Abstract
Context: There is currently no consensus regarding optimal dose or dose-range of buprenorphine (BUP) for treatment of opioid use disorder (OUD).
Objective: To elucidate the relationship between BUP dose and opioid receptor blockade, retention in treatment and illicit opioid drug use.
Methods: Systematic review of the scientific literature through searches in the databases MEDLINE and PubMed.
Results: The review of the opioid receptor blockade studies did not find evidence that a daily sublingual (SL) BUP tablet dose higher than 16 mg confers added blockade benefit, while doses under 8 mg are insufficient to produce opioid receptor blockade. The data are inconclusive regarding the relative effectiveness of an 8 mg SL BUP tablet dose versus a 16 mg SL BUP tablet dose in terms of opioid receptor blockade. The review did not establish any clear relationship between BUP dose and treatment retention or illicit opioid use.
Conclusions: The BUP dose in treatment of OUD should be individualized based on a continuous clinical benefit-risk assessment. Further research is needed to better understand the relationship between dose and efficacy over time in patients with this complex disorder.
Introduction
One of the fundamental maxims taught in most medical school curriculums in clinical pharmacology is ‘Primum Non Nocere’ - i.e. ‘First Do No Harm’. Although both the origins and applicability of this motto has been questioned, it still serves as a reminder that medical decisions carry the potential for harm and that selection of for instance the dose of medications should be based on a benefit risk assessment [Citation1]. The introduction in recent years of molecularly targeted agents, in particular in oncology, has increased the opportunities to establish optimal benefit risk balance and the concept of maximally tolerated dose (MTD) has now for many medications been replaced with dose optimization leading to use within expanded therapeutic windows [Citation2].
Buprenorphine (BUP) is a medication used for treatment of opioid use disorder (OUD) and its efficacy in reducing illicit opioid use and mortality rate among opioid dependent patients is well established [Citation3]. BUP is believed to exert its effects mainly through binding to mu opioid receptors (μOR) with high-affinity and slow dissociation kinetics while being a low-efficacy partial agonist [Citation4]. In treatment of OUD the binding and blockade of μORs by BUP suppresses opioid withdrawal and cravings, reduces illicit opioid use and blocks the effects of exogenous opioids, including respiratory depression [Citation4]. BUP also binds to delta and opioid-receptor-like 1 receptors and is a high-affinity kappa opioid receptor antagonist, but the relevance of these interactions in OUD treatment is unknown [Citation4].
The ability of BUP to block the effect of full opioid agonists on the μOR has been shown in pharmacological ‘blockade’ or ‘challenge’ studies where patients are first administered BUP at different doses and then challenged with drugs such as heroin or hydromorphone [Citation4]. There are some authors of scientific publications claiming that an adequate opioid blockade requires an ‘optimal exposure’ with BUP plasma concentrations of ≥2–3 ng/mL and that this is achieved with a daily sublingual (SL) dose of BUP or buprenorphine/naloxone (BUP-NLX) of 16 mg and higher [Citation5–7]. However, others suggest that further research is needed to understand dose-response relationships in patients with this complex disorder [Citation8,Citation9].
To investigate the scientific evidence behind dosing recommendations for the use of BUP in OUD we have performed a systematic review of the literature reporting effects of different BUP doses on opioid receptor blockade, retention in treatment and illicit opioid drug use.
Methods
A systematic search of the published literature regarding SL BUP dose and opioid blockade, retention in treatment and illicit opioid drug use in clinical trials and cohort studies was performed in the databases MEDLINE and PubMed using a broad panel of terms including ‘opioid dependence’, ‘opioid use disorder’ and ‘buprenorphine’ combined with ‘clinical trial’, ‘opioid blockade’, ‘opioid challenge’, ‘hydromorphone’, ‘mu-opioid receptor’, ‘withdrawal’, ‘craving’, ‘retention’, ‘opioid use’, and ‘urine samples’ with no search restrictions. As the indexing using these databases of articles was not accurate enough to systematically and comprehensively capture all relevant published studies, the searches had to be complemented with inclusion of publications referenced by those found in the searches and with publications known to the authors. The publications were divided into blockade studies and randomized clinical trials. The randomized clinical trials were limited to those with a duration of at least 24 weeks in order to capture all the treatment phases induction, stabilization and maintenance in this chronic relapsing remitting disease generally requiring long-term therapy. Furthermore, a clinical efficacy trial duration of 24 weeks is required by regulatory agencies for approval of new medications for treatment of OUD [Citation7,Citation10]. The clinical trials selected were also limited to those conducted in Europe and North America.
The publications identified in the searches included studies of both oral liquid and SL tablet formulations of BUP. Pharmacokinetic studies have found the relative bioavailability of SL tablets versus oral liquid to be 64% [Citation11] and 71% [Citation12]. In order to make comparisons between the studies, a conversion factor of 1.5 was therefore used to calculate the approximate BUP SL tablet doses corresponding to the oral liquid BUP formulation doses. In two of the opioid blockade studies, the liquid formulation was administered subcutaneously and in the absence of pharmacokinetic data to inform a corresponding SL tablet dose, these studies have been excluded from the analysis.
The review of outcome measures in the opioid blockade studies focused on subjective opioid effects or suppression of withdrawals or cravings as those were consistently assessed in all studies. For the randomized clinical trials, the efficacy outcomes of retention in treatment and illicit opioid drug use were selected as these, in contrast to withdrawal and cravings, were consistently assessed in almost all publications. Analysis of the lowest effective ceiling dose for BUP was only conducted for the blockade studies. The review was not focused on safety outcomes, such as hepatic toxicity and cardiotoxicity, neither on misuse nor diversion, which are other factors that might contribute to BUP dose benefit-risk assessment.
A statistical analysis using weighted correlation coefficients was performed to describe the association between retention in treatment and illicit opioid use and the dose. The number of patients per study was used as weights.
Results
Opioid blockade in relation to buprenorphine dose
There were 15 opioid blockade studies with BUP included in the systematic review () [Citation13–28]. Six of these studies were conducted with the oral liquid formulation of BUP, which provides higher plasma exposures per milligram dose than the BUP SL tablet formulation [Citation11,Citation12]. Two of the studies were conducted with subcutaneously administered BUP and therefore excluded from the analysis. Many of the studies included a small number of subjects and the BUP run-in time and the time since last BUP dose prior to opioid challenge varied considerably between the studies. The highest challenge dose of the full μOR agonist also varied among the studies. Many of the studies did not include the full dose-range of BUP, e.g. most studies did not include an 8 mg SL BUP dose. Only 7 studies included a BUP dose over 16 mg and among those 5 did not include any dose between 16 and 32 mg.
Table 1. Opioid blockade studies with BUP.
The lowest ceiling dose observed for blockade of subjective opioid effects or suppression of withdrawals or cravings ranged between 3 mg and 18 mg as follows: 3 mg (1 study), 8 mg (3 studies), 12 mg (4 studies), 16 mg (4 studies) and 18 mg (1 study). Most studies found doses under 8 mg to be ineffective in producing opioid receptor blockade. There were 10 studies that included a dose of 16 mg or higher and 5 of these found a dose below 16 mg to be at least equivalent to higher doses in producing blockade effects. The studies that reported a dose of 16 mg or higher to be most effective generally included higher challenge doses of a μOR agonist compared to the studies where a dose under 16 mg was found to be most effective. Only 3 studies included both tablet doses of 8 mg and 16 mg and in two of these studies the 8 mg dose was found to be as effective as the 16 mg dose. None of the 6 studies that included a BUP tablet dose of 32 mg or higher reported these doses to be more effective than doses of 16 mg or lower in blocking the subjective effects of opioids and suppressing withdrawal and cravings.
Retention and illicit opioid use in relation to buprenorphine dose
There were 15 randomized controlled trials included in the systematic review () [Citation29–44]. The definition of retention varied across the studies. The incentives for the patients to remain in treatment and for investigators to retain the patients also varied between the studies, but as this is not consistently described it is difficult to report. For the analysis of illicit drug use, there were variations in the sensitivity and specificity of the urine drug screen assays used, the timepoints for urine drug screenings, and whether or not imputation of missing samples was performed. Furthermore, it is not possible to make any comparisons between fixed versus variable doses and supervised versus unsupervised dosing with regards to retention between these studies as these factors were not consistently reported.
Table 2. Retention and illicit opioid use in prospective clinical trials with BUP.
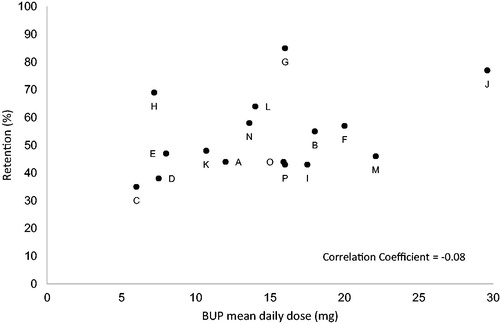
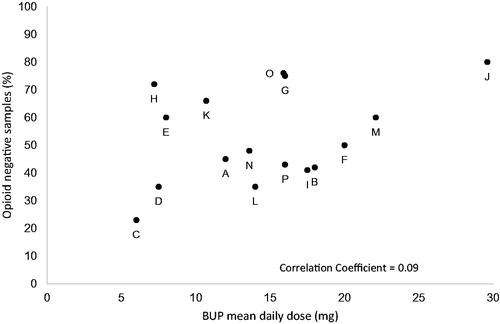
The range of BUP/BUP-NLX dose investigated varied among the studies, but only two studies included doses higher than 24 mg. The mean dose in the studies varied with an average dose of 12.9 mg across all studies (). The number of patients treated with BUP/BUP-NLX in the studies ranged from 20 to 738. There was no clear correlation between BUP/BUP-NLX dose and the reported treatment retention and illicit opioid use during the study periods ( and and ). The correlation coefficients weighted for sample size of the studies for the outcomes in relation to dose were -0.08 for retention and 0.09 for illicit opioid use.
Discussion
This systematic review of opioid receptor blockade studies and randomized clinical trials with BUP with a duration of 24 weeks or more indicates that the currently available data do not enable any conclusions with regards to the optimal dose for either opioid receptor blockade, retention in treatment or reduction in illicit opioid use. For opioid receptor blockade, most studies indicate that a dose under 8 mg is not effective. As a full dose range was not included in most studies, firm conclusions on dose-response relationship and a minimum effective dose for opioid receptor blockade are difficult to draw. Only three studies included for instance both the doses 8 mg and 16 mg, and only two studies included a dose between 16 and 32 mg BUP.
In the two most recently conducted opioid receptor blockade studies with SL BUP there were no differences between 8 mg and 32 mg [Citation26,Citation27]. One of these studies used a highest challenge dose of 12 mg hydromorphone, which is lower than the challenge doses of μOR agonists used in most studies where a BUP dose of 16 mg was the most effective in producing opioid receptor blockade. It is difficult to determine the corresponding BUP concentration provided by the 8 mg SL tablets in these two studies as no PK samples were taken, but the corresponding mean average BUP plasma concentrations are likely below 2 ng/ml. A recently published study with an extended-release formulation of BUP has shown that opioid receptor blockade can occur with BUP plasma concentrations below 2 ng/ml. Blockade of opioid effects after a challenge with 18 mg of hydromorphone administered intramuscularly was observed in this study at a mean BUP plasma concentration of approximately 1.25 ng/ml [Citation45]. In contrast, opioid receptor blockade, based on a modeling exercise from two brain imaging studies with positron emission tomography (PET) of 5 and 10 subjects, respectively [Citation25,Citation28], has been hypothesized to require a BUP plasma concentration of 2-3 ng/ml associated with at least 70% μOR occupancy [Citation5]. In these studies, the 8 mg SL BUP tablet dose was not examined, and the absence of a full dose range makes conclusions on the optimal dose difficult.
In the randomized clinical trials with a duration of at least 24 weeks, there was a wide variation in retention in treatment and no clear relationship between dose and retention could be established. A large variation in retention was also found in another recent systematic review [Citation46]. In contrast, a further systematic review indicates that daily SL BUP doses of ≥ 16 mg results in higher retention compared to lower doses [Citation47]. A systematic Cochrane review indicates that daily doses ≥ 2 mg results in higher retention compared to placebo and that doses ≥ 8 mg results in retention rates that are comparable to treatment with methadone [Citation48]. The reasons for the conflicting results in these reviews might be related to the inclusion of studies of different durations, with the two latter reviews including mostly studies with shorter durations under six months. A major limitation of the present systematic review is that shorter studies, that may be relevant for defining the most effective doses for retention during the initial phases of treatment, are excluded. The dose response relationship examined in shorter studies will to a greater extent be influenced by retention during the induction phase of BUP treatment, i.e. the treatment phase in which the drop-out rate is highest. This was demonstrated in a recent large induction study with SL BUP-NLX which reported that approximately 25% of patients withdrew from treatment during the first 15 days [Citation49]. In addition, approximately 24% of patients withdrew during the 7–14 day SL BUP run-in period in a trial of an extended-release BUP formulation [Citation7]. That doses of 16 mg and higher may be associated with lower drop-out during the induction phase has been shown in analyses of outcome trajectories from the START clinical trial in the US [Citation40,Citation50]. Interestingly, in this study the statistically significant difference in retention for patients treated with ≥16 mg BUP versus <16 mg BUP was found only for drop-out during the first 28 days, and not for drop out during the later stage of the trial [Citation50]. The importance of a dose of 16 mg daily SL BUP or higher during the induction phase was also demonstrated in an observational cohort study from Italy, where a dose of 16 mg in the induction phase was associated with better outcomes than lower doses [Citation51]. In contrast, long-term prospective observational studies from Italy, UK and Germany show no correlation between retention and higher mean BUP doses [Citation52–54], supporting the findings in this systematic review of longer randomized controlled trials with a duration of over 24 weeks.
The present systematic review did not establish a dose-dependent reduction of illicit opioid use with increasing BUP dose. The variation in illicit drug use reported was high across studies and might be influenced by a number of factors such as the sensitivity and specificity of the urine drug screen assay used, the timepoints for urine drug screens, and whether missing samples were imputed. In addition, the results might differ between studies depending on the demographics of the study populations and the treatment settings. However, despite high mean doses in some studies, which result in BUP plasma exposures at steady-state well over 3 ng/ml conferring a high level of opioid receptor blockade, there was still considerable use of illicit opioids in a large proportion of the patients. The continued use of illicit opioids despite high doses of BUP is also shown in the recently published studies with extended-release formulations using flexible [Citation10] or high fixed doses of BUP [Citation7]. Reduction in opioid cravings conferred by opioid receptor blockade might not be sufficient to stop illicit drug use in all patients and this is further supported by a recent finding of low correlation between opioid cravings and opioid use in patients treated with BUP [Citation55].
In treatment of OUD, dosing of the full opioid receptor agonist, methadone, is tailored to the individual to provide their optimum dose, a practice that is based on both clinical studies and real world evidence [Citation56,Citation57]. For BUP treatment this has not always been the case although a recent cohort study from Spain investing dose adequacy in patients receiving SL BUP indicates that the dose should be adjusted individually to the patient [Citation58,Citation59].
Conclusions
In summary, this systematic review has not found evidence that a daily SL BUP tablet dose of higher than 16 mg confers added opioid receptor blockade benefit. Daily doses below 8 mg are not sufficient to provide opioid receptor blockade in most patients. The data are inconclusive with regards to the relative effectiveness of a daily 8 mg versus a 16 mg SL BUP tablet dose to provide μOR blockade. The present review does not report any firm conclusions regarding BUP dose and treatment retention or illicit opioid use, partly due to the different methodologies used in the studies included in the review. Overall, the findings indicate that the BUP dose in treatment of OUD should be individualized based on a continuous benefit-risk assessment, as is currently the case with methadone. Further research is needed to better understand the relationship between dose and efficacy over time in patients with this complex disorder.
Disclosure statement
The authors are employees of Camurus AB. The authors declare no conflicts of interest.
Additional information
Funding
References
- Smith CM. Origin and uses of primum non nocere – above all, do no harm! J Clin Pharmacol. 2005;45(4):371–377.
- Lu D, Lu T, Stroh M, et al. A survey of new oncology drug approvals in the USA from 2010 to 2015: a focus on optimal dose and related postmarketing activities. Cancer Chemother Pharmacol. 2016;77(3):459–476.
- Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2018;24:1868–1883.
- Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93–103.
- Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1–11.
- Nasser AF, Greenwald MK, Vince B, et al. Sustained-release buprenorphine (RBP-6000) nlocks the effects of opioid challenge with hydromorphone in subjects with opioid use disorder. J Clin Psychopharmacol. 2016;36(1):18–26.
- Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778–790.
- Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760–1772.
- Pendergrass SA, Crist RC, Jones LK, et al. The importance of buprenorphine research in the opioid crisis. Mol Psychiatry. 2019;24(5):626–632.
- Lofwall MR, Walsh SL, Nunes EV, et al. Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: a randomized clinical trial. JAMA Intern Med. 2018;178(6):764–773.
- Schuh KJ, Johanson C-E. Pharmacokinetic comparison of the buprenorphine sublingual liquid and tablet. Drug Alcohol Depend. 1999;56(1):55–60.
- Compton PJ, Ling W, Moody D, et al. Pharmacokinetics, bioavailability and opioid effects of liquid versus tablet buprenorphine. Drug Alcohol Depend. 2006;82(1):25–31.
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35(4):501–516.
- Mello NK, Mendelson JH, Kuehnle JC. Buprenorphine effects on human heroin self-administration: an operant analysis. J Pharmacol Exp Ther. 1982;223(1):30–39.
- Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207(4431):657–659.
- Bickel WK, Stitzer ML, Bigelow GE, et al. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther. 1988;247(1):47–53.
- Rosen MI, Wallace EA, McMahon TJ, et al. Buprenorphine: duration of blockade of effects of intramuscular hydromorphone. Drug Alcohol Depend. 1994;35(2):141–149.
- Walsh SL, Preston KL, Bigelow GE, et al. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274(1):361–372.
- Strain EC, Walsh SL, Preston KL, et al. The effects of buprenorphine in buprenorphine-maintained volunteers. Psychopharmacology. 1997;129(4):329–338.
- Schuh KJ, Walsh SL, Stitzer ML. Onset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humans. Psychopharmacology. 1999;145(2):162–174.
- Greenwald MK, Johanson CE, Schuster CR. Opioid reinforcement in heroin-dependent volunteers during outpatient buprenorphine maintenance. Drug Alcohol Depend. 1999;56(3):191–203.
- Comer SD, Collins ED, Fischman MW. Buprenorphine sublingual tablets: effects on IV heroin self-administration by humans. Psychopharmacology. 2001;154(1):28–37.
- Strain EC, Walsh SL, Bigelow GE. Blockade of hydromorphone effects by buprenorphine/naloxone and buprenorphine. Psychopharmacology. 2002;159(2):161–166.
- Greenwald MK, Schuh KJ, Hopper JA, et al. Effects of buprenorphine sublingual tablet maintenance on opioid drug-seeking behavior by humans. Psychopharmacology. 2002;160(4):344–352.
- Greenwald MK, Johanson CE, Moody DE, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28(11):2000–2009.
- Comer SD, Walker EA, Collins ED. Buprenorphine/naloxone reduces the reinforcing and subjective effects of heroin in heroin-dependent volunteers. Psychopharmacology. 2005;181(4):664–675.
- Correia CJ, Walsh SL, Bigelow GE, et al. Effects associated with double-blind omission of buprenorphine/naloxone over a 98-h period. Psychopharmacology. 2006;189(3):297–306.
- Greenwald M, Johanson CE, Bueller J, et al. Buprenorphine duration of action: mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry. 2007;61(1):101–110.
- Ling W, Wesson DR, Charuvastra C, et al. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch Gen Psychiatry. 1996;53(5):401–407.
- Schottenfeld RS, Pakes JR, Oliveto A, et al. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54(8):713–720.
- Fischer G, Gombas W, Eder H, et al. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction. 1999;94(9):1337–1347.
- Pani PP, Maremmani I, Pirastu R, et al. Buprenorphine: a controlled clinical trial in the treatment of opioid dependence. Drug Alcohol Depend. 2000;60(1):39–50.
- Fudala PJ, Bridge TP, Herbert S, et al. Buprenorphine/Naloxone Collaborative Study Group. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949–958.
- Kakko J, Svanborg KD, Kreek MJ, et al. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361(9358):662–668.
- Marsch LA, Bickel WK, Badger GJ, et al. Buprenorphine treatment for opioid dependence: the relative efficacy of daily, twice and thrice weekly dosing. Drug Alcohol Depend. 2005;77(2):195–204.
- Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365–374.
- Kakko J, Grönbladh L, Svanborg KD, et al. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry. 2007;164(5):797–803.
- Soyka M, Zingg C, Koller G, et al. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11(5):641–653.
- Rosenthal RN, Ling W, Casadonte P, et al. Buprenorphine implants for treatment of opioid dependence: randomized comparison to placebo and sublingual buprenorphine/naloxone. Addiction. 2013;108(12):2141–2149.
- Hser YI, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87.
- Gryczynski J, Mitchell SG, Jaffe JH, et al. Retention in methadone and buprenorphine treatment among African Americans. J Subst Abuse Treat. 2013;45(3):287–292.
- Mitchell G, Gryczynski J, Schwartz RP, et al. A randomized trial of intensive outpatient (IOP) vs. standard outpatient (OP) buprenorphine treatment for African Americans. Drug Alcohol Depend. 2013;128(3):222–229.
- Hoffman K, Peyton ML, Sumner M. Safety of a rapidly dissolving buprenorphine/naloxone sublingual tablet (BUP-NLX-RDT) for treatment of opioid dependence: a multicenter, open-label extension study. J Addict Med. 2017;11(3):217–223.
- Lee JD, Nunes E, Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309–318.
- Walsh SL, Comer SD, Lofwall MR, et al. Effect of Buprenorphine weekly depot (CAM2038) and hydromorphone blockade in Individuals with opioid use disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(9):894–902.
- Timko C, Schultz NR, Cucciare MA, et al. Retention in medication-assisted treatment for opiate dependence: a systematic review. J Addict Dis. 2016;35(1):22–35.
- Fareed A, Vayalapalli S, Casarella J, et al. Effect of buprenorphine dose on treatment outcome. J Addict Dis. 2012;31(1):8–18.
- Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207.
- Gunderson EW, Hjelmström P, Sumner M. Effects of a higher-bioavailability buprenorphine/naloxone sublingual tablet versus buprenorphine/naloxone film for the treatment of opioid dependence during induction and stabilization: a multicenter, randomized trial. Clin Ther. 2015;37(10):2244–2255.
- Jacobs P, Ang A, Hillhouse MP, et al. Treatment in opioid dependent patients with different buprenorphine/naloxone induction dosing patterns and trajectories. Am J Addict. 2015;24(7):667–675.
- Leonardi C, Hanna N, Laurenzi P, et al. Multi-centre observational study of buprenorphine use in 32 Italian drug addiction centres. Drug Alcohol Depend. 2008;94(1–3):125–132.
- Vigezzi P, Guglielmino L, Marzorati P, et al. Multimodal drug addiction treatment: a field comparison of methadone and buprenorphine among heroin- and cocaine-dependent patients. J Subst Abuse Treat. 2006;31(1):3–7.
- Pinto H, Maskrey V, Swift L, et al. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39(4):340–352.
- Apelt SM, Scherbaum N, Gölz J, et al. Safety, effectiveness and tolerance of buprenorphine-naloxone in the treatment of opioid dependence: results from a nationwide non-interventional study in routine care. Pharmacopsychiatry. 2013;46:94–107.
- Serre F, Fatseas M, Denis C, et al. Predictors of craving and substance use among patients with alcohol, tobacco, cannabis or opiate addictions: commonalities and specificities across substances. Addict Behav. 2018; 83:123–129.
- Willenbring ML, Hagedorn HJ, Postier AC, et al. Variations in evidence-based clinical practices in nine United States Veterans Administration opioid agonist therapy clinics. Drug Alcohol Depend. 2004;75(1):97–106.
- Trafton JA, Minkel J, Humphreys K. Determining effective methadone doses for individual opioid-dependent patients. PLoS Med. 2006;3(3):e80.
- González-Saiz F, Rojas OL, Trujols J, et al. Evidence of validity and reliability of the Opiate Dosage Adequacy Scale (ODAS) in a sample of heroin addicted patients in buprenorphine/naloxone maintenance treatment. Drug Alcohol Depend. 2018;183:127–133.
- Alcaraz S, González-Saiz F, Trujols J, et al. A cluster-analytic profiling of heroin-dependent patients based on level, clinical adequacy, and patient-desired adjustment of buprenorphine dosage during buprenorphine-naloxone maintenance treatment in sixteen Spanish centers.Drug Alcohol Depend. 2018;187:278–284.