs, which include aldrin, chlordane, dieldrin, endrin, heptachlor, DDT and its metabolites, keltan, α-HCH, β-HCH, γ-HCH. They can spread through the air or water over long distances from the place of their application. Various studies show global presence of pesticide pollution around the world. [Citation1–5] OCPs can pass along the food chain and accumulate in the adipose tissues of living organisms and humans. They harm human health and can cause cancer, cardiovascular, autoimmune and nervous diseases. [Citation6–9] OCPs can trigger disorders of reproductive and immune systems, and also affect the processes of individual development. The danger of OCPs is caused by their toxicity to non-target organisms, even at low concentrations. [Citation10–13] Besides, some initially nontoxic pesticides’ compounds can be transformed in soil into persistent toxic metabolites. Accumulating in the soil over a very long period, they can reach the toxic concentration.
OCPs are currently banned, however stockpiles of non-utilized banned pesticides are still available in many countries of the world. Tons of obsolete pesticides accumulate around the globe.[Citation14] Moreover, some countries still produce and use OCPs, for example, to fight malaria.
After the collapse of the Soviet Union in 1991 the regulation of obsolete pesticides was not properly established in Kazakhstan. This led to the storehouses with obsolete pesticides being abandoned, leaving obsolete pesticides and their containers unattended and open to the environment. Most of the obsolete pesticides were moved to other storage areas, taken by citizens for individual use, resold, or released into the surrounding environment with no indication of their potential danger to local residents. The majority of the resold pesticides were first repackaged in unlabeled or mislabeled containers. People living around the storehouses often use the surrounding land for pastures, allotments, and play areas for children, and as a source of construction materials. Kazakhstan signed the Stockholm Convention on POPs in 2001 and ratified the treaty in 2007. According to the International HCH & Pesticides Association, the exact quantity of obsolete pesticides in countries of the former Soviet Union, including Kazakhstan, was not adjusted and still varies widely. [Citation15] According to IPEN, Kazakhstan has stockpiles of obsolete pesticides, lots of equipment containing PCB (some of which is in exploitation), as well as PCB-contaminated territories. As of July 2012, about 6,931,440 tons of obsolete, prohibited and unusable pesticides were stored. [Citation16] Due to the violation of storage standards and damage to containers, the obsolete pesticides often form a mixture which contains OCPs.
This work is part of a comprehensive study to assess the impact of undisposed, banned pesticides on the genetic status and health of the population living in the Almaty region. 4 villages in the Talgar district of the Almaty region containing storage facilities of undisposed pesticides were investigated in this study. Many of the storehouses were destroyed. Due to inappropriate storage conditions, pesticides from destroyed storehouses get into the soil and water. This contaminated water can then be used to irrigate gardens and allotments. The soil pesticides get into plants grown both in household plots and in pastures. Some varieties of forage plants can accumulate organochlorine pesticides. When livestock is grazing in these areas, pesticides get into the bodies of domestic animals. In addition, animals can drink water from located nearby natural reservoirs, which are contaminated with pesticides. People consume milk and meat of these animals, which may also cause undesirable effects. This is how the territories of the former pesticide storehouses became the dangerous sources of toxic pollutants.
The objectives of our study were the following: to determine the OCP contamination level for the natural and drinking water and for the soil in the immediate vicinity of storage sites with undisposed pesticides; to evaluate their mutagenic effect using four different model systems: Salmonella typhimurium, Drosophila melanogaster, human and animal lymphocyte cultures; to assess the potential risk of pesticide contamination to human health.
Materials and methods
Studied sites
A total of four former storehouses were studied in the Talgar district of the Almaty region in Kazakhstan (Kyzylkairat, Beskainar, Belbulak, Amangeldy villages), where the obsolete pesticide burial sites were identified. All storehouses were either partially, or completely destroyed. The inventory included description of the state of storehouses; assessment of the main stocks and containers with obsolete pesticides and an inspection of storehouses and surrounding areas potentially contaminated with pesticides. 450 kg of obsolete pesticides were detected in the Talgar district (Amangeldy village). There were no obsolete pesticides in other storehouses. People lived near the storehouses, there was an active construction of new cottages, a plant for the production of animal feed and also greenhouses were found within the area. Local people grew vegetables and fruits. Animals (horses, donkeys, cows) grazed near the storehouses. Some people used former pesticide containers as drinking bowls for animals.
The Basshi village of the Kerbulak district was taken as a control site. According to official data of the Ministry of Ecology for the Republic of Kazakhstan, no pesticides were found in this region. In accordance with World Reference Base,[Citation17] the research soil belonged to the group of chernozem.
GPS coordinates of the studied sites are given in .
Table 1. GPS coordinates of the surveyed sites.
Soil, surface natural water and drinking tap water sampling were carried out in the studied sites.
The collected samples were used for chemical analysis to determine the concentration of OCPs, as well as to determine the genotoxic effect of the collected samples in different biological models.
Soil sampling
The soil sampling was done at a depth of 0,2 m using the envelope method, and soil was sifted through a sieve with a diameter of 2 mm and later through a 1 mm sieve. Then, the soil was thoroughly homogenized, air-dried and stored at 15 °C till the experiment.[Citation18]
Water sampling
Samples of drinking tap and surface natural water were collected in the studied sites. The drinking water samples were collected from a tap in each surveyed village located near the pesticide storehouse sites. This water was used for drinking and cooking. The surface natural water samples were collected from natural water basins located near the former storehouses (lakes in Kyzylkairat, Beskainar and Basshi. And rivers in other villages). Animals drank this water, and it was also used for watering gardens and allotments. In total, 15 samples of natural water, and 30 samples of drinking water were taken from each site.[Citation19]
Determination of OCPs in soil, drinking and natural water samples by GC-ECD
The chemical analysis of pesticide content in soil, drinking and natural water samples was carried out in a certified laboratory of the Scientific Analytical Center LLP (Accreditation certificate No.KZ.T.02.0926 from 08.24, 2018) in accordance with international and Kazakhstani standards.[Citation20,Citation21] Analysis of pesticide residues and their decay products was performed by capillary gas chromatography with an electron capture detector (gas chromatography Agilent Technologies 6890 N) equipped with the Combi-PAL autosampler (CTC Analytics AG, Switzerland). For quantitative determination of OCPs, calibration plots were obtained over the concentration range of 1 − 500 ng/L−1. Standard solutions of OCPs were analyzed using the GC-ECD method. The chromatography results were processed using MSD ChemStation software E.02.02 SP1.
Water and soil samples used for genotoxic effect evaluation were prepared as described below.
Preparation of water and soil samples for analysis in model test systems
For the Ames test the samples of natural and drinking water (500 mL) were evaporated by boiling to obtain a dry precipitate. The precipitate was then dissolved in 2 mL of DMSO.
For other short-term screening tests (SSTs) twice autoclaved water samples were used.
Benzene extracts from the soil samples were used for the Ames test and for the Drosophila tests. To prepare benzene extracts, 10 g of soil without extraneous inclusions was ground in a porcelain mortar, sifted through a sieve with a diameter of 0.5 mm, then added to 300 mL of benzene and left in a shaker for 6 hours. After that, the solution was filtered through a red stripe filter. Then it was dried in a vacuum evaporator at 40–50 °C. This precipitate was dissolved in 2 mL of DMSO and used for analysis.[Citation22]
Since the protocol for culturing lymphocytes does not allow the use of benzene extracts of soil, the aqueous extracts were prepared. For aqueous soil extracts preparation, 10 g of soil was mixed with 50 mL of distilled water and kept in the refrigerator for 10 days where it was periodically stirred. After this the aqueous fraction was collected, centrifuged and sterilized. Then 5 or 10% of each sample was added to the lymphocyte culture medium.
The estimation of genotoxic effects using model objects
To assess the genotoxic effect of contaminated samples, model organisms of different levels of biological organization were studied: S.typhymurium, D.melanogaster, sheep lymphocytes cultures and human lymphocytes cultures. Samples of soil, drinking and natural water were added to cultural medium for each system.
The estimation of mutagenic activity in the Ames test
The mutagenic activity was evaluated by the Salmonella-microsome assay without (−S9) metabolization using the pre-incubation method. [Citation23] Salmonella typhimurium tester strains TA98 and TA100 were used. In each experiment variant three Petry dishes were used. The experiment was set in 3 replications. The number of reverse mutations (reversions) from histidine auxotrophy to prototrophy was taken into account. The presence of mutagenic activity was judged by the excess of revertants above the level of spontaneous mutation: 2–10 times exceeding was classified as a weak effect, 10–100 times - moderate effect, 100–1000 times – strong effect.
Screening of Drosophila melanogaster recessive lethal mutations of the X chromosome and autosomes
The autoclaved water samples from monitoring points were added to standard medium at concentration 3%, 5%, and 10%. Benzene extracts of soil samples dissolved in DMSO were added to the medium at concentration 0.1%, 0.3% and 0.5%, as higher concentrations of DMSO are toxic to Drosophila. In control experiments, a saline solution of 1xPBS (130 mM NaCl, 7 mM Na2HPO4, 3 mM NaH2PO4, pH 7.0) was added to the medium instead of water samples or DMSO instead of soil samples at the same concentration values. Control without treatment was also used.
5 males and 5 females of the wild Oregon R flies were placed on the medium and progeny F0 from these flies were used in further experiments.
Screening of recessive sex-linked mutations in Drosophila was carried out according to conventional approaches using the double yellow attached-X strain, that is, two X chromosomes that share a single centromere containing linked X chromosomes.[Citation24]
In addition to lethal mutations, we calculated the percentage of pupae/larva deaths and sterility percentage of males (males were considered sterile in the absence of larvae for two weeks during which the nutrient medium was refreshed at least twice.
Screening of recessive lethal mutations in autosomes was carried out by conventional methods with authorial modifications based on the use of Cy/Pm; D/Sb balance line, allowing to record recessive lethal mutations in the second and the third autosomes simultaneously.[Citation25]
In order to determine whether the mutations were allelic (the mutation was the same) or non-allelic (different), an allelism test was performed. [Citation24]
Estimation of teratogenic and carcinogenic effects of water and soil samples
To determine a teratogenic effect (morphogenetic disorders in ontogenesis) all flies appeared in F0 were screened. All phenotypic changes were recorded, and the percentage of changes was calculated and compared with the control.
To elucidate the carcinogenicity of the analyzed samples a histological analysis was performed. The third-instar larvae from previously isolated lines of recessive lethal mutations were used. Histological slides were prepared according to a standard procedure. [Citation26]
Preparation of slides and cytogenetic analysis of sheep peripheral blood lymphocytes
Blood samples from healthy sheep were collected from an environmentally friendly zone (Mynbaevo settlement, Zhambyl district, Almaty region). Peripheral blood of animals was collected from the jugular vein using a vacuum system for blood collection into heparinized tubes and transported in closed cold containers. Heparinized blood samples were processed and cultured immediately upon the arrival to laboratory (no more than 24 hours after collection). The cultivation of lymphocytes and preparation of the slides was carried out according to the standard procedure. [Citation27] Cytological slides were stained according to Romanovsky-Giemsa. The stained chromosome preparations were analyzed using an oil immersion with the “Axioscop-40” microscope providing magnification 20 × 100. According to general recommendations, 300-600 metaphases were analyzed for each animal. All types of chromosome aberrations, detected during routine staining of chromosome plates were considered.
Preparation of slides and cytogenetic analysis of human peripheral blood lymphocytes
The studies were carried out according to ethical standards approved by the local ethical commission of the Kazakh-Russian Medical University (protocol No. 52 of September 5, 2017). Peripheral blood samples were collected from volunteers who signed the letters of consent.
To estimate the mutagenicity of drinking water samples the healthy donors from Almaty were used. Peripheral blood was collected using a vacuum system for blood collection into heparinized tubes and transported in closed cold containers. Heparinized blood samples were processed and cultured immediately upon arrival to laboratory (no more than 24 hours after collection). The cultivation of lymphocytes and preparation of the slides was carried out according to the standard procedure.[Citation28] Cytological slides were stained with 4% Giemsa solution. The stained chromosome plates were analyzed using an oil immersion with “Leica” and “Zeiss” microscopes (Germany), providing magnification 10 × 100. According to general recommendations, 300-500 metaphases were analyzed for each individual. All types of chromosome aberrations, detected during routine staining of chromosome plates, were considered.
Data analysis
Statistical analysis was conducted using STATISTICA 10 (StatSoft, Russia) software. Mathematically processed results were presented in the form M ± SE, where M was the arithmetic mean, S - standard mistake.
In tests with Drosophila, chi-square test was used to determine the significance of differences. When conducting a cytogenetic analysis of human lymphocytes, the following statistical values were indicated: average percentage of cells with chromosome aberrations, average error (mр), error of differences (md), significance of differences (tst) between two compared values by Student criterion, Pearson correlation coefficient. [Citation29] Statistical analysis, was performed using Microsoft Excel.
Results
Screening of contaminated sites and levels of soil pollution by OCPs
The article analyses the concentration of 17 organochlorine pesticides from the soil around the former pesticide storehouses (α-HCH, γ-HCH, β-HCH, δ-HCH, DDT, 4.4-DDE, 2.4-DDD, 4.4-DDD, keltan, endrin, chlordane; organochlorine insecticides - endosulfan, methoxychlor, heptachlor, heptachlorepoxide; organochlorine acaricides – chlorobenzilate). The total content of pesticides in the soil exceeded the maximum permissible concentration (MPC) for all selected samples. The main soil contaminants around the former storehouses were identified as DDT and its metabolites, β-HCH, aldrin, dieldrin, endrin and chlorobenzilate ().
Table 2. Residual organochlorine pesticides in soil around former pesticide storehouses.
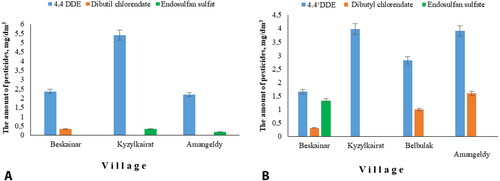
Analysis of pesticides in natural water, from sources located near the former pesticide storehouses showed that studied water samples had a highly toxic metabolite 4.4-DDE. The residual amount of metabolite 4.4-DDE in natural water varied from 2.4 ± 0.3 to 5.4 ± 0.9 mg/L−1. MPC for 4.4-DDE in water is 2 mg/L−1. The analysis of drinking water showed similar results. All studied samples contained a highly toxic metabolite 4.4-DDE. The residual amount of metabolite 4.4-DDE varied in drinking water from 1.7 ± 0.5 to 3.9 ± 1.0 mg/L−1. In addition to 4.4-DDE the analysis of drinking water samples also showed residual amounts of dibutylchlorendate and endosulfan sulfate in Beskainar village; dibutylchlorendate in Belbulak and Kyzylkairat villages; endosulfan sulfate in Amangeldy village. illustrates the residual level of pesticides in water samples.
Figure 1. The residual concentration of pesticides in water samples collected near the former pesticide’s storehouses: A – natural water; B – drinking water.
Thus, the analysis indicated that water and soil around the former chemical storehouses was contaminated with organochlorine pesticides. The concentration of OCPs in soil exceeded the MPC by tens to thousands of times and poses a danger to the environment and humans. The intensity of pesticides in the soil was alarming as they contaminated natural and drinking water, thereby getting into the food chain and having a negative impact on human health.
A study of the genotoxicity of organochlorine pesticides identified in the soil in natural and drinking water around the former pesticide storehouses
Short screening tests (SST) were conducted to estimate the mutagenic potential of water and soil samples, collected next to the banned undisposed OCPs storage places. The tests used the following models: Salmonella typhimurium, Drosophila melanogaster, sheep lymphocyte culture and human lymphocyte culture. The results for each model system are presented below.
Assessment of genotoxic potential using Ames test
The Ames test has been widely used to study mutagenic activity of different samples. Three types of controls were used: initial, positive and negative. The mutagenic effect was evaluated from the number of revertant colonies per plate. The results are presented in .
Table 3. Mutagenic activity of drinking water samples, natural water samples and soil samples by using the standard Ames method.
Samples from Basshi (control) showed the absence of mutagenic activity. Low mutagenic potency for water and soil samples from Kyzylkairat, Amangeldy and Belbulak was detected. Sample of natural water from. Beskainar also showed low mutagenic activity. The highest mutagenic activity was observed for natural water samples from Kyzylkairat (5.5 times) and Belbulak (5.1 times). In total, the Ames test showed low mutagenic activity for 10 out of 15 tested samples.
Assessment of genotoxic potential using Drosophila model
Four different tests using D.melanogaster were performed: screening of recessive lethal mutations in X-chromosome and in autosomes and the assessment of teratogenic and carcinogenic effects. Screening of recessive lethal mutations in X-chromosome of Drosophila revealed the occurrence of lethal mutations when using soil samples from Amangeldy (2 mutations) and drinking water samples from Belbulak (2 mutations). The greatest number of mutations was recorded when using all types of samples from Kyzylkairat (5 mutations). When using samples from other villages, lethal mutations were not registered. Control variants also didn’t show lethal mutations. Difference between experimental and control group was statistically unreliable. In addition to lethal mutations, effects on Drosophila ontogenesis, such as the induction of male sterility and increased death of pupae (more than 3%), was observed in these experiments. Increased death of pupae was observed for samples from Amangeldy. High sterility of males was recorded for soil samples from Beskainar, Kyzylkairat and natural water samples from Amangeldy. In the control variants, the death of pupae and the sterility of the males were low, with the exception of the soil sample at 0.5% concentration from Basshi (5.26%). This can be explained by the toxic effect of DMSO used for preparation of the soil extract. illustrates disorders such as death of pupae, sterility of males, frequency of autosomal lethal mutations, and frequency of morphoses recorded in Drosophila tests.
Table 4. Results of accounting for abnormalities in Drosophila under the influence of water and soil samples contaminated by pesticides.
The results of screening of recessive lethal mutations in Drosophila autosomes showed the statistically reliable increase of mutation rate for all investigated villages. Some variations were observed for different probe concentrations in nutrient medium. It should be noted that in some cases mutagenic effect had no direct dependence on sample concentration in the medium. The highest number of lethal mutations was registered for all types of samples (soil, drinking and natural water) from Amangeldy. When using 0.5% soil extract, significant differences were obtained only for the control without treatment, while for the DMSO 0.5% and Basshi 0.5% differences were unreliable. The high percentage of mutation in these control variants was believed to be caused by the DMSO toxicity. In total the moderate mutagenic effect to D. melanogaster autosomes was registered for 17 out of 48 tested experimental variants.
Assessment of teratogenic and carcinogenic effect
The screening of morphogenetic abnormalities in imagoes F0 revealed different morphological changes, with frequencies between 2.3 ± 0.9 and 14.1 ± 1.4% (). A high percentage of morphological changes was registered when using soil samples from Basshi. This was probably due to the toxic effect of DMSO, since the difference from the control with the corresponding DMSO concentrations turned out to be unreliable. The spectrum of wings changes included crumpled, shortened, spread, curved and unfolded wings, various folds and notches on the wings. The most commonly found were wing and tergit changes. When 3% of drinking water from Kyzylkairat was added to the nutrient medium, some changes in the structure of the eyes were registered: reduced eyes and notches of different shapes and sizes, as well as growths on the eyes ().
Figure 2. Morphological changes of imago. a- antenna, tv – tergit violation, wn - notch of wing, en – notch of eyeA-C – wing and tergit violations: А – spread wing and tergit violation 0.5% soil sample, Belbulak, 1 × 16; B – spread wing with notch, 0.5% soil sample Beskainar, 1 × 16; C – swollen wing, 3% drinking water sample, Amangeldy, 1 × 16. D-F – eye mutations: D – reduced eye, 3% natural water sample, Кyzylkairat, 1 × 32; E – eye with notch and antenna on the eye, 3% drinking water sample, Кyzylkairat, 1 × 32; F – eye with notch, 3% drinking water sample, Кyzylkairat, 1 × 32.
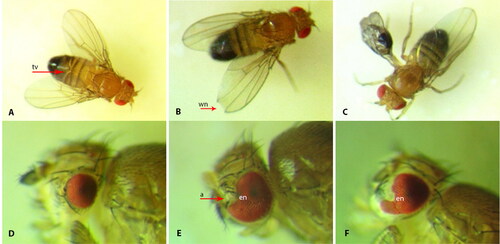
An analysis of the heritability of the identified morphological changes showed that the observed changes of wings and tergites were non-inherited, i.e., morphoses. However, eye changes were passed on from generation to generation and at that moment were showing heritability in thirty generations. It should be noted that in the other SSTs conducted by us earlier, all changes were non-inheritable, and their frequency was lower.[Citation30,Citation31]
Thus, we can say that OCPs increase the frequency of morphoses in imago Drosophila. Moreover, OCPs are chemical mutagens and can cause heritable changes - mutations in the model object D. melanogaster.
To analyze the carcinogenic properties of water and soil samples, a histological analysis of larvae, containing previously isolated autosomal recessive lethal mutations, was performed. As a result, no abnormalities were found in the structure of the larval tissues. Thus, water and soil samples from investigated villages did not cause a carcinogenic effect in Drosophila.
Assessment of mutagenic effect in sheep cell cultures
Sheep lymphocytes cultures were used as the next research model. Samples of natural water and aquatic extracts of soil were used in the experiments. In total, 7749 metaphase plates from five studied villages were analyzed. The frequency of cells with chromosomal aberration and genomic mutations was counted. The most commonly found were single and double fragments. Single rings and isolated dicentrics were rare. The results of the analysis are presented in .
Table 5. Evaluation of the mutagenic effect of natural water and aquatic soil extracts from different villages of Talgar district of Almaty region in sheep lymphocyte cultures.
Water samples from Belbulak, Beskainar and Kyzylkairat villages taken in 10% concentration, showed significantly increased (p < 0.003) frequency of chromosomal aberrations in sheep lymphocytes compared to the control. Water samples from Amangeldy and Basshi villages also showed increase in frequency of chromosomal aberrations by two times. However, the statistical differences did not reach the confidence level (p > 0.05).
10% soil extracts from all investigated villages, including Basshi led to significant increase of cells with chromosomal aberrations. The average frequency of chromosomal aberrations was 7.0 ± 1.3%.
When 5% of water or soil extracts was added to cultural medium, the frequencies of chromosome aberrations in lymphocytes decreased (4.7 ± 0.5% and 6.3 ± 1.2%, respectively). This decrease was not statistically significant.
During cytogenetic analysis, the frequency of tetraploid cells was also taken into account. Tetraploid cells were found in all variants of the experiment. The highest frequency of polyploid cells was observed when 5-10% of water from Beskainar was added to the cultural medium. This increase was statistically significant.
Assessment of mutagenic effect in human cell cultures
The human lymphocytes cultures were used as the last research model. Samples of drinking water and aquatic extracts of soil were used in the experiments. In total, about 7950 metaphase plates from five studied villages were analyzed. The frequency of cells with chromosomal aberrations and genomic mutations was counted. The most commonly found types of registered aberrations were single breaks or fragments. In rare cases, acentric rings and translocations were registered. The results of the analysis are presented in .
Table 6. Evaluation of the mutagenic effect of drinking water and aquatic soil extracts from different villages of Talgar district of Almaty region in human lymphocyte cultures.
Water samples from Amangeldy, taken in 10% concentration, showed significantly increased (P < 0.001) frequency of chromosomal aberrations in human lymphocytes compared to the control. Water samples from Belbulak and Beskainar showed increase in frequency of chromosomal abnormalities in human lymphocytes cultures by 2 times, from Basshi and Kyzylkairat – by 1.5 times compared to control. However, these differences did not reach the confidence level (p ≥ 0.1). The addition of 5% water from investigated villages revealed only a tendency of frequency increase for chromosomal aberrations in human lymphocytes cultures, since the differences with the control were not statistically significant (p ≥ 0.1).
When 10% of soil samples was added to the human lymphocytes cultures, the statistically significant mutagenicity was demonstrated for samples from Amangeldy and Belbulak. (P < 0.001). The aquatic extracts of soil samples from Basshi showed increase in frequency of chromosomal aberrations by 2 times compared to control (p ≥ 0.05).
Statistically increased frequency of polyploid cells was noted when using the 10% concentration soil samples from Beskainar and 10% water sample from Belbulak. This indicated a violation of mitosis, particularly the segmentation spindle violation.
Analysis of the chromosomal aberrations’ spectrum showed that there were aberrations of both chromosomal and chromatid types, which were mostly represented by single breaks or fragments. Acentric rings and translocations were registered rarely. Such a picture of structural damage in chromosomes is specific when exposed to chemical genotoxicants.
Discussion
Comparison of mutagenic effect according to the results of different test systems
Based on the research, a summary table demonstrating the presence or absence of a mutagenic effect in different model systems (see ) was compiled.
Table 7. Testing of water and soil samples for toxic effects using various model systems.
As it is shown in , water and soil samples polluted with POP were able to increase mutation rate in each model system. However, not all model systems responded equally to pesticide exposure, that is, they had different sensitivity to pesticides. For example, soil samples from Beskainar did not show mutagenicity in the Ames test and did not increase the frequency of lethal mutations in Drosophila chromosomes. However, they caused moderate teratogenic effect in Drosophila and strong mutagenic effect in sheep lymphocyte cultures, while in human lymphocyte cultures they showed only a tendency to increase the number of chromosome aberrations. Similar differences were registered for other samples. This suggests that there is no single method to assess the ability to induce different types of mutations. To assess the mutagenic properties of pesticides, it is necessary to use a set of methods with different test objects.
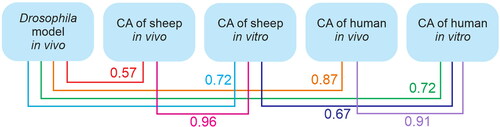
Correlation analysis between the results obtained from different test systems was carried out. A stable correlation was established between the soil samples’ testing results on Drosophila and cultures of human and sheep lymphocytes ().
Figure 3. Correlation between the results received in the studied villages from the different test systems.
Sensitivity studies and correlations between the test systems used to analyze the potential hazard of pesticides, are fundamental for accurate assessment of environmental risks and also for extrapolation of data for other non-target organisms, including humans. The sensitivity of the applied test systems differs and depends not only on their resolution. For example, in mice, carbosulfan caused increase in frequency of chromosomal abnormalities by 7 times (5 mg/kg body weight), it also caused increase in frequency of polychromatophilic erythrocytes with micronuclei by 3.5 times for the same dose. [Citation32] These results are consistent with the fact that for a single test system, mutagenic activity is detected in approximately 40-50% of the studied pesticides, while five test systems detect mutagenic activity in more than 90% of the studied pesticides. [Citation33] Therefore, there is no single method to unambiguously assess the genotoxic potential of the studied agent. It is necessary to use a set of methods performed on different test objects in vitro and in vivo (from microorganisms and higher plants to cell cultures of humans and animals). Despite correlation between some test objects, the use of different test systems is important for richer assessment of various aspects of the agent's action. This helps to get a complete overview of the agent’s effects and their severity.
Also of great importance is the composition of components for the substance and the joint use of various drugs, as is usually the case in practice. This often leads to the synergistic action of pesticides. Thus, deltamethrin and thiacloprid induced a significant increase in the frequency of micronuclei and chromosomal aberrations of rat bone marrow cells. Their combination caused an even greater genotoxic effect than each of them individually. [Citation34] Similarly, previously widely used pesticides - parathion-methyl and carbofuran did not exert a genotoxic effect on human keratinocyte cells in vitro as separate compounds. However, their combination caused a pronounced synergistic genotoxic effect when evaluated by the comet-test method. [Citation35]
In our studies, a synergistic effect also may take place, since several pesticides were found in all soil samples. A test system using models of different levels of organization is also preferable for evaluating the synergistic effect of a mixture containing different hazardous pesticides. This helped us to show that a multicomponent environment may not have a definite answer across all tests, however an effect may be more visible in one test or in another. And we believe that even if one of the tests has a mutagenic effect, it can be dangerous for humans.
Due to the synergistic action of the pesticides in the mixture we studied, it was not possible to determine exactly which of the pesticides plays a decisive role in the occurrence of the effects we observed. However, we believe that DDT and its metabolites DDE and DDD make the largest contribution, since their concentrations in our samples were exceeded by tens, and sometimes by hundreds of times. Even though enough time has passed (since the ban) for DDT to breakdown, it’s metabolites, DDE and DDD, also maintain environmental stability, high toxicity, accumulate in living organisms, possess the ability to biomagnify and exhibit mutagenic properties. [Citation36]
Problems related to the influence of pesticides are associated not only with their general toxic effect, but also with long-term consequences, the spectrum of which is constantly expanding in the scientific literature. In addition to mutagenic and carcinogenic effects, immunotoxic effects are also reported, [Citation37] as well as epigenetic changes.[Citation38,Citation39] At the same time, even micro doses of pesticides that do not exceed the MPC under chronic conditions suppress the vital functions of the body,[Citation40] and the absence of intoxication does not mean the absence of changes at a more subtle level.
The most dangerous long-term effect of unused organochlorine pesticides is the toxic effect on nearby biotopes, plants, animals and humans.
A study conducted in the same villages (by our colleagues) showed the accumulation of pesticides in plants such as cucumbers, tomatoes, bell peppers, apples and pears. The content of pesticides in animal products (meat, milk, honey) was also determined. A relationship between the content of pesticides in food and the level of chromosomal aberrations (CA) for the population of the studied villages was established. [Citation41] An increase in the level of chromosomal aberrations in the lymphocytes of sheep and cattle was also shown. [Citation42]
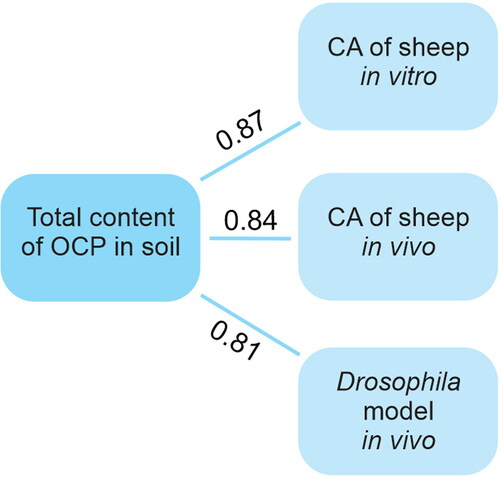
We analyzed the total content of pesticides in the soil of the studied sites and found a correlation with the level of mutations in Drosophila, as well as the level of chromosomal aberrations in the lymphocyte cultures of sheep in vitro and sheep kept in subsidiary farms in vivo [Citation42] (). A significant correlation between the level of CA in cultures of sheep lymphocytes and sheep in vivo was also found. In addition, a comparative analysis of our results for cultures of human lymphocytes in vitro with the human cytogenetic analysis results in vivo, obtained by our colleagues Djangalina et al.,[Citation41] was performed. Strong correlation was observed between CA level in vivo and in vitro ().
Figure 4. Correlation between the total content of pesticides in soil and results for the different test systems.
Thus, the use of model systems makes it possible to competently assess the mutagenic effect of contaminated samples and can be a prognostic criterion for assessing the risk of pesticide exposure to humans.
Conclusion
Thus, water and soil samples from the studied villages are contaminated with unused organochlorine pesticides. These pesticides have a genotoxic effect on model organisms of different levels of organization: Salmonella, Drosophila, human and sheep lymphocytes. Studies performed on model systems correlate with in vivo studies. These studies reflect the actual soil contamination from unused pesticides. The obtained results indicate the genotoxic effect of unused pesticides on the organisms of animals and people living in the immediate vicinity of the warehouses. These pesticides clearly pose a threat to their health.
Despite the OCPs not being used in agriculture for a long time, (more than 10 years) the former warehouses of the obsolete pesticides continue to be the sources of environmental pollution. They pose a threat to ecosystems, as they can migrate through soil and water into plants, animals and humans. It is therefore necessary to timely solve the problems of utilization of obsolete pesticides, and also to study the possibilities of soil and water rehabilitation at storage sites. In this regard, constant monitoring and strict control measures are necessary for hazardous substances.
To conclude we would like to encourage a thorough and comprehensive testing of new pesticides and their effects on various biological models. It cannot be overlooked that in future the currently used pesticides might be found harmful for the environment and living organisms, including humans. As we know, DDT was considered quite safe for humans in the middle of the 20th century and only at a later date additional research revealed its negative influence. This is why a preference should be given to biological methods of weed, pest and plant disease control in order to maintain an ecosystem balance and minimize any potentially dangerous impact of pesticides.
Acknowledgments
Studies were carried out as part of the programs "Development and application of new genomic technologies to protect organisms from mutagenic influence, increase the productivity of natural resources, and improve the quality of life of the population" and "Comprehensive assessment of the undisposed banned pesticides’ impact on the genetic status and health of the Almaty region’s population".
Additional information
Funding
References
- Li, C.; Yang, L.; Shi, M.; Liu, G. Persistent Organic Pollutants in Typical Lake Ecosystems. Ecotoxicol. Environ. Saf. 2019, 180, 668–678. DOI: https://doi.org/10.1016/j.ecoenv.2019.05.060.
- Chaiyarat, R.; Sookjam, C.; Eiam-Ampai, K.; Damrongphol, P. Bioaccumulation of Organochlorine Pesticides in the Liver of Birds from Boraphet Wetland, Thailand. Sci Asia 2014, 40, 198–203. 1513-1874.2014.40.198. DOI: https://doi.org/10.2306/scienceasia.
- Dhananjayan, V. Organochlorine Pesticides and Polychlorinated Biphenyls in Various Tissues of Water-Birds in Nalabana Bird Sanctuary, Chilika Lake, Orissa, India. Bull. Environ. Contam. Toxicol. 2012, 89, 197–201. DOI: https://doi.org/10.1007/s00128-012-0640-9.
- Musa, U.; Hati, S. S.; Adamu, Y. I.; Mustapha, A. Pesticides Residues in Smoked Fish Samples from North-Eastern Nigeria. J. Appl. Sci. 2010, 10, 975–980. DOI: https://doi.org/10.3923/jas.2010.975.
- Tsygankov, V. Organochlorine Pesticides in Pacific Salmon, Seabirds and Marine Mammals from the Okhotsk and the Bering Seas. Thesis for PhD, 2016. 21 p. DOI: https://doi.org/10.13140/RG.2.2.16050.81609/1.
- Mrema, E. J.; Rubino, F. M.; Colosio, C. Obsolete Pesticides – a Threat to Environment, Biodiversity and Human Health. In Environmental Security Assessment and Management of Obsolete Pesticides in Southeast Europe, NATO Science for Peace and Security. Series C: Environmental Security. Springer Science + Business Media: Dordrecht, 2013; pp 1–21.
- Bassil, K. L.; Vakil, C.; Sanborn, M.; Cole, D. C.; Kaur, J. S.; Kerr, K. J. Cancer Health Effects of Pesticides. Systematic Review. Can. Fam. Physician 2007, 53, 1704–1724.
- Mostafalou, S.; Abdollahi, M. Pesticides: An Update of Human Exposure and Toxicity. Arch. Toxicol. 2017, 91, 549–599. DOI: https://doi.org/10.1007/s00204-016-1849-x.
- Strbac, S. R.; Stolic, N. S.; Pucarevic, M. M.; Balic, B. S. Organochlorine Pesticides in the Tisza River (Serbia): Distribution and Risk Assessment. Matica Srpska. J. Nat. Sci. Novi Sad. 2019, 136, 113–122.
- Chen, S.; Edwards, C.; Subler, S. A Microcosm Approach for Evaluating the Effects of the Fungicides Benomyl and Captan on Soil Ecological Process and Plant Growth. Appl. Soil Ecol. 2001, 18, 69–82. DOI: https://doi.org/10.1016/S0929-1393(01)00135-4.
- Crecchio, C.; Curci, M.; Pizzigallo, M.; Ricciuti, P.; Ruggiero, P. Molecular Approaches to Investigate Herbicide-Induced Bacterial Community Changes in Soil Microcosms. Biol. Fertil. Soils 2001, 33, 460–466. DOI: https://doi.org/10.1007/s003740100352.
- Sagiv, S. K.; Thurston, S. W.; Bellinger, D. C.; Tolbert, P. E.; Altshul, L. M.; Korrick, S. A. Prenatal Organochlorine Exposure and Behaviors Associated with Attention Deficit Hyperactivity Disorder in School-Aged Children. Am. J. Epidemiol. 2010, 171, 593–601. DOI: https://doi.org/10.1093/aje/kwp427.
- UNEP. The hazardous Chemicals and Waste Conventions. WHO/UNEP/FAO; 2013. http://www.OCP’s.int/documents/background/hcwc.pdf.
- FAO. Baseline study on the problem of obsolete pesticides stocks. Viale delle Terme di Caracalla, Rome, 2001, 9. http://www.fao.org/docrep/003/x8639e/x8639e00.htm#TopOfPage.
- Weber, R.; Schlumpf, M.; Nakano, T.; Vijgen, J. The Need for Better Management and Control of POPs Stockpiles. Environ. Sci. Pollut. Res. Int. 2015, 22, 14385–14390. DOI: https://doi.org/10.1007/s11356-015-5162-7.
- Implementation of the Stockholm, Rotterdam and Basel conventions in Kazakhstan. 2013; pp. 53. http://www.greenwomen.kz/pdf/stok.pdf
- World Reference Base for Soil Resources. Update 2015. ISSN 0532-0488. 192 p.
- GOST ISO 11464-2015. Soil quality. Preliminary preparation of samples for physical and chemical analysis. 2015. 12 p.
- GOST 31861-2012. Water. General requirements for sampling. 2013. 36 p.
- ST RK 2131-2011. Soil quality. Determination of the content of organochlorine pesticides and polychlorinated biphenyls. Gas chromatographic method with an electron capture detector. 64 p.
- GOST 31858-2014. Drinking water. Method for determination of the content of organochlorine pesticides by gas-liquid chromatography. 16 p.
- Piotr, J.; Kołwzan, B. Genotoxicity of Soil Pollutants Extracted with Different Solvents. Pol. J. Environ. Stud. 2013, 22, 141–147.
- Maron, D. M.; Ames, B. N. Revised Methods for the Salmonella Mutagenicity Test. Mutat. Res. 1983, 113, 173–215. DOI: https://doi.org/10.1016/0165-1161(83)90010-9.
- Roberts, D. B., Eds. Drosophila in a Practical Approach; Oxford University Press: Oxford, New-York, Tokyo, 1998.
- Djansugurova, L. B.; Tazhin, O. T.; Bersimbaev, R. I. Workshop on the Drosophila Genetics Almaty. Kazakh University: Almaty, 1998. 25 p.
- Lillie, R. D. Histopathologic Technic and Practical Histochemistry; Blakiston Division, McGraw-Hill: New York, 1965.
- Sharipov, I. K. Mammalian Chromosome Analysis Methods. Kazakh University: Almaty, 1998.
- Moorhead, P. S.; Nowell, P. C.; Mellman, W. J.; Battips, D. M.; Hungerford, D. A. Chromosome Preparations of Leucocytes Cultured from Human Peripheral Blood. Exp. Cell Res. 1960, 20, 613–616. DOI: https://doi.org/10.1016/0014-4827(60)90138-5.
- Rokitsky, P. F. Introduction to Statistical Genetics; High School: Minsk, 1978.
- Mit, N. V.; Amirgalieva, A. S.; Zhapbasov, R.; Zh Tashenova, A.; Cherednichenko, O. G.; Baimukhamedova, M. Kh. Determination of the Genotoxic Potential of Priority Pollutants for Terrestrial and Aquatic Ecosystems in the Ili-Balkhash Region. Collection of Scientific Papers "Actual Issues of Ecology and Nature Management. 2012, 14, 168–176. Moscow: RUDN.
- Amirgalieva, A. S.; Begmanova, M. O.; Mit, N. V.; Djansugurova, L. B. Assessment of the Genotoxic Potential of Priority Pollutants in the Atyrau Region of the Caspian Region. Bull Tidings MES RK. A Series of Biological and Medical 2016, 6, 74–80.
- Giri, S.; Giri, A.; Sharma, G.; Prasad, S. Mutagenic Effect of Carbosulfan, a Carbamate Pesticide. Mutat. Res. 2002, 519, 75–82. DOI: https://doi.org/10.1016/j.fct.2009.09.041.
- Fedorov, L. A.; Yablokov, A. V. Pesticides - a Toxic Blow to the Biosphere and Human. Nauka: Moscow, 1999.
- Şekeroğlu, V.; Şekeroğlu, Z. A.; Kefelioğlu, H. Cytogenetic Effects of Commercial Formulations of Deltamethrin and/or Thiacloprid on Wistar Rat Bone Marrow Cells. Environ. Toxicol. 2013, 28, 524–531. DOI: https://doi.org/10.1002/tox.20746.
- Abhishek, A.; Ansari, N. G.; Shankhwar, S. N.; Jain, A.; Singh, V. In Vitro Toxicity Evaluation of Low Doses of Pesticides in Individual and Mixed Condition on Human Keratinocyte Cell Line. Bioinformation 2014, 10, 716–720. DOI: https://doi.org/10.6026/97320630010716.
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine Pesticides, Their Toxic Effects on Living Organisms and Their Fate in the Environment. Interdiscip. Toxicol. 2016, 9, 90–100. DOI: https://doi.org/10.1515/intox-2016-0012.
- Gerunov, T. V.; Redkin, Y. V.; Gerunova, L. K. Immunotoxicity of Pesticides: A Role in the Pathology of Animals and Humans. Succs Modern Biol. 2011, 131, 474–482.
- Chopra, D.; Tanzi, R. Supergenes: Unlock the Astonishing Power of Your DNA for Optimum Health and Well-Being. Harmony Books: New York, 2017.
- Lind, P. M.; Salihovic, S.; Lind, L. High Plasma Organochlorine Pesticide Levels Are Related to Increased Biological Age as Calculated by DNA Methylation Analysis. Environ. Int. 2018, 113, 109–113. DOI: https://doi.org/10.1016/j.envint.2018.01.019.
- Ilyushina, N. A.; Egorova, O. V.; Masal’Tsev, G. V.; Averyanova, N. S.; Revazova, Y. A. Mutagenecity and Carcinogenicity of Pesticides, Danger to Human Health. Jour. 2019, 61, 96–102. DOI: https://doi.org/10.18821/0044-197Х-2017-61-2-96-102.
- Djangalina, E.; Altynova, N.; Bakhtiyarova, Sh.; Kapysheva, U.; Zhaksymov, B.; Shadenova, E.; Baizhanov, M.; Sapargali, O.; Garshin, A.; Seisenbayeva, A.; Delannoy, M.; et al. Comprehensive Assessment of Unutilized and Obsolete Pesticides Impact on Genetic Status and Health of Population of Almaty Region. Ecotoxicol. Environ. Saf. 2020, 202, 110905. DOI: https://doi.org/10.1016/j.ecoenv.2020.110905.
- Zhapbasov, R.; Zhomartov, A. M.; K.Zh, D.; Kornilova, A. A.; Musabaeva, G. K.; Akhmetjan, S. T.; Djansugurova, L. B.; Nurzhanova, A. A.; Bekmanov, B. O. Guidelines for Cytogenetic Testing of Farm Animals for Genotoxicity of Unused and Prohibited Pesticides on the Territory of the Almaty Region. Kazakh university: Almaty, 2020.162 p.
