ABSTRACT
The United States has a small net-pen salmon industry dating back over 40 years and a nascent net-pen industry for other marine fish. The United States net-pen aquaculture sector has improved its resource efficiency in terms of the amount of fish meal and fish oil used in feeds and reduced its environmental impacts in terms of the mass loading and impact of nutrient discharge on the receiving ecosystem, the incidence and treatment of fish diseases, the use of antibiotics, and the number and impact of fish escapes, while increasing production. These changes can be attributed to a combination of advances in science and technology, rising cost of fish meal/oil, improved management, and informed regulatory practices. Net-pen aquaculture has become an efficient food production system. Existing laws and regulations in the United States effectively address most of the potential adverse environmental effects of net-pen aquaculture.
RESUMEN
Los Estados Unidos de Norteamérica (EE. UU.) poseen una pequeña industria de acuacultura de salmones mediante jaulas de red que data desde hace cuatro décadas y una incipiente industria de cultivo con jaulas de otras especies de peces marinos. El sector de la acuacultura con jaulas de red en los EE.UU. ha mejorado la eficiencia de sus recursos en términos de cantidad de carne y aceite de pescado utilizados para la engorda y en cuanto a la reducción de sus impactos negativos: el aumento de la producción versus la carga de masa y el impacto de la descarga de nutrientes en los ecosistemas receptores, la incidencia y tratamiento de enfermedades de peces, uso de antibióticos y el impacto del escape de peces. Estos cambios son atribuibles a la combinación de avances científicos y tecnológicos, el incremento en el costo de la carne y aceite de pescado, un mejor manejo y prácticas regulatorias informadas. La acuacultura con jaulas de red se ha convertido en un sistema eficiente de producción de alimentos. Las leyes y regulaciones existentes en los EE.UU. abordan de forma efectiva los efectos adversos potenciales de la acuacultura con jaulas de red.
INTRODUCTION
Aquaculture is likely to supply most of the projected increased need for seafood over the next few decades (CitationUnited Nations 2011; CitationFood and Agriculture Organization of the United Nations [FAO] 2012; CitationWorld Bank 2013). With available land and freshwater becoming scarce, marine aquaculture (finfish, shellfish, and seaweeds) will be an increasingly important contributor to the world's future food supply (CitationWorld Bank 2013; CitationOrganization for Economic Co-operation and Development [OECD]/FAO 2014). Aquaculture is well established in many countries and continues to grow worldwide (CitationFAO 2012). The United States is a global leader in aquaculture technologies and scientific advances (CitationNatale et al. 2012) but has a relatively small aquaculture industry (CitationNational Oceanic and Atmospheric Administration [NOAA] 2012; CitationWorld Bank 2013), providing less than 5% of the seafood consumed nationally (CitationNOAA 2012). We estimate that the U.S. net-pen salmon industry (Atlantic Salmon Salmo salar and steelhead Oncorhynchus mykiss) produced about 12,000 tons (live weight) in Maine (US$78 million) and around 8,000 tons in Washington State ($52 million) in 2010. In the same year, the United States also imported over 280,000 tons of farmed salmon (CitationNOAA 2012). We estimate that another 500–1,000 tons of various marine species were produced in net-pens from the remaining states ().
Marine finfish aquaculture in the United States represents an opportunity to provide healthy, domestic seafood (CitationMerino et al. 2012), create jobs, contribute to coastal economies, and help reduce the trade deficit (CitationNational Research Council [NRC] 1978; CitationRubino 2008; CitationKite-Powell et al. 2013). The United States has one of the largest areas of exclusive economic zone that is environmentally and economically suitable for net-pen culture (CitationKapetsky et al. 2013). Given this potential, why is the marine finfish aquaculture industry not expanding? The reasons may lie, in part, with environmental concerns expressed about the salmon net-pen aquaculture industry (CitationNaylor et al. 1998, Citation2000; CitationNaylor and Burke 2005). Specific issues include impacts on water quality (CitationBoyd et al. 2007), degradation of the seafloor under net-pens (CitationBridger and Costa-Pierce 2003; CitationBeveridge 2004), the effect of fish escapes on the genetic diversity of wild populations (CitationWaples et al. 2012), the sustainability of using fish meal and fish oil for feeds (CitationNaylor et al. 1998, Citation2000; CitationAdler et al. 2008), the use of antibiotics (CitationSmith and Samuelsen 1996), and the potential transfer of diseases from farmed to wild populations (CitationJohansen et al. 2011). These concerns have been widely publicized beyond the scientific community (CitationKnapp et al. 2007; CitationBaron 2010; CitationKnapp 2012) and generate negative public perceptions that, in turn, reduce social acceptance for many types of aquaculture (CitationMoffitt 2006; CitationAmberg and Hall 2008; CitationMazur and Curtis 2008). Once established, negative public preconceptions may overshadow recognition of the progress made in the netpen fish farming industry and other forms of aquaculture. A lack of social acceptance hinders efforts to simplify a complex and uncertain regulatory process (CitationGibbs 2009; CitationChu et al. 2010). In turn, regulatory and economic barriers to entry (e.g., onerous, lengthy, and uncertain permitting; high costs of coastal land, labor, and other inputs) reduce the ability of the United States to compete in the global farmed seafood market (CitationKite-Powell et al. 2013).
The last 40+ years have seen significant advances in fish farming technology and management practices focused on decreasing the environmental footprint and increasing economic performance (CitationKaiser and Stead 2002; CitationTveterås 2002; CitationAsche 2008). Regulations have been developed to set performance standards in all jurisdictions of the United States where net-pen aquaculture occurs (see Box 1 and ). Numerous organizations have developed purchasing policies, standards, and labeling programs that promote responsible aquaculture, creating financial incentives for producers to improve practices and become part of the responsible aquaculture movement (CitationBoyd et al. 2007). How do these pressures translate to impacts of netpen farming?
This article examines the current resource efficiency and environmental performance of U.S. marine net-pen finfish farming, considering the roles that administrative controls (regulation, economic, and management) and structural controls (science and technology) play in shaping a sustainable industry (CitationBoyd et al. 2007; CitationBelle and Nash 2008). We discuss issues related to feed, water quality and benthic effects, animal health, and potential genetic effects of fish escapes.
FEED AND FEEDING
The use of fish meal and fish oil in aquaculture feeds has been highlighted as a major sustainability issue and a limitation to the growth of carnivorous species aquaculture (CitationNaylor et al. 1998, Citation2000; CitationKristoffersson and Anderson 2006). Yet raising fish, including carnivores, has efficiency advantages over terrestrial animals (see Box 2), and no animal has a nutritional requirement for fish meal or fish oil (CitationNRC 1983, Citation1984, Citation2011). Further, formulated feeds with no fish meal or fish oil have been used experimentally to feed farmed Atlantic Salmon, resulting in growth and survival similar to those obtained with feeds containing fish meal and fish oil (CitationTorstensen et al. 2008; CitationBurr et al. 2012). The same is true of Rainbow Trout Oncorhynchus mykiss (K. J. CitationLee et al. 2002; CitationBarrows et al. 2007; CitationGaylord et al. 2007), Red Sea Bream Pagrus major (CitationTakagi et al. 2000), Grouper Cromileptes altivelis (CitationShapawi et al. 2007), White Sea Bass Atractoscion nobilis (CitationTrushenski et al. 2013), Cobia Rachycentron canadum (CitationWatson et al. 2012, Citation2013), and Pacific Whiteleg Shrimp Litopenaeus vannamei (CitationSookying 2010; CitationOlmos et al. 2011). Modern fish feeds are formulated from a variety of ingredients in carefully determined proportions to provide a balanced mix of essential nutrients and energy at the lowest practical cost (CitationHardy and Barrows 2002; CitationNRC 2011). Sources for these nutrients and energy are not limited to fish meal and fish oil, nor are there essential nutrients unique to fish meal or fish oil (CitationGatlin et al. 2007; CitationBarrows et al. 2008; CitationNRC 2011).
Traditionally, fish feeds have contained a high percentage of fish meal and fish oil because these ingredients provided a cost-effective means to satisfy the nutritional requirements of fish (CitationHardy and Barrows 2002). The balance of nutrients in fish meal and fish oil closely resembles and fulfills most nutritional requirements of fish with very few antinutritional factors (compounds that negatively impact the nutritional value of the feed). Alternative nutrient sources typically need to be treated, blended, and/or supplemented to adjust for missing nutrients, improve palatability, or remove antinutrients (CitationGatlin et al. 2007; CitationBarrows et al. 2008). Partial or total replacement of fish meal and fish oil in fish feeds is fast becoming the norm, but the research to develop and the effort to apply these modifications adds cost to the feed and requires investment in research, processing, and infrastructure (CitationGatlin et al. 2007; CitationBarrows et al. 2008; CitationNaylor et al. 2009).
Over the past several decades, the supply of fish meal and oil coming from targeted fisheries has been more or less constant, whereas fed aquaculture has increased (See Box 3). The proportion of the world's supply of fish meal and fish oil going into aquaculture feeds increased by displacing use from terrestrial animal agriculture until it consumed an estimated 68% of world fish meal and 88% of world fish oil in 2005 (CitationTacon and Metian 2008, Citation2009; CitationFAO 2011). However, by 2008, the amount of fish meal in aquaculture had fallen 13% from 2005 (CitationFAO 2011; CitationInternational Fish meal and Fish oil Organization 2013). Some stocks previously fished for producing fish meal and oil are increasingly being redirected toward human consumption (CitationJackson 2012; CitationOECD/FAO 2014). Likewise, fish oil is increasingly being used as a human dietary supplement (CitationTacon and Metian 2009; CitationFAO 2012; CitationJackson 2012). CitationTacon et al. (2011) and CitationJackson (2012) predicted that the percentage and the absolute amount of fish meal and fish oil consumed by aquaculture will continue to decrease as they become a smaller component of fish feeds, largely due to the development of lower cost alternative sources of protein (CitationGatlin et al. 2007; CitationBarrows et al. 2008) and oil (CitationRust et al. 2011; CitationRuiz-Lopez et al. 2014). Similarly, CitationTorrissen et al. (2011) reported that the Norwegian salmon farming industry has dramatically reduced the content of fish meal and fish oil in salmon feeds from >60% to <25% of the diet, largely by replacement with plant proteins and oils. Use of fish meal and fish oil in aquaculture has responded to the economic realities of the past few decades (see Box 3 and ), with increasing price differentials between fish meal/oil and other protein/oil sources leading to development of substitutes. Use of these substitutes is causing a decoupling of fed aquaculture and the harvest of stocks for fish meal and oil. Development of ingredient choice continues to be one of the most active areas of research in aquaculture nutrition.
BOX 1. Regulatory Requirements for U.S. Net-Pen Aquaculture
Multiple U.S. federal, state, and tribal government agencies regulate marine fish farms. Although aquaculture permitting processes can be complex and lengthy, federal and state local laws and regulations provide a comprehensive suite of requirements to address the environmental effects of fish farms outlined in this article. lists the federal laws that apply to environmental sustainability of marine net-pen aquaculture in the United States and the agencies responsible for their implementation. State governments often impose requirements that are more stringent than these federal requirements.
For net-pen aquaculture, the key federal permits related to the issues discussed in this article are issued by the U.S. Army Corps of Engineers (USACE) to authorize the placement of structures in navigable waters and by the U.S. Environmental Protection Agency (USEPA) to authorize discharges into the environment. These permits are typically issued in coordination with state agencies; however, in the case of National Pollutant Discharge Elimination System (NPDES) permits, the USEPA vests the states with the authority to issue permits in state waters in accordance with the Clean Water Act. Before issuing permits, the USACE and USEPA are required to consult with the National Marine Fisheries Service (NMFS) and/or with the U.S. Fish and Wildlife Service on issues related to protection of habitat, endangered species, and marine mammals. Aquaculture operations must also comply with permitting, monitoring, and reporting requirements for aquatic animal health under regulations of the U.S. Department of Agriculture's APHIS. Regulations pertaining to chemical application require permits from the USEPA, whereas aquatic animal drugs and feed manufacture require approvals from the U.S. Food and Drug Administration (CitationUSFDA 2014). Fish feeds and ingredients are regulated for safety by the USFDA under the Federal Food, Drug, and Cosmetic Act and the Food Safety Modernization Act. The USFDA requires animal feed to be “pure and wholesome, to be produced under sanitary conditions, to contain no harmful substances, and to be truthfully labeled.” Only approved ingredients can be used in animal feeds, and feed mills have to follow quality control plans. To be approved by the USFDA for use in animal feeds, ingredients must demonstrate utility and safety to both the target animal (fish) and to the humans consuming them. Harvest levels of fish species used in making fish meal and fish oil in the United States are determined by fishery management regulations under the provisions of the Magnuson-Stevens Fishery Conservation and Management Act and state laws. Fact sheets on all of these federal laws as they relate to aquaculture can be found at websites run by the Fish Culture Section of the CitationAmerican Fisheries Society (2013) and the CitationNational Association of State Aquaculture Coordinators (2013).
Currently, all commercial net-pen aquaculture production takes place in state waters. Commercial salmon net-pen farming is well established in Maine and Washington, which have correspondingly well-developed regulatory programs to authorize and oversee these operations. For example, Washington State laws and regulations specific to marine aquaculture give the Washington Department of Ecology and Washington Department of Fish and Wildlife regulatory authority over marine net-pens, disease, fish transfer, escapement, and best management practices (Lori LeVander, Washington Department of Ecology, personal communication). Hawaii has been authorizing and overseeing commercial-scale operations using submerged net-pens for more than 10 years. New Hampshire has done the same with smaller-scale research facilities, and a commercial facility recently started operations in New York. As interest in commercial production of finfish in marine waters expands, it is likely that additional states will become more actively engaged in the regulation of the net-pen aquaculture industry. In addition, NOAA is preparing regulations for a Fishery Management Plan for offshore aquaculture in the Gulf of Mexico, designed to allow NOAA to issue permits for finfish aquaculture of managed species in federal waters. Other regional fishery management councils may adopt similar plans, which would result in additional federal rules to regulate fish farming in additional regions.
BOX 2. Relative Efficiency of Aquatic and Terrestrial Animals
Farmed fish are more efficient protein and energy converters than terrestrial livestock (CitationBartley et al. 2007; CitationBrooks 2007). This is because fish generally do not use energy to maintain body temperature and they do not need to support their own weight against gravity (R. R. CitationSmith et al. 1978; CitationTalbot 1993). Fish also invest less energy and body mass in a skeletal system compared to terrestrial animals (CitationMoffitt 2006). CitationSmil (2002) compared the protein efficiencies (the amount of protein in the product/protein fed × 100) of different farmed animals and found that carp had higher protein conversion efficiency (30%) than land animals (5% for beef, 13% for pork, and 25% for chicken). Salmon, trout, and other carnivorous fish have protein conversion rates that can range between 30% and 50%, depending on diet and other conditions (CitationRefstie et al. 2004; CitationSoto et al. 2007).
U.S. consumers often prefer boneless meat and fish products. Because fish have relatively small skeletal systems, they have a higher percentage of edible portions than animals with larger skeletal systems. For example, as much as 68% of the weight of farmed salmon is edible compared to about 44% in cows, 52% in pigs, 46% in chicken, and 38% in sheep (CitationBjørkli 2002; CitationBrooks 2007; CitationHall et al. 2011). CitationTorrissen et al. (2011) suggested that Atlantic Salmon could be among the most efficient domesticated farm animals because 100 kg of dry feed yields 65 kg of boneless salmon fillets, compared to only 20 kg of edible product from poultry or 12 kg from pork.
BOX 3. Fish Meal Supply and Demand
The world's annual supply of fish meal and fish oil has averaged 4 to 5 million metric tons of meal and around 1+ million metric tons of oil for the last 20 years (CitationInternational Fish Meal and Fish Oil Organization 2013). Of these total quantities, currently, about 70% originates from “reduction” fisheries targeted at small, wild pelagic fish, such as sardine, anchovy, menhaden, and capelin. The remainder originates from processing wastes from both wild and farmed fish (CitationJackson 2012; CitationFAO 2012; CitationOECD/FAO 2014). Stocks historically used for reduction fisheries are more and more being used for human consumption, and processing wastes that were historically discarded are now being used for fish meal and oil production (CitationJackson 2012; CitationWorld Bank 2013; CitationOECD/FAO 2014).
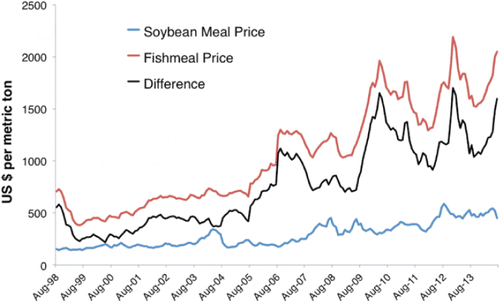
Increased demand with fixed supply caused prices of fish meal () and fish oil to increase dramatically over the last decade (CitationAdler et al. 2008; CitationJackson 2012; CitationOECD/FAO 2014). This increasing cost differential relative to other protein and oil sources spurred development of replacements for fish meal and fish oil in aquaculture feeds (CitationGatlin et al. 2007; CitationTacon and Metian 2009; CitationTacon et al. 2011) and a greater recovery of fish trimmings from aquaculture and wild captured seafood (CitationShepard et al. 2005; CitationJackson 2012; CitationOECD/FAO 2014). Prior to 2004, the price of fish meal was less than US$1,000/ton and was closely constrained by the prices of substitute proteins. After mid-2006, fish meal prices increased to $1,000–$1,500/ton, and by late 2009, they had further increased to $1,500–$1,800/ton. In 2012, for the first time, they peaked above $2,000/ton and were at $2,400/ton at the end of 2014. In comparison, during this same period, soybean meal, a leading substitute protein, increased from about $200 to a peak at $550/ton before settling down around $500/ton, widening the price gap between the two protein sources from less than $500/ton prior to 2004 to $1,000–$1,500/ton by 2009. Since 2002, the cost gap between soy protein and fish meal has increased almost fourfold. This provided the financial incentive to justify spending for the extra processing and supplementation needed to use increased amounts of alternative proteins and oils in fish feeds. Because feed accounts for more than 50% of the total operating costs in net-pen aquaculture and ingredients account for about 70% of the cost of making feed, there are strong economic incentives to use the most cost-effective mix of ingredients.
Figure 2. Cost in U.S. dollars per metric ton of 65% crude protein fish meal (Peru), 48% crude protein soybean meal (United States), and the difference between the two. Source: www.indexmundi.com (August 2014)
NUTRIENT IMPACTS TO WATER QUALITY AND BENTHOS
Deleterious effects to water quality and the benthos around net-pen fish farms can occur when nutrient inputs exceed the physical, chemical, and biological capacity of the ecosystem to assimilate them (CitationPearson and Rosenberg 1978). Excess organic nutrients and suspended solids can lead to eutrophication and sedimentation in receiving waters (CitationBoyd et al. 2007). Uneaten feed and fish wastes are the main sources of excess organic nutrients from net-pens. Because nutrients are discharged directly to the ocean, effluent treatment is not feasible. Instead, farms seek to manage nutrient waste with farm practices, efficient feeds and feeding (), optimal pen configurations and farm orientation in order to optimize fish growth, waste distribution, and nutrient assimilation by the food web. Modeling interactions between farm production and environmental processes can guide decisions about sustainable farming (CitationAguilar-Manjarrez et al. 2010) and prevent exceeding the site's ecological carrying capacity.
Water Quality
Impacts to water quality at farm sites, including increased nitrogen, phosphorus, lipids, and turbidity, or oxygen depletion, have lessened significantly over the last 20 years (CitationSoto and Norambuena 2004; CitationMcKinnon et al. 2008; CitationPrice and Morris 2013). These improvements are attributable to a combination of better understanding of siting requirements, improved feeding, better feed formulation, and better farm management practices. Good management practices include siting farms in well-flushed areas with adequate current (mean of >7 cm/s) and depth (CitationBelle and Nash 2008). When net-pens are properly sited, water quality impacts are typically not detectable beyond 30 m from the pens (CitationMantzavrakos et al. 2005; CitationNash et al. 2005; CitationTlusty et al. 2005). Though a phytoplankton response to nutrient loading has been reported at some fish farms, this is generally considered low risk (CitationNordvarg and Hakanson 2002; CitationSoto and Norambuena 2004; CitationApostolaki et al. 2007).
Figure 3. Control room for a salmon farm in Washington State. Fish feeding, behavior, and health are monitored using underwater video and water quality data are collected and displayed on computer screens. Feeding is done based on a computer-controlled system, feedback from the video, and the operator's experience. Photo by Laura Hoberecht

Causal linkages have not been established between fish farming and eutrophication (CitationPitta et al. 2005; CitationModica et al. 2006) or phytoplankton blooms (CitationSilvert 2001; CitationAnderson et al. 2008). In Maine and Washington, other factors besides nutrients, such as light availability and water temperature, often control natural variability in primary productivity. Naturally occurring nutrient fluxes from coastal ocean upwelling, or from land- and ocean-based sources, are often high relative to loads from aquaculture. Because nutrients may be flushed away from the immediate cage area and dispersed into the surrounding water body, it is difficult to assess whether far-field primary production is being affected over large areas and at longer timescales. The occurrence of many anthropogenically derived nutrients in coastal marine waters makes it difficult to attribute nutrification and phytoplankton response to any one source, including aquaculture.
Benthic Impacts
Benthic impacts result where organic nutrients in uneaten feed and fish waste accumulate on the seafloor (CitationPearson and Black 2001; CitationChamberlain and Stucchi 2007; CitationBelle and Nash 2008) and do not decompose quickly enough by natural aerobic bacterial processes to keep up with the supply from the farm. In this case, sediments shift toward anaerobic conditions, and the benthic species diversity declines, with perturbation-tolerant generalists becoming dominant (CitationHargrave 2003; CitationHolmer et al. 2005; CitationHargrave et al. 2008).
Benthic impacts from U.S. net-pens have reduced dramatically over the last few decades, due to improved siting and better management practices. Indicators to assess benthic condition include total organic carbon, redox potential, free sulfides, and abundance and diversity of marine organisms. Electrochemical and image analysis methods are also used (CitationSchaaning and Hansen 2005; CitationWildish et al. 2003). These indicators inform site management decisions, such as when to fallow (leave a site empty of fish for a period of time) or to adjust feeding and harvest. Because feed typically accounts for more than half the operating costs, farmers have the financial incentive to use underwater cameras to monitor and regulate feeding to minimize wasted feed ().
Accumulation of particulate waste is unlikely at farms over erosional seafloors (CitationKalantzi and Karakassis 2006). Under dispersive conditions, particulate wastes are spread away from the immediate farm footprint, aerobically decomposed, and assimilated by benthic organisms (CitationHolmer et al. 2005; CitationPhillips 2005; CitationGiles 2008). Farm discharge can enhance productivity of macro-algae, invertebrates, and fish (CitationKatz et al. 2002; CitationDempster et al. 2005; CitationRensel and Forster 2007). Conversely, depositional sites tend to accumulate organic waste. In this case, fallowing allows chemical and biological recovery of sediments (CitationWildish and Pohle 2005; CitationTucker and Hargreaves 2008; CitationBorg and Massa 2011). Fallowing takes months to years for bottoms to return to pre-farm conditions depending on the site's flushing characteristics and level of accumulation (CitationBrooks et al. 2003, Citation2004; CitationLin and Bailey-Brock 2008).
Modeling and Monitoring Water and Benthic Impacts
U.S. fish farms must monitor discharges to the benthos and water column to meet the standards of the Clean Water Act, which established the National Pollutant Discharge Elimination System (NPDES). In 2004, the U.S. Environmental Protection Agency (USEPA) developed a national effluent rule for net-pen aquaculture (CitationUSEPA 2004), establishing effluent limitations for aquaculture facilities into waters of the United States. Environmental impact models now allow regulators to assess the suitability of sites, understand the potential risks and benefits of proposed net-pen operations, and estimate the limits of acceptable farm biomass before they are permitted (CitationRensel et al. 2007; CitationBlack et al. 2008).
Monitoring data collected from U.S. marine fish farms (CitationAlston et al. 2005; CitationLee et al. 2006; CitationLangan 2007) and from other countries (CitationHargrave 2003; CitationWildish et al. 2004) often indicate few significant or persistent water quality or benthic issues (CitationPrice and Morris 2013). Such data help to validate and improve models to inform siting and management of current and future farms. In Maine and Washington, improved siting and pen configurations, better feeds, and improved feeding practices have decreased benthic deposition at salmon farms (CitationNash 2001; CitationLangan 2007). Washington State regulations require no net increases in benthic nutrients (Lori LeVander, Washington Department of Ecology, personal communication). In Maine, the standard is “the habitat must be of sufficient quality to support all species of fish indigenous to the receiving waters and maintain the structure and function of the resident biological community” (Jon Lewis, Maine Department of Marine Resources, personal communication). Fish farms are required to have regular monitoring by independent third-party scientists with results reviewed by state agencies and made available to the public.
FISH HEALTH AND DISEASE TRANSFER
Disease is a fact of life in all animal populations and production systems. Water moves freely between net-pens and the open marine environment, allowing the transmission of pathogens between wild and farmed fish (CitationKent 2000). Fisheries managers are concerned about the risk of pathogen amplification on farms followed by transmission of pathogens from farmed to wild fish, as well as the introduction of nonnative pathogens and parasites when live fish are moved from region to region. Culturists have incentive to work with resource managers and regulators to ensure that fish health is optimized on the farm and not negatively affecting wild populations. Robust health of farmed fish is economically advantageous to fish farmers, who depend on high survival rates and marketing healthy fish.
Experience and observation of disease outbreaks in farmed salmon (CitationHastein and Lindstad 1991; CitationJones and Beamish 2011) and hatcheries (CitationAmos and Thomas 2002) provide information on disease risks to wild populations. Fish diseases occur naturally in the wild, but their effects often go unnoticed because moribund or dead animals quickly become prey for other aquatic animals. Clinical disease occurs only when sufficient numbers of pathogens encounter susceptible fish under environmental conditions that are conducive to disease (CitationRose et al. 1989). Observable disease events may occur in net-pens because (1) fish are reared at higher densities than those found in nature, increasing rate of contact between individual fish within the pen; (2) infected fish are not removed from the farm population as they would be in nature by predators; and (3) the farm population is easily observed. Therefore, pathogens that normally exist in low numbers and do not cause disease in the wild may result in disease and observable mortality in farmed fish (CitationRaynard et al. 2007).
Managers of aquaculture facilities prevent and control disease events with biosecurity measures, effective vaccines, appropriate nutrition, genetically improved lines of organisms, appropriate rearing densities and other proven aquatic animal health measures, and therapeutants. In addition, regulatory bodies have implemented rules to prevent introduction of exotic pathogens into new regions/zones and transmission of endemic pathogens among animals within an area. Common health risks for and from farmed salmon include bacterial and viral diseases and parasites. Principles to prevent and treat these health risks developed by the farmed salmon industry are also applicable for other species and are specified by the World Organization for Animal Health (CitationOIE 2013) and the U.S. Department of Agriculture's Animal and Plant Health Inspection Service (CitationAPHIS 2008).
Bacterial and Viral Diseases
Although bacterial infections are common in farmed salmon and caused significant mortality in the early years of salmon farming, a number of measures, including vaccines, probiotics, limiting culture density, high-quality diets, and judicious use of antibiotics are effective at preventing and controlling bacterial diseases. Antibiotics are considered a method of last resort and are being replaced by other aforementioned management approaches (see Box 4 and ).
The management of viruses is focused on monitoring for diseases and maintaining culture conditions that provide for healthy fish able to resist disease through good nutrition, genetics, and low stress husbandry approaches. When a reportable virus is discovered, farms are typically depopulated (see Box 5).
Parasites
Much of what we understand about risks associated with parasites on farmed fish comes from work done to control sea lice on salmonids, and this is still an active area of research (see Box 6). Controlling the level of parasites on farms significantly reduces the potential for transfer to wild salmon and trout (D. CitationJackson et al. 2002; CitationJones and Beamish 2011; CitationRogers et al. 2013) and the health of the cultured stock. Significant integrated lice management programs that have been instituted in Norway (CitationMinistry of Fisheries and Coastal Affairs 2009) and Ireland include treatment of lice on farmed fish with approved therapeutants, fallowing of farm sites, and zonal single year-class management strategies. Cleaner wrasses and other species have been used commercially with success to reduce the lice load on salmon in pens and are an important part of integrated pest control programs there (CitationTorrissen et al. 2013).
BOX 4. Antibiotic Use in Salmon Net-Pens
In the Norwegian salmon farming industry, antibiotic use has decreased by approximately 95% in the past 20 years due to the introduction and use of efficacious vaccines (CitationMidtlyng et al. 2011). During that same period, salmon production in Norway has increased from about 180,000 to 1,000,000 metric tons (CitationFAO 2013). Similar numbers are available for British Columbia (CitationDepartment of Fisheries and Oceans, Canada 2014). In the United States, three antibiotics are approved and labeled to treat specific diseases in specific aquatic species. The majority of these labels are for freshwater applications. Any use by species, conditions, or diseases other than those listed on the label must be done via extra-label use that requires a licensed veterinarian to approve (CitationUSFDA 2012). As in Norway, effective vaccines have significantly reduced the use of antibiotics in U.S. salmon farming. In Maine, no antibiotic use was reported in net-pen salmon farms starting from 2007 (). This trend has continued and no antibiotic use has been reported for salmon net-pens in Maine from 2007 to 2012 (the last year records are available; Jon Lewis, MDMR, Aquaculture Environmental Coordinator, personal communication). This contrasts with approximately 13,500 metric tons of antibiotics being used in 2010 for all animals used for human consumption in the United States (CitationUSFDA 2011).
BOX 5. Dealing with IHN and ISA Viral Diseases
Infectious hematopoietic necrosis (IHN) is an acute disease of salmon caused by the virus of the same name (CitationWorld Organization for Animal Health 2012). It occurs naturally in the Pacific Northwest and causes varying degrees of mortality in wild salmon (CitationTraxler et al. 1997). Atlantic Salmon are farmed in the Pacific Northwest, but they have little resistance to IHN and are particularly sensitive to this virus. This has resulted in significant outbreaks of IHN in marine salmon pens in British Columbia (CitationSaksida 2006) and a recent event in Washington State (J. Kerwin, Washington Department of Fish and Wildlife, personal communication). However, there is no evidence that historic IHN outbreaks in farmed Atlantic Salmon have impacted wild Pacific salmon in the Pacific Northwest. Returning adult wild salmon populations did not appear to be affected in years in which significant IHN outbreaks occurred in farmed salmon in British Columbia (CitationPacific Salmon Commission 1993). Furthermore, in controlled water-borne transmission studies with IHN virus, researchers were unable to cause an infection in Chinook Salmon O. tshawytscha or Sockeye Salmon O. nerka but caused infection leading to a 10% mortality rate in Atlantic Salmon (CitationTraxler et al. 1993). There is an IHN vaccine that has been used in the Pacific Northwest on Atlantic Salmon but with variable success.
Likewise, infectious salmon anemia (ISA) is a serious viral disease of farmed Atlantic Salmon. Although ISA has been observed in Atlantic Salmon farms in Europe, Chile, New Brunswick, and Maine (CitationGustafson et al. 2007), the OIE reports that there are no confirmed cases of this disease or causative virus in the Pacific Northwest in wild or farmed salmon (CitationOIE 2013). Nevertheless, agencies and industry in the United States and Canada carry on an active surveillance program for the ISA virus. Attempts to induce ISA disease in Pacific salmon using water-borne laboratory challenges have been unsuccessful, suggesting that Pacific salmon are resistant to the ISA virus (CitationRolland and Winton 2003). Recent reports in British Columbia suggest that the ISA virus, but not the ISA disease, was found in wild Pacific salmon (CitationSimon Fraser University 2011). However, the Canadian Food Inspection Agency has been unable to confirm these findings (CitationCanadian Food Inspection Agency 2012). Because ISA is a very serious disease for Atlantic Salmon, increased surveillance and research is currently being undertaken by Canadian and U.S. agencies to determine whether ISA virus is truly present in the Pacific Northwest. To date, this surveillance in 2012 and 2013 in the Northwest has failed to demonstrate the presence of ISA disease or the ISA virus (J. Whaley, USDA/APHIS, personal communication; J. Constantine, Canadian Food Inspection Agency, personal communication).
Management of viral diseases is focused on monitoring for the diseases and maintaining culture conditions that provide for healthy fish through good nutrition and low stress husbandry changes. When viral diseases are discovered, farms are depopulated. In the future, we may see vaccines for viral diseases, but so far they remain experimental.
BOX 6. Sea Lice Impact Is Still an Active Area of Scientific Research
Sea lice have varying effects on wild and farmed fish depending on geographic location, ocean salinity, temperature, and infected fish populations in the vicinity (CitationJackson et al. 2012). Extensive studies conducted in Europe (CitationTorrissen et al. 2013) showed that lice transmission initiates from wild to farmed fish and then can be transmitted back to wild fish (CitationRaynard et al. 2007). Detrimental effects have been described for both wild and farmed hosts. The impact of sea lice from farmed salmon on wild fish has been reported to be substantial in some cases (e.g., wild Sea Trout in Ireland; CitationTully and Whelan 1993). Conversely, a 10-year study by D. CitationJackson et al. (2013) a decade later indicated that overall survival of out-migrating juvenile Atlantic Salmon in Ireland was only slightly impacted by sea lice, accounting for about 1% mortality compared to approximately 94% mortality for all other causes (5% survival to spawn). This study suggests that lice from salmon farms have a relatively small impact on wild Atlantic Salmon.
Observations by some researchers suggest that sea lice originating at salmon farms in British Columbia have caused infections and significant mortality in wild juvenile Pacific salmon (CitationKrkosek et al. 2005; CitationMorton and Routledge 2005). These authors postulated that marine salmon farms were responsible for depressions in wild Pacific salmon populations, including a low return of adult Pink Salmon Oncorhynchus gorbuscha to the Broughton Archipelago. In contrast, other research (CitationBeamish et al. 2006; CitationJones et al. 2006; CitationJones and Beamish 2011) indicates that sea lice populations fluctuate due to climatic and water conditions and that wild Three-Spine Sticklebacks Gasterosteus aculeatus and wild salmon act as natural carriers and reservoirs of infection for other wild fish. After reviewing 20 years of data on sea lice in farmed Atlantic Salmon in British Columbia and its relationship with wild Pink Salmon survival (Pink Salmon are potentially the most impacted because of their relative small size upon entry to sea water as compared to other salmon species), CitationMarty et al. (2010) concluded that wild salmon productivity was not associated with farmed fish production or prevalence of sea lice.
Researchers have investigated the use of vaccines and genetic resistance of hosts to combat lice. Although both approaches show promise in the laboratory, to date they have provided limited commercial success.
Similar approaches are used to manage sea lice on salmon farms in Maine and British Columbia, Canada (CitationRogers et al. 2013). In 2002, Maine salmon farmers and state resource agencies implemented an integrated pest management plan that includes monitoring, coordinated stocking of defined bay management areas, and a 3-year production cycle to include 8–12 months of fallowing between salmon harvest and restocking (CitationMaine Department of Marine Resources [MDMR] 2007). A permit from the MDMR is required to stock a bay management area during the first year of the production cycle after fallow periods are met. The MDMR also monitors the movements and prevalence of sea lice on wild salmon smolts (CitationMDMR 2007). Conversely, in Washington, significant sea lice infestation of farmed salmon has never been an issue because net-pens are located in areas where the salinity is too low for lice proliferation (CitationNash et al. 2005); therefore, treatment has not been necessary.
These approaches appear to have reduced the shedding and potential impacts of sea lice from salmon farms (D. CitationJackson et al. 2002; CitationTorrissen et al. 2013). Common elements of successful lice control programs that are in use and are successful both in Europe and North America include treatment of severe infestations with appropriate and approved therapeutants (such as hydrogen peroxide and emamectin benzoate), rearing a single year-class of fish at a marine pen site or zone, fallowing sites between production cycles to minimize cross-infection between groups, and general management practices that ensure the health of aquatic animals (CitationTorrissen et al. 2013). It is important that research continues to optimize and improve lice control on farmed salmon.
Prevention of Fish Disease Transfer
Most states have comprehensive aquatic animal health regulations that are prescriptive in preventing the introduction of diseased animals into the state and methods for managing disease events should they occur. In Maine, for example, laboratory fish health certification and a transfer permit from the MDMR are required prior to any movement of fish. Similar requirements are in place in other states. In addition, the federal agencies that have a role to play in fish health (U.S. Department of Agriculture [USDA], NOAA, and U.S. Fish and Wildlife Service) have developed a National Aquatic Animal Health Plan (CitationAPHIS 2008). Evidence from salmon farming indicates that operations that follow structured disease prevention programs and best management practices do not amplify pathogens sufficiently to cause disease in wild populations (D. CitationJackson et al. 2002). Effective programs include (1) routine health exams by aquatic animal health specialists; (2) health inspections prior to movement of fish between regions or health management zones; (3) accurate record keeping by the farmer to include mortalities, growth, and feed conversion; (4) implementation of a biosecurity plan for each farm site; and (5) use of preventive medicine such as vaccines and probiotics. Such programs are already in place for U.S. salmon farms.
Another concern is that escaped farmed fish could be vectors of disease transmission to wild stocks or produce other demographic impacts, such as competition with or predation on wild stocks. Should escapees carry a disease agent, the risk of them being the source of an outbreak in wild fish is low because (1) native pathogens are already a part of the environment where wild fish are routinely exposed and have developed some natural immunity; (2) escapees are unlikely to generate an infectious dose (or infective pressure) sufficient to result in disease in a healthy, wild population; (3) the mere presence of a pathogen alone will not cause disease without environmental factors that play a large role in triggering disease events (CitationMcVicar 1997; CitationMoffitt et al. 1998; CitationAmos, Appeby et al. 2001); and (4) most escaped farmed fish have low fitness for the wild and quickly become easy victims of predators such as marine mammals, other fish, and birds (CitationAmos, Thomas, and Stewart 2001). Nevertheless, escapes should be minimized, and cultured stock health should be maximized for both ecological and economic reasons.
Table 1. Main issues associated with marine net-pen aquaculture addressed by federal laws and the agencies responsible for their implementation.
Table 2. Annual use of therapeutants by Maine marine fish farms from 2001 to 2008. The use of trade names does not imply endorsement (reproduced from CitationMaine Department of Marine Resources 2009).
BOX 7. Understanding Genetic Risks and Benefits—Make Them Different or Keep Them the Same?
Understanding genetic risks from escaped fish to wild populations comes primarily from studies of farmed and wild populations of Atlantic Salmon (CitationMcGinnity et al. 1997, Citation2003; CitationHindar et al. 2006) and studies of hatchery released and wild populations of Pacific salmon (CitationFord 2002; CitationAraki et al. 2008). Genetic and fitness risks from interbreeding of farmed and wild fish include loss of genetic diversity within and among populations and loss of fitness (CitationFord 2002; CitationNaylor et al. 2005; CitationWaples et al. 2012). Loss of diversity within a population or among populations may occur when cultured animals with low genetic diversity escape and interbreed at very high levels with locally adapted wild populations, making the next generation of the wild population more homogenous. Loss of fitness can occur when cultured fish genetically suited to survival in captivity interbreed with wild populations and the resulting offspring have reduced ability to survive and reproduce in the wild (CitationFleming et al. 2000; CitationMcGinnity et al. 2009; CitationAraki et al. 2008).
Genetic selection in aquaculture is usually viewed in terms of increased profitability through gains in traits of commercial importance, such as growth rate, disease resistance, feed conversion, or product quality. However, genetic selection can also have implications on resource efficiency and environmental sustainability. Selected organisms may use less feed, produce less waste, pose less of a disease risk, and/or be more efficient at converting animal feed into human food than wild counterparts. Specific selection objectives in aquaculture that relate to environmental sustainability include better feed utilization to reduce waste and improved ability to utilize plant products to reduce dependency on fishmeal.
Managing for risks associated with loss of diversity and the benefits of selected breeding may involve trade-offs. For example, choosing unselected local wild broodstock, thereby keeping the genetic makeup of the cultured animals as similar as possible to that of the wild population, may minimize the impacts of escapes once they interbreed. However, this negates the ecological advantages of selective breeding for traits with commercial and environmental benefits (CitationGjoen and Bentsen 1997; CitationGjedrem et al. 2012). One approach could be to use highly domesticated animals with reduced survival and reproductive success in the wild. These fish may have low or no direct genetic impact on wild populations (CitationBaskett et al. 2013) because such animals are less likely to breed and pass on genes to their wild counterparts and, therefore, less likely to influence the long-term genetic makeup of wild populations. Offspring from those that do breed successfully are also less likely to survive and so on as natural selection impacts future generations of wild fish. The loss of fitness in cultured animals for life in the wild generally increases with increasing number of captive-bred generations (CitationAraki et al. 2008; CitationChristie et al. 2011). Although domesticated organisms often have reduced reproductive success in the wild, when highly domesticated animals do breed successfully in the wild, the genetic impact on the natural populations could be greater than if undomesticated (wild-type) organisms were involved ().
This dichotomy results in two opposite strategies to manage genetic risks: (1) strong domestication or make-them-different and (2) minimal or no domestication or keep-them-similar. Both strategies may have environmental merit depending on the specific situations and considerations.
GENETICS AND ESCAPES
Managing risks associated with escapes requires clear delineation of the risks, followed by measures to reduce escapes and their effects () A variety of approaches based on analysis of risks and critical control points exist for reducing the number of escapes and their potential harm to wild stocks, including advances in infrastructure, veterinary science, and breeding (CitationNaylor et al. 2005; CitationJensen et al. 2010; CitationLaikre et al. 2010).
Fish may escape from net-pens in large numbers during singular events like severe storms, in small losses through damaged nets (CitationMorris et al. 2008; CitationJensen et al. 2010), or during harvest operations (CitationDempster et al. 2002). Although catastrophic losses may be easily identified, more attention is needed to identify and prevent chronic, low-level escapes. Efforts to reduce escapes in salmon farming in Washington State and British Columbia, Canada, have been successful, as shown in . In the 10-year period from 1987 to 1996, the average annual escape rate was 3.7% of annual harvest, whereas more recently (2000–2009) escape rate averaged 0.3%. Similar trends are evident in Maine (unpublished) and in Chile (CitationSepulveda et al. 2013). Farm operators in the United States are required to develop best management practices for the prevention of escapes, have recovery plans if escapes should occur, mark all farmed salmon, and report any escapes.
Even with this improving trend, prevention of all escapes is unlikely; therefore, understanding the biological significance of escapes and dealing with the risks posed by escapes is necessary. The primary concern of escaped fish is the potential for them to interbreed with wild conspecifics and reduce the long-term fitness of the wild population (see Box 7).
Risks are typically species, site, and operation specific. The magnitude and type of genetic risk associated with the escape of farmed fish on wild counterparts is a function of (1) the number and survival of escapes relative to the population of wild conspecifics, (2) the difference in genetic makeup between the escaped farmed and wild fish, (3) the reproductive fitness of the escaped fish (CitationFord 2004; CitationWaples et al. 2012), and (4) the opportunity for reproduction with wild fish. As domestication advances, survival and reproductive success in the wild decreases (items 1, 3, and 4) tending to reduce the risk, while genetic difference increases (item 2), which tends to increase the risk.
The approach used to deal with the trade-offs between selectively breeding cultured animals to be genetically different (make-them-different strategy) or maintaining wild broodstock (keep-them-similar strategy) may depend on the specific situation (CitationLorenzen et al. 2012). Comparison of alternate genetic strategies reveals varied degrees of consequences depending on the relative timing of natural selection, density dependence, and time of escape during the life cycle of the fish (CitationBaskett and Waples 2013). For example, the make-them-different strategy can be a viable alternative to the keep-them-similar strategy, reducing risk if natural selection is significant between when escapes occur and reproduction happens. In addition, if selection in the captive environment is minimal, then demographic (e.g., competition and natural selection) effects outweigh fitness effects; if selection is significant, then fitness effects dominate (CitationBaskett and Waples 2013).
Mitigation strategies to minimize the risk of genetic impacts include improved containment through better management and design of net-pen systems and antipredator nets; shore-based rearing for part of the grow-out period; improved fish handling practices during stocking, rearing, and harvesting; and use of sterile fish to eliminate reproduction (see Box 8). Maintaining large and healthy wild stocks, or choosing species for culture that have large, healthy populations, also decreases risk by decreasing the ratio of escapes to wild fish ().
The trade-offs in genetic management and operational parameters of aquaculture can be complex, and one approach does not fit all species and locations. Models have been developed and are being refined to understand and manage risks to promote good management under a range of conditions (CitationTufto 2010; CitationBaskett et al. 2013; CitationNOAA 2014). Much of what we know about the underlying conservation genetics and mitigation strategies comes from modeling work done to understand and create genetic management plans for hatcheries producing fish for restocking programs (CitationFord 2002); that information is applicable to the management of escapes from commercial aquaculture operations (CitationHindar et al. 2006; CitationBesnier et al. 2011). For example, salmon hatchery program managers can use models to simulate how changes in hatchery practices impact the genetics of enhanced populations (CitationPaquet et al. 2011). Quantitative models provide insight for commercial operators and public hatchery managers to focus attention on risk reduction, for scientists to focus research efforts, and for resource managers to focus on monitoring and regulation.
CONCLUSIONS
Advances in technology and regulation over the last few decades now allow net-pen marine fish farms to produce significant amounts of seafood sustainably. Fish are very efficient converters of feed into human food, but as with other animal farming, care must be exercised to avoid harming the environment. In the United States, the Salmon farming industry and the government agencies that regulate aquaculture have had decades to develop the science, technology, management options, and regulations to successfully address key environmental concerns.
Table 3. General approaches for mitigating risks from aquaculture escapes.
BOX 8. Making Farmed Fish Sterile?
Research to produce sterile farmed fish may eliminate the direct genetic risks and reduce some of the demographic effects of escapes. Sterilization of cultured fish is more compelling as a risk reduction strategy and more effective when significant genetic differences exist between farmed and wild populations and escapees are still reproductively fit. Sterilization of fish may also benefit industry by allowing companies who invest in selective breeding some control over the use of proprietary high-performance domesticated lines. Sterilization of fish by inducing triploidy has been effective, with some triploids exhibiting survival and growth similar to diploids (CitationTaylor et al. 2011). Research has also explored repressible sterile fish (fish that require dietary additives for maturation), which would be unable to reproduce if they escaped (CitationThresher et al. 2009). Even though these approaches are promising, much work remains to develop a cost-effective method of reliably producing sterile fish.
Progress over the last four decades has been significant. Research has produced feeds that contain reduced amounts of fish meal and fish oil, opening the door for carnivorous fish farming systems to become net producers of fish oil and fish meal. Vaccines, improved nutrition, and better health management have greatly reduced the need for antibiotics and the risks associated with diseases. Proper siting and feeding has greatly reduced negative impacts of nutrients on ecosystems. Escapement has been reduced by improved net-pen engineering and management, and our understanding of the genetic consequences of escaped fish has advanced to the point where models can be used to identify and manage the risks.
Table 4. Escaped farmed salmon in Washington State (WA), United States, and British Columbia (BC), Canada. BC salmon production levels in tons were used to estimate percentage of escapes for each year.
Marine fish farms are required to comply with regulations similar to those of other food-producing and marine industries. Existing U.S. regulations address the environmental effects of net-pen aquaculture effectively. Technological progress, better monitoring, and adaptive oversight of the U.S. net-pen aquaculture industry have resulted in sustainable, affordable, and domestically produced seafood.
ACKNOWLEDGMENTS
The authors thank Dave Alves, Jess Beck, Susan Bunsick, Alan Everson, Brian Fredieu, John Hargreaves, Dennis Hedge-cock, Laura Hoberecht, Nicole Kirchhoff, Lori LeVander, Jon Lewis, Patti Marraro, Jack Rensel, Jill Rolland, Paul Sandifer, Jeff Schaeffer, Jesse Trushenski, Diane Windham, and anonymous reviewers for valuable review and contributions.
REFERENCES
- Adler, J., B. Campbell, V. Karpouzi, K. Kaschner, and D. Pauly. 2008. Forage fish: from ecosystems to markets. Annual Reviews of Environment and Resources 33: 153–166.
- Aguilar-Manjarrez, J., J. M. Kapetsky, and D. Soto. 2010. The potential of spatial planning tools to support the ecosystem approach to aquaculture. Food and Agriculture Organization of the United Nations, Fisheries and Aquaculture Proceedings 17, Rome.
- Alston, D. E., A. Cabarcas, J. Capella, D. D. Benetti, S. Keene-Meltzoff, J. Bonilla, and R. Cortes. 2005. Report on the environmental and social impacts of sustainable offshore cage culture production in Puerto Rican waters. Final Report to the National Oceanic and Atmospheric Administration, NA16RG1611, Washington, D.C. Available: www.lib.noaa.gov/retiredsites/docaqua/reports_noaaresearch/finaloffshorepuertorico.pdf. (April 2014).
- Amberg, S. M., and T. E. Hall. 2008. Communicating risks and benefits of aquaculture: a content analysis of U.S. newsprint representations of farmed salmon. Journal of the World Aquaculture Society 39: 143–157.
- Amos, K. H., A. Appleby, J. Thomas, and D. Seiler. 2001. Atlantic Salmon (Salmo salar) in the Pacific Northwest: assessing the risk of impact on wild Pacific salmon. Pages 193–201 in C. J. Rodgers, editor. Proceedings of an international conference on risk analysis in aquatic animal health. World Organization for Animal Health, Paris.
- Amos, K. H., and J. Thomas. 2002. Disease interactions between wild and cultured fish: observations and lessons learned in the Pacific Northwest. Bulletin of the European Association of Fish Pathologists 22: 95–102.
- Amos, K. H., J. Thomas, and B. Stewart. 2001. Pathogen transmission between wild and cultured salmonids: risk avoidance in Washington State, United States of America. Pages 83–89 in C. J. Rodgers, editor. Proceedings of an international conference on risk analysis in aquatic animal health. World Organization for Animal Health, Paris.
- Anderson, D. M., J. M. Burkholder, W. P. Cochlan, P. M. Glibert, C. J. Gobler, C. A. Heil, R. M. Kudela, M. L. Parsons, J. E. J. Rensel, D. W. Townsend, V. L. Trainer, and G. A. Vargo. 2008. Harmful algal blooms and eutrophication: examining linkages from selected coastal regions of the United States. Harmful Algae 8: 39–53.
- APHIS (Animal and Plant Health Inspection Service). 2008. National aquatic animal health plan for the United States. Animal and Plant Health Inspection Service, Department of Agriculture, Washington, D.C. Available: www.aphis.usda.gov/animal_health/animal_dis_spec/aquaculture/downloads/naahp.pdf. (April 2014).
- Apostolaki, E. T., T. Tsagaraki, M. Tsapaki, and I. Karakassis. 2007. Fish farming impact on sediments and macrofauna associated with seagrass meadows in the Mediterranean. Estuarine, Coastal and Shelf Science 75: 408–416.
- Araki, H., B. Berejikian, M. J. Ford, and M. S. Blouin. 2008. Fitness of hatchery-reared salmonids in the wild. Evolutionary Applications 1: 342–355.
- Asche, F. 2008. Farming the sea. Marine Resource Economics 23: 527–547.
- Baron, N. 2010. Escape from the ivory tower: a guide to making your science matter. Island Press, Seattle.
- Barrows, F. T., D. Bellis, A. Krogdahl, J. T. Silverstein, E. M. Herman, W. M. Sealey, M. B. Rust, and D. M. Gatlin III. 2008. Report of the plant products in aquafeed strategic planning workshop: an integrated, interdisciplinary research roadmap for increasing utilization of plant feedstuffs in diets for carnivorous fish. Reviews in Fisheries Science 16: 449–455.
- Barrows, F. T., G. Gaylord, D. J. Stone, and C. E. Smith. 2007. Effect of protein sources and nutrient density on growth efficiency of Rainbow Trout (Oncorhynchus mykiss). Aquaculture Research 38: 1747–1758.
- Bartley, D. M., C. Brugere, D. Soto, P. Gerber, and B. Harvey, editors. 2007. Comparative assessment of the environmental costs of aquaculture and other food production sectors. Food and Agriculture Organization of the United Nations, Rome.
- Baskett, M. L., S. C. Furgess, and R. S. Waples. 2013. Assessing strategies to minimize unintended fitness consequences of aquaculture on wild populations. Evolutionary Applications 6: 1090–1108.
- Baskett, M. L., and R. S. Waples. 2013. Evaluating alternative strategies for minimizing unintended fitness consequences of cultured individuals on wild populations. Conservation Biology 27: 83–94.
- Beamish, R. J., S. Jones, C. Neville, R. Sweeting, G. Karreman, S. Saksida, and E. Gordon. 2006. Exceptional marine survival of Pink Salmon that entered the marine environment in 2003 suggests that farmed Atlantic Salmon and Pacific Salmon can coexist successfully in a marine ecosystem on the Pacific Coast of Canada. ICES Journal of Marine Science 63: 1326–1337.
- Belle, S. M., and C. E. Nash. 2008. Better management practices for net pen aquaculture. Pages 261–330 in C. S. Tucker and J. Hargreaves, editors. Environmental best management practices for aquaculture. Blackwell Publishing, Ames, Iowa.
- Besnier, F., K. A. Glover, and O. Skaala. 2011. Investigating genetic change in wild populations: modeling gene flow from farm escapees. Aquaculture Environment Interactions 2: 75–86.
- Beveridge, M. 2004. Cage aquaculture, 3rd edition. Fishing News Books, Blackwell Science, London.
- Bj⊘rkli, J. 2002. Protein ogenergiregnskap hos laks, kylling, grisog lam [Protein and energy account in salmon, chicken, pig, and lamb]. Master's thesis. Norwegian University of Life Sciences (UMB), Oslo, Norway.
- Black, K. D., P. K. Hansen, and M. Holmer. 2008. Salmon aquaculture dialogue: working group report on benthic impacts and farm siting. World Wildlife Fund, Washington, D.C.
- Borg, J. A., and F. Massa. 2011. Site selection and carrying capacity in Mediterranean marine aquaculture: key issues. Draft report to the General Fisheries Commission for the Mediterranean (GFCM), Rome. Available: www.GfcmWebSite/GFCM/35/GFCM_XXXV_2011_Dma.9.pdf. (April 2014).
- Boyd, C. E., C. Tucker, A. McNevin, K. Bostick, and J. Clay. 2007. Indicators of resource use efficiency and environmental performance in fish and crustacean aquaculture. Reviews in Fisheries Science 15: 327–360.
- Bridger, C. J., and B. A. Costa-Pierce. 2003. Open ocean aquaculture: from research to commercial reality. The World Aquaculture Society, Baton Rouge, Louisiana.
- Brooks, K. M. 2007. Assessing the environmental costs of Atlantic Salmon cage culture in the Northeast Pacific in perspective with the costs associated with other forms of food production. Pages 137–182 in D. M. Bartley, C. Brugère, D. Soto, P. Gerber, and B. Harvey, editors. Comparative assessment of the environmental costs of aquaculture and other food production sectors: methods for meaningful comparisons. Food and Agriculture Organization of the United Nations, Fisheries and Aquaculture Proceedings Number 10, Rome.
- Brooks, K. M., A. R. Stierns, and C. Backman. 2004. Seven year remediation study at the Carrie Bay Atlantic Salmon (Salmo salar) farm in the Broughton Archipelago, British Columbia, Canada. Aquaculture 239: 81–123.
- Brooks, K. M., A. R. Stierns, C. V. W. Mahnken, and D. B. Blackburn. 2003. Chemical and biological remediation of the benthos near Atlantic Salmon farms. Aquaculture 219: 355–377.
- Burr, G. S., W. R. Wolters, F. T. Barrows, and R. W. Hardy. 2012. Replacing fishmeal with blends of alternative proteins on growth performance of Rainbow Trout (Oncorhynchus mykiss), and early or late stage juvenile Atlantic Salmon (Salmo salar). Aquaculture 334–337: 110–116.
- Canadian Food Inspection Agency. 2012. Salmon disease surveillance in British Columbia. Available: www.inspection.gc.ca/about-the-cfia/newsroom/news-releases/salmon-disease/eng/1330100767319/1330100801573. (April 2014).
- Chamberlain, J., and Stucchi, D. 2007. Simulating the effects of parameter uncertainty on waste model predictions of marine finfish aquaculture. Aquaculture 179: 127–140
- Christie, M. R., M. L. Marine, R. A. French, and M. S. Blouin. 2011. Genetic adaptation to captivity can occur in a single generation. Proceedings of the National Academy of Sciences 109: 238–242.
- Chu, J., J. L. Anderson, F. Asche, and L. Tudur. 2010. Stakeholders’ perceptions of aquaculture and implications for its future: a comparison of the USA and Norway. Marine Resource Economics 25: 61–76.
- Dempster, T., D. Fernandez-Jover, P. Sanchez-Jerez, F. Tuya, J. Bayle-Sempere, A. Boyra, and R. J. Haroun. 2005. Vertical variability of wild fish assemblages around sea-cage fish farms: implications for management. Marine Ecology Progress Series 304: 15–29.
- Dempster, T., P. Sanchez-Jerez, J. T. Bayle-Sepmere, F. Gimenez-Casalduero, and C. Valle. 2002. Attraction of wild fish to sea-cage fish farms in the south-western Mediterranean Sea: spatial and short-term temporal variability. Marine Ecology Progress Series 242: 237–252.
- Department of Fisheries and Oceans, Canada. 2014. Fish health management. Available: www.pac.dfo-mpo.gc.ca/aquaculture/reporting-rapports/health-sante-eng.html. (July 2014).
- FAO (Food and Agriculture Organization of the United Nations). 2011. World aquaculture 2010. Food and Agriculture Organization of the United Nations, Fisheries and Aquaculture Department, Technical Paper 500/1, Rome.
- FAO (Food and Agriculture Organization of the United Nations). 2012. The state of world fisheries and aquaculture 2012. Food and Agriculture Organization of the United Nations, Fisheries and Aquaculture Department, Rome.
- FAO (Food and Agriculture Organization of the United Nations). 2013. National aquaculture sector overview. Food and Agriculture Organization of the United Nations, Fisheries and Aquaculture Department, Norway. Available: www.fao.org/fishery/countrysector/naso_norway/en. (April 2014).
- FAO (Food and Agriculture Organization of the United Nations) and WHO (World Health Organization of the United Nations). 2011. Report of the joint FAO/WHO expert consultation on the risks and benefits of fish consumption. FOA/WHO, Rome, and Geneva, Switzerland.
- Fish Culture Section, American Fisheries Society. 2013. Federal aquaculture regulations. Available: www.sites.google.com/site/fishculturesection/federal-aquaculture-regulations. (April 2014).
- Fleming, I. A., K. Hindar, I. B. Mj⊘lner⊘d, B. Jonsson, T. Balstad, and A. Lamberg. 2000. Lifetime success and interactions of farm salmon invading a native population. Proceedings of the Royal Society B: Biological Sciences 267: 1517–1523.
- Ford, M. J. 2002. Selection in captivity during supportive breeding may reduce fitness in the wild. Conservation Biology 16: 815–825.
- Ford, M. J. 2004. Conservation units and preserving diversity. Pages 338–357 in A. P. Hendry and S. C. Stearns, editors. Evolution illuminated: salmon and their relatives. Oxford University Press, Oxford, England.
- Gatlin, D. M., F. T. Barrows, D. Bellis, P. Brown, K. Daborwski, T. G. Gaylord. R. W. Hardy, E. M. Herman, G. Hu, A. Krogdahl, R. Nelson, K. Overturf, M. B. Rust, W. M. Sealey, D. Skonberg, E. J. Souza, D. Stone, R. Wilson, and E. Wurtele. 2007. Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquaculture Research 38: 551–579.
- Gaylord, T. G., F. T. Barrows, A. M. Teague, K. A. Johansen, K. E. Overturf, and B. Shepherd. 2007. Supplementation of taurine and methionine to all-plant protein diets for Rainbow Trout (Oncorhynchus mykiss). Aquaculture 269: 514–524.
- Gibbs, M. T. 2009. Implementation barriers to establishing a sustainable coastal aquaculture sector. Marine Policy 33: 83–89.
- Giles, H. 2008. Using Bayesian networks to examine consistent trends in fish farm benthic impact studies. Aquaculture 274: 181–195.
- Gjedrem, T., N. Robinson, and M. Rye. 2012. The importance of selective breeding in aquaculture to meet future demands for animal protein: a review. Aquaculture 353: 117–129.
- Gjoen, H. M., and H. B. Bentsen. 1997. Past, present, and future of genetic improvement in Salmon aquaculture. ICES Journal of Marine Science 54: 1009–1014.
- Gustafson, L. L., S. K. Ellis, M. J. Beattie, B. D. Chang, D. A. Dickey, T. L. Robinson, F. P. Marenghi, P. J. Moffett, and F. H. Page. 2007. Hydrographics and the timing of infectious Salmon anemia outbreaks among Atlantic Salmon (Salmo salar L.) farms in the Quoddy region of Maine, USA and New Brunswick, Canada. Preventive Veterinary Medicine 78: 35–56.
- Hall, S. J., A. Delaporte, M. J. Phillips, M. Beveridge, and M. O'Keefe. 2011. Blue frontiers: managing the environmental costs of aquaculture. The World Fish Center, Penang, Malaysia.
- Hardy, R. W., and F. T. Barrows. 2002. Diet formulation and manufacture. Pages 505–600 in J. E. Halver and R. W. Hardy, editors. Fish nutrition, 3rd edition. Academic Press, San Diego, California.
- Hargrave, B. T. 2003. A scientific review of the potential environmental effects of aquaculture in aquatic ecosystems, volume 1. Fisheries and Oceans Canada, Canadian Technical Report of Fisheries and Aquatic Sciences 2450, Ottawa. Available: www.dfo-mpo.gc.ca/science/enviro/aquaculture/sok-edc/volume1/hargrave-eng.htm. (July 2014).
- Hargrave, B., M. Holmer, and C. Newcombe. 2008. Towards a classification of organic enrichment in marine sediments based on biogeochemical indicators. Marine Pollution Bulletin 56: 810–824.
- Hastein, T., and T. Lindstad. 1991. Diseases in wild and cultured Salmon: possible interaction. Aquaculture 98: 277–288.
- Hindar, K., I. A. Fleming, P. McGinnity, and O. Diserud. 2006. Genetic and ecological effects of salmon farming on wild Salmon: modeling from experimental results. ICES Journal of Marine Science 63: 1234–1247.
- Holmer, M., D. Wildish, and B. Hargrave. 2005. Organic enrichment from marine finfish aquaculture and effects on sediment biogeochemical processes. Pages 181–206 in B. T. Hargrave, editor. Handbook of environmental chemistry, volume 5M, Springer-Verlag, Berlin.
- Index Mundi, Commodity Prices www.indexmundi.com (August 2014). International Fish meal and Fish oil Organization. 2013. Is aquaculture growth putting pressure on feed fish stocks and is the growth of aquaculture being restricted by finite supplies of fishmeal and fish? Available: www.iffo.net/default.asp?contentID=817. (September 2013).
- Jackson, A. 2012. Fishmeal and fish oil and its role in sustainable aquaculture. International Aquafeed 15 (5): 18–21.
- Jackson, D., D. Cotter, J. Newell, S. McEvoy, P. O'Donohoe, F. Kane, T. McDermott, S. Kelly, and A. Drumm. 2013. Impact of Lepeophtheirus salmonis infestations on migrating Atlantic Salmon, Salmo salar L., smolts at eight locations in Ireland with an analysis of lice-induced marine mortality. Journal of Fish Diseases 36: 273–281.
- Jackson, D., D. Hassett, and L. Copley. 2002. Integrated lice management on Irish salmon farms. Fish Veterinary Journal 6: 28–38.
- Jackson, D., P. O'Donohoe, K. S. Kelly, T. McDermott, A. Drumm, K. Lyons, and G. Nolan. 2012. Result of an epidemiological study of sea lice infestation in South Connemara, West of Ireland. Aquaculture 365: 118–123.
- Jensen, O., T. Dempster, E. B. Thorstad, I. Uglem, and A. Fredheim. 2010. Escapes of fish from Norwegian sea-cage aquaculture: causes, consequences and methods to prevent escapes. Aquaculture Environment Interactions 1: 71–83.
- Johansen, L. H., I. Jensen, H. Mikkelsen, P. A. Bjorn, and O. Berg. 2011. Disease interaction and pathogen exchange between wild and farmed fish populations with special reference to Norway. Aquaculture 315: 167–186.
- Jones, S. R. M., and R. J. Beamish. 2011. Lepeophtheirus salmonis on salmonids in the northeast Pacific Ocean. Pages 307–329 in S. Jones and R. Beamish, editors. Salmon lice: an integrated approach to understanding parasite abundance and distribution. John Wiley & Sons, Chichester, UK.
- Jones, S., G. Prosperi-Porta, E. Kim, P. Callow, and N. B. Hargreaves. 2006. The occurrence of Lepeoptheirus salmonis and Caligus clemensi (Copepoda: Caligidae) on Three-Spine Stickleback Gasterosteus aculeatus in coastal British Columbia. Journal of Parasitiology 92: 473–480.
- Kaiser, M., and S. M. Stead. 2002. Uncertainties and values in European aquaculture: communication, management and policy issues in times of “changing public perceptions.” Aquaculture International 10: 469–490.
- Kalantzi, L., and L. Karakassis. 2006. Benthic impacts of fish farming: meta-analysis of community and geochemical data. Marine Pollution Bulletin 52: 484–493.
- Kapetsky, J. M., J. Aguilar-Manjarrez, and J. Jenness. 2013. A global assessment of offshore mariculture potential from a spatial perspective. Food and Agriculture Organization of the United Nations, Fisheries and Aquaculture Technical Paper 549, Rome.
- Katz, T., B. Herut, A. Genin, and D. L. Angel. 2002. Gray Mullets ameliorate organically enriched sediments below a fish farm in the oligotrophic Gulf of Aqaba (Red Sea). Marine Ecology Progress Series 234: 205–214.
- Kent, M. L. 2000. Marine net pen farming leads to infections with some unusual parasites. International Journal of Parasitology 30: 321–326.
- Kite-Powell, H. L., M. C. Rubino, and B. Morehead. 2013. The future of U.S. seafood supply. Aquaculture Economics & Management 17: 228–250.
- Knapp, G. 2012. The political economics of United States marine aquaculture. Bulletin of Fisheries Research Agency 35: 51–63.
- Knapp, G., C. A. Roheim, and J. L. Anderson. 2007. The great Salmon run: competition between wild and farmed Salmon. TRAFFIC North America, World Wildlife Fund, Washington, D.C.
- Kristoffersson, D., and J. L. Anderson. 2006. Is there a relationship between fisheries and farming? Interdependence of fisheries, animal production and aquaculture. Marine Policy 30: 721–725.
- Krkosek, M., M. A. Lewis, and J. P. Volpe. 2005. Transmission dynamics of parasitic sea lice from farm to wild salmon. Proceedings of the Royal Society B: Biological Sciences 272: 689–696.
- Laikre, L., M. K. Schwartz, R. S. Waples, N. Ryman, and the GeM Working Group. 2010. Compromising genetic diversity in the wild: unmonitored large-scale release of plants and animals. Trends in Ecology and Evolution 25: 520–529.
- Langan, R. 2007. Results of environmental monitoring at an experimental offshore farm in the Gulf of Maine: environmental conditions after seven years of multi-species farming. Pages 57–60 in C. S. Lee and P. J. O'Bryan, editors. Open ocean aquaculture—moving forward. Oceanic Institute, Waimanalo, Hawaii. Available: www.nsgl.gso.uri.edu/ocei/oceiw06001.pdf. (April 2014).
- Lee, H. W., J. H. Bailey-Brock, and M. M. McGurr. 2006. Temporal changes in the poly-chaete infaunal community surrounding a Hawaiian mariculture operation. Marine Ecology Progress Series 307: 175–185.
- Lee, K. J., K. Dabrowski, J. H. Blom, S. C. Bai, and P. C. Stromberg. 2002. Mixture of animal and plant protein sources can completely replace fish meal in a diet for juvenile Rainbow Trout (Oncorhynchus mykiss). Journal of Animal Physiology and Animal Nutrition 86: 201–213.
- Lin, D. T., and J. H. Bailey-Brock. 2008. Partial recovery of infaunal communities during a fallow period at an open-ocean aquaculture. Marine Ecology Progress Series 371: 65–72.
- Lorenzen, K. M., C. M. Beveridge, and M. Mangel. 2012. Cultured fish: integrative biology and management of domestication and interactions with wild fish. Biological Reviews 87: 639–660.
- Mantzavrakos, E., M. Kornaros, G. Lyberatos, P. Kaspiris, and T. D. Lekkas. 2005. Impacts of a marine fish farm in Argolikos Gulf on the water column and the sediment. Proceedings of the 9th International Conference on Environmental Science and Technology, Rhodes Island, Greece. Available: www.srcosmos.gr/srcosmos/showpub.aspx?aa=6625. (April 2014).
- Marty, G. D., S. M. Saksida, and T. J. Quinn. 2010. Relationship of farm salmon, sea lice, and wild salmon populations. Proceedings of the National Academy of Sciences of the United States of America 107: 22599–22604.
- Mazur, N. A., and A. L. Curtis. 2008. Understanding community perceptions of aquaculture: lessons from Australia. Aquaculture International 16: 601–621.
- McGinnity, D., E. Jennings, E. deEyto, N. Allott, P. Samuelsson, G. Rogan, K. Whelan, and T. Cross. 2009. Impact of naturally spawning captive-bred Atlantic Salmon on wild populations: depressed recruitment and increased risk of climate-mediated extinction. Proceedings of the Royal Society B: Biological Sciences 276: 3601–3610.
- McGinnity, P., P. Prodohl, A. Ferguson, R. Hynes, N. O. Maoileidigh, N. Baker, D. Cotter, B. O'Hea, D. Cooke, G. Rogan, J. Taggart, and T. Cross. 2003. Fitness reduction and potential extinction of wild populations of Atlantic Salmon, Salmo salar, as a result of interactions with escaped farm Salmon. Proceedings of the Royal Society B: Biological Sciences 270: 2443–2450.
- McGinnity, P., C. Stone, J. B. Taggert, D. Cooke, D. Cotter, R. Haynes, C. McCamley, T. Cross, and A. Ferguson. 1997. Genetic impact of escaped farmed Atlantic Salmon (Salmo salar L.) on native populations: use of DNA profiling to assess freshwater performance of wild, farmed and hybrid progeny in a natural river environment. ICES Journal of Marine Science 54: 998–1008.
- McKinnon, D., L. Trott, S. Duggan, R. Brinkman, D. Alongi, S. Castine, and F. Patel. 2008. Report on the environmental impacts of sea cage aquaculture in a Queensland context—Hinchinbrook Channel case study (SD57/06). Australian Institute of Marine Science, Final Report, Queensland, Australia. Available: www.aims.gov.au/c/document_library/get_file?uuid=965f17c9-b42b-4e41-a5a5-e568a37a5459&groupId=30301. (April 2014).
- McVicar, A. H. 1997. Disease and parasite implications of the coexistence of wild and cultured Atlantic Salmon populations. ICES Journal of Marine Science 54: 1093–1103.
- MDMR (Maine Department of Marine Resources). 2007. Sea lice and Salmon aquaculture in Maine. Available: www.maine.gov/dmr/aquaculture/reports/sealice.pdf. (April 2014).
- MDMR (Maine Department of Marine Resources). 2009. Information sheet on use of therapeutic agents in marine finfish aquaculture. Available: www.maine.gov/dmr/aquaculture/reports/therapeutants08.pdf. (April 2014).
- Merino, G., M. Barange, J. L. Blanchard, J. Harle, R. Holmes, I. Allen, E. H. Allison, M. C. Badjeck, N. K. Dulvy, J. Holt, S. Jennings, C. Mullon, and L. D. Rodwell. 2012. Can marine fisheries and aquaculture meet fish demand from a growing human population in a changing climate? Global Environmental Change 22,(4):795–806.
- Midtlyng, P., K. Grave, and T. E. Horsberg. 2011. What has been done to minimize the use of antibacterial and antiparasitic drugs in Norwegian aquaculture? Aquaculture Research 42: 28–34.
- Ministry of Fisheries and Coastal Affairs. 2009. Forskriftombekjempelseavlus i akvakulturanlegg (luseforskriften) [Directive on prevention of sea lice in aquaculture]. Ministry of Fisheries and Coastal Affairs, FOR-2009-08-18-1095, Oslo, Norway. Available: www.lovdata.no/cgi-wift/wiftldles?doc=/app/gratis/www/docroot/for/sf/fi/fi-20090818-1095.html. (April 2014).
- Modica, A., D. Scilipoti, R. La Torre, A. Manganaro, and G. Sara. 2006. The effect of mariculture facilities on biochemical features of suspended organic matter (southern Tyrrhenian, Mediterranean). Estuarine, Coastal and Shelf Science 66: 177–184.
- Moffitt, C. M. 2006. Environmental, economic and social aspects of animal protein production and the opportunities for aquaculture. Fisheries 30 (9): 36–38.
- Moffitt, C. M., B. Stewart, S. Lapatra, R. Brunson, J. Bartholomew, J. Peterson, and K. H. Amos. 1998. Pathogens and diseases of fish in aquatic ecosystems: implications in fisheries management. Journal of Aquatic Animal Health 10: 95–100.
- Morris, M. R. J., D. J. Fraser, A. J. Heggelin, F. G. Whoriskey, J. W. Carr, S. F. O'Neil, and J. A. Hutchings. 2008. Prevalence and recurrence of escaped farmed Atlantic Salmon (Salmo salar) in eastern North American rivers. Canadian Journal of Fisheries and Aquatic Sciences 65: 2807–2826.
- Morton, A. B., and R. Routledge. 2005. Mortality rates for juvenile Pink Salmon (Oncorhynchus gorbuscha) and Chum (O. keta) Salmon infested with sea lice Lepeoptheirus salmonis in the Broughton Archipelago. Alaska Fishery Research Bulletin 11: 146–152.
- Nash, C. E. 2001. The net pen Salmon farming industry in the Pacific Northwest. National Oceanic and Atmospheric Administration, Technical Memorandum NMFS-NWFSC-49, Silver Spring, Maryland.
- Nash, C. E., P. R. Burbridge, and J. K. Volkman. 2005. Guidelines for ecological risk assessment of marine fish aquaculture. National Oceanic and Atmospheric Administration, Technical Memorandum NMFS-NWFSC-71, Silver Spring, Maryland.
- Natale, F., G. Fiore, and J. Hofherr. 2012. Mapping the research on aquaculture. A bibliometric analysis of aquaculture literature. Scientometrics 90: 983–999.
- National Association of State Aquaculture Coordinators. 2013. Federal aquaculture regulatory fact sheets. Available: www.nasac.net. (April 2014).
- Naylor, R., and M. Burke. 2005. Aquaculture and ocean resources: raising tigers of the sea. Annual Review of Environment and Resources 30: 185–218.
- Naylor, R. L., R. J. Goldburg, H. Mooney, M. Beveridge, J. Clay, C. Folke, N. Kautsky, J. Lubchenco, J. Primavera, and M. Williams. 1998. Nature's subsidies to shrimp and Salmon farming. Science 282: 883–884.
- Naylor, R. L., R. J. Goldburg, J. H. Primavera, N. Kautsky, M. C. M. Beveridge, J. Clay, C. Folks, J. Lubchenco, H. Mooney, and M. Troell. 2000. Effect of aquaculture on world fish supplies. Nature 405: 1017–1024.
- Naylor, R. L., R. W. Hardy, D. P. Bureau, A. Chiu, M. Elliott, A. P. Farrell, I. Forster, D. M. Gatlin, R. J. Goldburg, K. Hua, and P. D. Nichols. 2009. Feeding aquaculture in an era of finite resources. Proceedings of the National Academy of Sciences 106: 15103–15110.
- Naylor, R. L., K. Hindar, I. Fleming, R. Goldburg, S. Williams, J. Volpe, F. Whoriskey J. Eagle, D. Kelso, and M. Mangel. 2005. Fugitive salmon: assessing the risks of escaping fish from net pen aquaculture. BioScience 55: 427–437.
- NOAA (National Oceanic and Atmospheric Administration). 2012. Fisheries of the United States 2011. National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Silver Spring, Maryland.
- NOAA (National Oceanic and Atmospheric Administration). 2014. Offshore mariculture escapes genetics assessment (OMEGA) model. National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Silver Spring, Maryland. Available: www.nmfs.noaa.gov/aquaculture/science/omega_model_homepage.html. (April 2014).
- Nordvarg, L., and L. Hakanson. 2002. Predicting the environmental response of fish farming in coastal areas of the Aland Archipelago (Baltic Sea) using management models for coastal water planning. Aquaculture 206: 217–243.
- NRC (National Research Council). 1978. Aquaculture in the United States: constraints and opportunities: a report of the Committee on Aquaculture, Board on Agriculture and Renewable Resources, Commission on Natural Resources, the National Research Council. National Academies Press, Washington, D.C.
- NRC (National Research Council). 1983. Nutrient requirements of warmwater fishes and shellfishes, nutrient requirements of domestic animals. National Academies Press, Washington, D.C.
- NRC (National Research Council). 1984. Nutrient requirements of coldwater fishes, nutrient requirements of domestic animals. National Academies Press, Washington, D.C.
- NRC (National Research Council). 2011. Nutrient requirements of fish and shrimp, animal nutrition series. National Academies Press, Washington, D.C.
- OECD (Organization for Economic Co-operation and Development) and FAO (Food and Agriculture Organization of the United Nation). 2014. OEDC/FAO agricultural outlook 2014–2013. OECD Publishing, Paris. Available: www.oecd.org/site/oecd-faoagriculturaloutlook/publication.htm. (July 2014).
- OIE (World Organization for Animal Health). 2012. Aquatic animal health code, world animal health database (WAHID). Available: www.oie.int/international-standard-setting/aquatic-code. (April 2014).
- OIE (World Organization for Animal Health). 2013. Aquatic animal health code, 16th edition. Available: www.oie.int/international-standard-setting/aquatic-code/access-online. (July 2014).
- Olmos, J., L. Ochoa, J. Paniagua-Michel, and R. Contreras. 2011. Functional feed assessment on Litopenaeus vannamei using 100% fish meal replacement by soy bean meal, high levels of complex carbohydrates and Bacillus probiotic strains. Marine Drugs 9: 1119–1132.
- Pacific Salmon Commission. 1993. 1992/93, 8th Annual Report, Vancouver. Available: www.psc.org/pubs/8thAnnualReport.pdf. (April 2014).
- Paquet, P. J., T. Flagg, A. Appleby, J. Barr, L. Blankenship, D. Campton, M. Delarm, T. Evelyn, D. Fast, J. Gislason, P. Kline, D. Maynard, L. Mobrand, G. Nandor, P. Seidel, and S. Smith. 2011. Hatcheries, conservation, and sustainable fisheries—achieving multiple goals: results of the Hatchery Scientific Review Group's Columbia River basin review. Fisheries 36: 547–561.
- Pearson, T. H., and K. D. Black. 2001. The environmental impacts of marine fish cage culture. Pages 1–31 in K. D. Black, editor. Environmental impacts of aquaculture. CRC Press, Boca Raton, Florida.
- Pearson, T. H., and R. Rosenberg. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology: Annual Review 16: 229–311.
- Phillips, S. 2005. Environmental impacts of marine aquaculture issue paper. Report to the Pacific States Marine Fisheries Commission, Portland, Oregon. Available: www.aquaticnuisance.org/wordpress/wp-content/uploads/2009/01/Issue%20–%20Aquaculture%20Environmental%20Impacts,%20Atlantic%20Salmon,.pdf. (April 2014).
- Pitta, P., E. T. Apostolaki, M. Giannoulaki, and I. Karakassis. 2005. Mesoscale changes in the water column in response to fish farming zones in three coastal areas in the Eastern Mediterranean Sea. Estuarine, Coastal and Shelf Science 65: 501–512.
- Price, C. S., and J. A. Morris, Jr. 2013. Marine cage culture and the environment: twenty-first century science informing a sustainable industry. National Oceanic and Atmospheric Administration, Technical Memorandum NOS-NCCOS-164, Silver Spring, Maryland.
- Raynard, R., T. Wahli, I. Vatsos, and S. Mortensen, editors. 2007. Review of disease interactions and pathogen exchange between farmed and wild finfish and shellfish in Europe. VESO, Oslo, Norway.
- Refstie, S., J. J. Ollic, and H. Standald. 2004. Feed intake, growth, and protein utilization by post-smolt Atlantic Salmon (Salmo salar) in response to graded levels of fish protein hydrolysate in the diet. Aquaculture 239: 331–349.
- Rensel, J. E., and J. R. M. Forster. 2007. Beneficial environmental effects of marine finfish mariculture. Final Report to the National Oceanic and Atmospheric Administration, #NA040AR4170130, Washington, D.C. Available: www.wfga.net/documents/marine_finfish_finalreport.pdf. (April 2014).
- Rensel, J. E. J., D. A. Kiefer, J. R. M. Forster, D. L. Woodruff, and N. R. Evans. 2007. Offshore finfish mariculture in the Strait of Juan de Fuca. Bulletin of Fisheries Research Agency 19: 113–129.
- Rogers, L. A., S. J. Peacock, P. McKenzie, S. DeDominicis, S. R. Jones, P. Chandler, M. G. Foreman, C. W. Revie, and M. Krkoşek. 2013. Modeling parasite dynamics on farmed Salmon for precautionary conservation management of wild salmon. PLoS ONE 8 (4): e60096.
- Rolland, J. B., and J. R. Winton. 2003. Relative resistance of Pacific Salmon to infectious Salmon anemia virus. Journal of Fish Diseases 26: 511–520.
- Rose, A. S., A. E. Ellis, and A. L. S. Munro. 1989. The infectivity by different routes of exposure and shedding rates of Aeromonas salmonicida subsp. salmonicida in Atlantic Salmon, Salmo salar L., held in sea water. Journal of Fish Diseases 12: 573–578.
- Rubino, M. C., editor. 2008. Offshore aquaculture in the United States: economic considerations, implications and opportunities. National Oceanic and Atmospheric Administration, Technical Memorandum NMFS F/SPO-103, Silver Spring, Maryland.
- Ruiz-Lopez, N., R. P. Haslam, J. A. Napier, and O. Sayanova. 2014. Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. The Plant Journal 77 (2): 198–208.
- Rust, M. B., F. T. Barrows, R. W. Hardy, A. Lazur, K. Naughten, and J. Silverstein. 2011. The future of aquafeeds: report to the NOAA/USDA Alternative Feeds Initiative. National Oceanic and Atmospheric Administration, Technical Memorandum NMFS F/SPO-124, Silver Spring, Maryland.
- Saksida, S. 2006. Infectious hematopoietic necrosis epidemics (2001 to 2003) in farmed Atlantic Salmon Salmo salar in British Columbia. Diseases of Aquatic Organisms 72: 213–223.
- Schaaning, M. T., and P. K. Hansen. 2005. The suitability of electrode measurements for assessment of benthic organic impact and their use in a management system for marine fish farms. Pages 381–408 in B. T. Hargrave, editor. Handbook of environmental chemistry, volume 5M, Springer-Verlag, Berlin.
- Sepulveda, M., I. Arismendi, D. Soto, F. Jara, and F. Farias. 2013. Escaped farmed salmon and trout in Chile: incidence, impacts, and the need for an ecosystem view. Aquaculture Environment Interactions 4: 273–283.
- Shapawi, R., W. K. Ng, and S. Mustafa. 2007. Replacement of fish meal with poultry by-product meal in feeds formulated for the Polka-Dot Grouper, Cromileptes altivelis. Aquaculture 273: 118–126.
- Shepard, C. J., I. H. Pike, and S. M. Barlow. 2005. Sustainable feed resources of marine origin. European Aquaculture Society Special Publication 35: 59–66. Available: www.iffo.net.769soon2b.co.uk/downloads/MarineRes.pdf. (April 2014).
- Silvert, W. 2001. Impact on habitats: determining what is acceptable. Pages 16–40 in M. Tlusty, D. Bengston, H. O. Halvorson, S. Oktay, J. Pearce, and R. B. Rheault, editors. Marine aquaculture and the environment: a meeting for stakeholders in the northeast. Cape Cod Press, Falmouth, Massachusetts.
- Simon Fraser University. 2011. Lethal atlantic virus found in Pacific Salmon. Public Affairs and Media Relations, Simon Fraser University, Vancouver. Available: www.sfu.ca/pamr/media-releases/2011/lethal-atlantic-virus-found-in-pacific-salmon.html. (April 2014).
- Smil, V. 2002. Nitrogen and food production: proteins for human diets. AMBIO 31: 126–131.
- Smith, P., and O. Samuelsen. 1996. Estimates of the significance of out-washing of oxytetracycline from sediments under Atlantic Salmon cages. Aquaculture 144: 17–26.
- Smith, R. R., G. L. Rumsey, and M. L. Scott. 1978. Heat increment associated with dietary protein, fat, carbohydrate, and complete diets in salmonids: comparative energetic efficiency. Journal of Nutrition 108: 1025–1032.
- Sookying, D. 2010. Development and application of soybean based diets for Pacific white shrimp Litopenaeus vannamei. Doctoral dissertation. Auburn University, Auburn, Alabama.
- Soto, D., and F. Norambuena. 2004. Evaluation of salmon farming effects on marine systems in the inner seas of southern Chile: a large-scale mensurative experiment. Journal of Applied Ichthyology 20: 493–501.
- Soto, D., F. J. Salazar, and M. A. Alfaro. 2007. Considerations for comparative evaluation of environmental costs of livestock and Salmon farming in southern Chile. Pages 121–136 in D. M. Bartley, C. Brugère, D. Soto, P. Gerber, and B. Harvey, editors. Comparative assessment of the environmental costs of aquaculture and other food production sectors: methods for meaningful comparisons. Food and Agriculture Organization of the United Nations, Fisheries Proceedings 10, Rome.
- Tacon, A. G. J., M. R. Hasan, and M. Metian. 2011. Demand and supply of feed ingredients for farmed fish and crustaceans trends and prospects. Food and Agriculture Organization of the United Nations, Fisheries Technical Paper 564, Rome.
- Tacon, A. G. J., and M. Metian. 2008. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: trends and future prospects. Aquaculture 285: 146–158.
- Tacon, A. G. J., and M. Metian. 2009. Fishing for feed or fishing for food: increasing global competition for small pelagic forage fish. AMBIO 38: 294–302.
- Takagi, S. T., H. Hosokawa, S. Shimeno, and M. Ukawa. 2000. Utilization of poultry by-product meal in a diet for Red Sea Bream Pagrus major. Nippon Suisan Gakkaishii 66: 428–438.
- Talbot, C. 1993. Some aspects of the biology of feeding and growth in fish. Proceedings of the Nutrition Society 52: 403–416.
- Taylor, J. F., A. C. Preston, D. Gut, and H. Migaud. 2011. Ploidy effects on hatchery survival deformities and performance in Atlantic Salmon (Salmo salar). Aquaculture 315: 61–68.
- Thresher, R., P. Grewe, J. G. Patil, S. Whyard, C. M. Templeton, A. Chaimongol, C. M. Hardy, L. A. Hinds, and R. Dunham. 2009. Development of repressible sterility to prevent the establishment of feral populations of exotic and genetically modified animals. Aquaculture 290: 104–109.
- Tlusty, M. F., V. A. Pepper, and M. R. Anderson. 2005. Reconciling aquaculture's influence on the water column and benthos of an estuarine fjord—a case study from Bay d'Espoir, Newfoundland. Pages 115–128 in B. T. Hargrave, editor. Handbook of environmental chemistry, volume 5M. Springer-Verlag, Berlin.
- Torrissen, O., S. Jones, F. Asche, A. Guttormsen, O. T. Skilbrei, F. Nilsen, T. E. Horsberg, and D. Jackson. 2013. Salmon lice—impact on wild salmonids and salmonaquaculture. Journal of Fish Diseases 36: 171–194.
- Torrissen, O., R. E. Olsen, R. Toresen, G. I. Hemre, A. G. J. Tacon, F. Asche, R. W. Hardy, and S. Lall. 2011. Atlantic Salmon (Salmo salar): the “super-chicken” of the sea? Reviews in Fisheries Science 19: 257–278.
- Torstensen, B. E., M. Espe, M. Sanden, I. Stubhaug, R. Waagb⊘, G. I. Hemre, R. Fontanillas, U. Nordgarden, E. M. Hevr⊘y, P. Olsvik, and M. H. G. Berntssen. 2008. Novel production of Atlantic Salmon (Salmo salar) protein based on combined replacement of fish meal and fish oil with plant meal and vegetable oil blends. Aquaculture 285: 193–200.
- Traxler, G. S., J. L. Roome, and M. L. Kent. 1993. Transmission of infectious hematopoietic necrosis virus in seawater. Diseases of Aquatic Organisms 16: 111–114.
- Traxler, G. S., J. R. Roome, K. A. Lauda, and S. LaPatra. 1997. The appearance of infectious hematopoietic necrosis virus (IHNV) and neutralizing antibodies in Sockeye Salmon (Oncorhynchus nerka) during their migration and maturation period. Diseases of Aquatic Organisms 28: 31–38.
- Trushenski, J., B. Mulligan, D. Jirsa, and M. Drawbridge. 2013. Sparing fish oil with soybean oil in feeds for White Seabass: effects of inclusion rate and soybean oil composition. North American Journal of Aquaculture 75: 305–315.
- Tucker, C. S., and J. A. Hargreaves, editors. 2008. Environmental best management practices for aquaculture. Wiley-Blackwell, Oxford, England.
- Tufto, J. 2010. Gene flow from domesticated species to wild relatives: migration load in a model of multivariate selection. Evolution 64: 180–192.
- Tully, O., and K. Whelan. 1993. Production of nauplii of Lepeophtheirus salmonis(Krřyer) (Copepoda: Caligidae) from farmed and wild salmon and its relation to the infestation of wild Sea Trout Salmo trutta L. of the west coast of Ireland in 1991. Fisheries Research 17: 187–200.
- Tveterås, R. 2002. Norwegian salmon aquaculture and sustainability: the relationship between environmental quality and industry growth. Marine Resource Economics 17: 121–132.
- United Nations. 2011. The millennium development goals report 2011. United Nations, New York.
- USEPA (U.S. Environmental Protection Agency). 2004. Aquaculture operations—laws, regulations, policies and guidance. Available: www.epa.gov/agriculture/anaqulaw.html#Effluent%20Guidelines. (April 2014).
- USFDA (U.S. Food and Drug Administration). 2011. 2010 summary report on antimicrobials sold or distributed for use in food-producing animals. U.S. Food and Drug Administration, Department of Health and Human Services, Silver Spring, Maryland. Available: www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActA-DUFA/UCM338170.pdf (April 2014).
- USFDA (U.S. Food and Drug Administration). 2012. Approved drugs for aquaculture. U.S. Food and Drug Administration, Department of Health and Human Services, Silver Spring, Maryland. Available: www.fda.gov/animalveterinary/developmentapprovalprocess/aquaculture/ucm132954.htm. (April 2014).
- USFDA (U.S. Food and Drug Administration). 2014. Food Safety Modernization Act and Animal Feed www.fda.gov/AnimalVeterinary/Products/AnimalFoodFeeds/ucm347941.htm#Overview_of_FSMA (October 2014).
- Waples, R. S., K. Hindar, and J. J. Hard. 2012. Genetic risks associated with marine aquaculture. National Oceanic and Atmospheric Administration, Technical Memorandum NMFS-NWFSC-119, Silver Spring, Maryland.
- Watson, A. M., F. T. Barrows, and A. Place. 2013. Taurine supplementation of plant derived protein and n-3 fatty acids are critical for optimal growth and development of Cobia, Rachycentron canadum. Lipids 48: 899–915.
- Watson, A. M., G. W. Kissil, F. T. Barrows, and A. Place. 2012. Developing a plant-based diet for Cobia, Rachycentron canadum. International Aquafeed 15: 34–38.
- Wildish, D. J., M. Dowd, T. F. Sutherland, and C. D. Levings. 2004. Near-field organic enrichment from marine finfish aquaculture. Canadian Technical Report of Fisheries and Aquatic Sciences 2450 (3). Available: www.dfo-mpo.gc.ca/Library/285141.pdf (April 2014).
- Wildish, D. J., B. T. Hargrave, C. MacLeod, and C. Crawford. 2003. Detection of organic enrichment near finfish net pens by sediment profile imaging at SCUBA-accessible depths. Journal of Experimental Marine Biology and Ecology 285–286: 403–413.
- Wildish, D. J., and G. Pohle. 2005. Benthic macrofaunal changes resulting from finfish mariculture. Pages 239–251 in B. T. Hargrave, editor. Handbook of environmental chemistry, volume 5M. Springer-Verlag, Berlin.
- World Bank. 2013. Fish to 2030: prospects for fisheries and aquaculture. Agriculture and environmental services, discussion paper 3. World Bank Group, Report 83177-GLB, Washington, D.C.




