?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
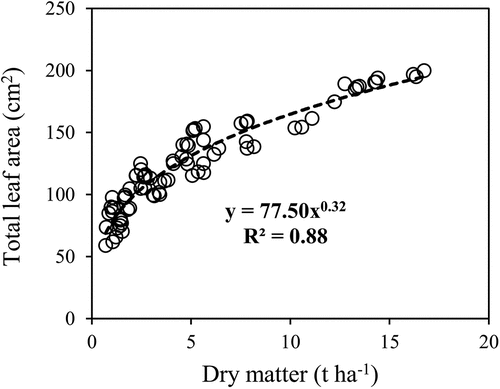
Considering the environmental issues linked to over fertilization, optimizing N fertilizer use is crucial for sustainable agriculture. Assessing the crop N status through calculating the N nutrition index (NNI) on the basis of critical N concentration (Nc) concept could help determine the minimum amount of N fertilizer to ensure maximum crop production. The present study aimed to develop, validate and compare three new critical N dilution curves (CNDCs) on the basis of lettuce total dry matter (TDM), total leaf area (TLA) and leaf area duration (LAD). Greenhouse experiments were conducted with six N application rates at the University of Tehran, Iran. Aboveground TDM, TLA and N concentration were measured at weekly intervals during the lettuce growth cycle. Relationships between Nc and TDM, TLA and LAD were described by three power functions (%Nc = 4.84 TDM−0.11, %Nc = 7.73 TLA−0.11, %Nc = 9.40 LAD−0.11). Furthermore, an allometric relationship between lettuce TDM and TLA (TLA = 0.75 TDM0.34) was obtained. The developed TLA and LAD-based CNDCs have the benefit of being non-destructive if TLA could be measured by portable devices. Overall, the CNDCs developed in the present research could provide accurate N status diagnosis for lettuce N management under greenhouse conditions.
Introduction
Nitrogen (N) is an essential element that restricts crop physiological processes. Application of N fertilizer in cropping systems is necessary to meet crop nutritional requirements and enhance agricultural production. Commonly, in intensive vegetable production, N fertilizers are applied in large quantities in order to achieve higher yields (Rodríguez et al. Citation2020). Most of the time, N application rates have surpassed the agronomic optimal levels, resulting in reduced environmental sustainability (Dong et al. Citation2020). Hence, a better understanding of crop N demand and status during the growing season can substantially improve N management in the agricultural systems (Huang et al. Citation2018; Zhang et al. Citation2022).
The concept of critical N concentration (Nc) (minimum N concentration required to attain maximum biomass) has been used extensively to determine the N status of crops (Ulrich Citation1952). Following the concept of Nc concentration, the N nutrition index (NNI: the ratio between actual and critical N concentration) has been established as an efficient and plant-based indicator to monitor crop N status during the growing season (Plénet and Lemaire Citation2000). Theoretically, values of Nc concentration are calculated as a function of aboveground biomass by a simple mathematical model called the critical N dilution curve (CNDC) (Plénet and Lemaire Citation2000). Various CNDCs have been developed for different crop species including maize (Plénet and Lemaire Citation2000), wheat (Justes et al. Citation1994), tomato (Tei et al. Citation2002), rice (Ata-Ul-Karim et al. Citation2013), basil (Rahimikhoob et al. Citation2020b) and barley (Zhao Citation2014) among others.
In all related studies (Justes et al. Citation1994; Plénet and Lemaire Citation2000; Ata-Ul-Karim et al. Citation2013; Zhao et al. Citation2018), it has been proved that the CNDCs can effectively distinguish between N-limiting and non-N-limiting treatments. However, the adaptation of dry matter-based CNDCs has several limitations in modern agriculture (Zhao et al. Citation2018). Crop dry matter (DM) measurement is commonly destructive and time consuming. Furthermore, the development of DM-based CNDCs in some crop species cannot assist in specifying the relationships among light interception and photosynthesis rate (Wang et al. Citation2017). Developing a CNDC based on variables other than DM provides detailed knowledge of the concept of N dilution and crop growth.
Leaf area (LA) is conceptually connected with the radiation fluxes through plant canopies, mass and energy exchange of carbon and water vapor (Woodgate et al. Citation2015). Leaf area (LA) has been reported to be a key variable in agronomic, physiological and horticultural research (Kumar et al. Citation2017; Qiang et al. Citation2019), and more specifically, it can be used in abiotic and biotic stresses impact assessment, growth monitoring and crop modelling studies. Furthermore, the use of LA in agricultural research has the advantage that it can be measured or estimated using portable rapid instruments (e.g. LI-3000C) or other non-destructive methods (e.g. remote sensing technology). Therefore, studying crop photosynthesis and growth rate in response to N availability is more practicable when expressed on the basis of LA.
Qiang et al. (Citation2019), established a LAI-based CNDC for winter wheat in Northwest China and indicated that the developed equation has the potential for diagnosing N status during the crop growth period. Also, an almost new CNDC for summer maize has been developed based on LAI data in China (Zhao et al. Citation2018). Their results demonstrated an approximately linear relationship between LAI and N uptake during the growth period. The developed LAI-based CNDC was identified as a valuable tool for maize N status diagnosis and monitoring. Another research was also conducted in China for construction of CNDC based on LAI of Japonica rice (Ata-Ul-Karim et al. Citation2014). They indicated that the LAI can be used as a reliable index to assess rice N status during the growing season.
Other than LAI-based CNDCs, Wang et al. (Citation2017) demonstrated that developing the CNDC based on leaf area duration (LAD) could better represent crop photosynthesis and DM accumulation as continuous processes. The product of the canopy leaf area and the leaves lifespan is defined as LAD. LAD is the integral of LA over time (Hunt Citation1978), incorporating general characteristics of TLA- and TDM-based CNDCs.
Previous studies have mainly focused on determining LAI and LAD-based CNDCs in cereals, there are few studies on vegetable crops, while these crops have a relatively lower N use efficiency compared to cereal crops (Rahimikhoob et al. Citation2020b). Moreover, agricultural producers commonly apply high amount of N fertilizers to ensure maximum biomass/yield of marketable products in vegetable crops. Therefore, in order to reduce the negative environmental impact of excessive nitrogen fertilization in vegetable production, it is imperative to adopt an efficient method to quantify nitrogen status throughout the growing period.
Lettuce (Lactuca sativa L.) belonging to the Compositae family is the most important and valuable leafy vegetable in the world (Paim et al. Citation2020). Despite its high nutritional value, lettuce can accumulate nitrates to potentially toxic and harmful concentrations (Renseigné et al. Citation2007). Therefore, developing a comprehensive approach to diagnose the lettuce N status is essential to control the rate of N fertilizer application and improve the quality of lettuce for human consumption. So far, two studies have been conducted to determine lettuce CNDC based on DM yield under Mediterranean climate conditions. In central Italy, a CNDC () was developed to monitor N nutrition of butterhead type of lettuce (Tei et al. Citation2003). Also, another research was carried out in southern Italy in order to determine butterhead (
) and crisphead (
) lettuce CNDCs (Conversa and Elia Citation2019). Since these studies have been conducted in the field, the developed curves could not be adapted to protected environments and therefore need evaluation. Controlled environment agriculture such as cultivation under greenhouse conditions, is an effective management technique for producing horticulture and high-value crops (Baudoin et al. Citation2017; Rahimikhoob et al. Citation2020a). Therefore, it seems worth doing research about developing a diagnostic approach for monitoring N nutrition of greenhouse crops. In addition, since both LA expansion and DM accumulation are influenced by the same N-regulatory mechanism (Lemaire et al. Citation2007), this raises the question of whether there is a theoretical relationship between lettuce TDM and TLA. The aims of this study were (i) to establish the lettuce CNDC based on TDM, TLA and LAD under greenhouse conditions and (ii) to compare the newly established dilution curves with previously developed ones.
Materials and methods
Experimental design
Two greenhouse experiments were conducted at the College of Agriculture and Natural Resources, University of Tehran, Karaj, Iran (50° 57′ E, 35° 48′ N, elevation 1292.9 m). The greenhouse had a multi-span structure with area of 8 × 5 m2 and its roof was covered by polycarbonate film. Meteorological parameters were measured inside the greenhouse from November 2021 to April 2022. Internal values of mean daily temperature and relative humidity during the experimental period were 20.5°C and 24.3%, respectively. Both trials were carried out in pots (diameter and depth of 25 and 17 cm, respectively) made of compact plastic filled by a loam textured soil (46% sand, 20% clay, 34% silt). The soil characteristics were 2.4% organic matter, pH 8.1, 0.2% total N, 52.2 mg kg−1 total K, 280 mg kg−1 total P, 1.33 g cm−3 bulk density, 21% field capacity and 10% permanent wilting point. Each experiment was arranged in a completely randomized design with 6 N fertilizer treatments and 3 replications.
Lettuce seedlings (Romaine) with 4 to 5 true leaves were transplanted into each pot (one seedling in a pot) and then well irrigated. The pots were placed about 40 cm apart. Information about dates of transplanting, harvest and sampling are given in . Five N fertilizer rates (0 (N0), 50 (N1), 100 (N2), 150 (N3), 200 (N4) and 250 (N5) kg ha−1) were considered for extracting the lettuce N dilution curve. Urea fertilizer was applied in 3 splits: 30,30 and 40% of the total amount at 17, 22 and 30 in the first and 14, 19 and 27 days after transplanting (DAT) in the second experiment. Four weighing micro-lysimeters were used to estimate irrigation requirements. Initially, micro-lysimeters were saturated and the soil surface was totally covered to prevent evaporation. Then, they were weighted in short time intervals until observations showed negligible weight loss. After about 72 hours, the measured values were considered as the pot capacity. Lettuce seedlings were also transplanted into the micro-lysimeters and the daily irrigation water requirement was calculated as follows:
Table 1. Information about two experiments conducted in the research greenhouse. (DAT: days after transplanting).
where i stands for the number of micro-lysimeters, is the irrigation water requirement,
is the weight of micro-lysimeter at pot capacity, and Wi is the weight of micro-lysimeter before irrigation.
Sampling and measurement
Crop samples were collected during the crop growth period in order to measure total dry matter (TDM), total leaf area (TLA, leaf area per plant), and total N content. Samples were oven dried at 70°C until constant weight. The dried samples (total biomass) were ground and then passed through a 1 mm sieve for total N determination. The N content in samples was quantified by Kjeldahl method (Kjeldahl Citation1883). Lettuce LA was measured by WinDIAS leaf area meter.
Values of LAD were calculated using EquationEquation 2(2)
(2) from the integration of LA over time (Peltonen-Sainio Citation1997).
The simplified approximation of LAD was obtained from EquationEquation 3(3)
(3)
where and
are the LA values calculated from EquationEquation 3
(3)
(3) at the sampling time t1 and t2, respectively.
The collected data from the first and second experiments were used for model development and validation, respectively.
Statistical analysis
The data for determining the critical N concentration (Nc) were analyzed according to the methodology described by Justes et al. (Citation1994). For the first experiment and in each sampling date, the amounts of TDM, TLA, LAD and total N concentration were subjected to analysis of variance (ANOVA) in IBM SPSS Version 25.0 (IBM Corporation, Chicago, IL, U.S.A.). The significant impacts of treatment on mean TDM, TLA and LAD were examined by using least significant difference (LSD 0.05) test, and the results were used to determine the breaking point between N-limiting and non-N-limiting treatments.
Critical N dilution curve and nitrogen nutrition index
Data from the first experiment were used to establish the CNDCs following the method of Justes et al. (Citation1994). The TDM, TLA and LAD-based CNDCs were expressed as follows (where a and b are the equation coefficients):
The maximum and minimum of CNDCs were generated by using data points from non-N-limiting and N-limiting treatments, respectively. It should be noted that data from the first sampling of the first experiment were not considered, since the TDM was less than 1 t ha−1. Also, the average %Nc values between two consecutive samplings were used to develop LAD-based CNDC.
The NNI at each sampling date was calculated by dividing the actual N concentration (Na) by Nc (Lemaire and Gastal Citation1997):
Values of NNI close to 1 represent optimum N nutrition. Values of NNI < 1 indicate N-limiting, while values of NNI > 1 correspond to luxury N consumption.
Since, LAD is an integrated variable, values of NNI (from LAD-based CNDC) was also integrated. The integrated NNI (NNIint) values were calculated by the weighted mean of NNI during the growth period as follows (Lemaire et al. Citation2008):
where, NNIint is the integrated NNI, T is length of growing period (days or growing degree-days), is the instantaneous NNI at different sampling dates and dt is the interval between two samplings.
Results
TDM, TLA, LAD and N concentration at different N levels
N application rate showed a significant impact on TDM, TLA and LAD during both lettuce growing periods. As shown in , the values of TDM, TLA, and LAD increased with increasing N application rate. Lettuce aboveground TDM increased continuously from 0.36 to 5.62 and 1.05 to 16.20 t ha−1 during the first and second experiments, respectively. Total LA values ranged from 25 to 192 and 52 to 238 cm2 during the first and second cultivations, respectively. Also, there was an increase in LAD from 244 to 1253 and 428 to 1607 cm2 days−1 in the first and second experiments, respectively. In contrast, dry matter N concentration gradually declined during both experiments. The comparison between the first and second experiment showed higher TDM production, TLA and LAD in the second trial since the second experiment was carried out in March and April when the solar radiation was much higher.
Figure 1. Changes of total dry matter (TDM), total leaf area (TLA), leaf area duration (LAD) and accumulated nitrogen (N) concentration under different N treatments during the first and second experiments.
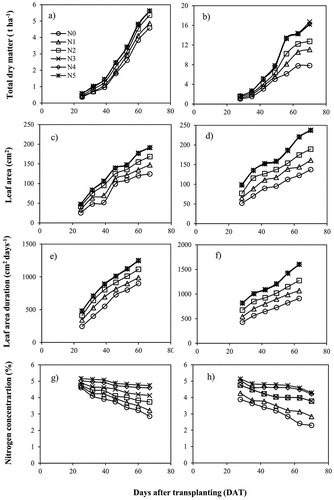
During the first experiment, TDM, TLA, and LAD increased significantly from N0 to N3 treatments (0–150 kg N ha−1) but similar response was observed in N3 and higher (>150 kg N ha−1), and this determines the breaking point (N3 treatment) between N-limiting and non-N-limiting treatments. The following statistical inequalities from the LSD test were observed among different N treatments during the first experiment:
where TDM0, TDM1, TDM2, TDM3, TDM4 and TDM5 are the total dry matter values of treatments 0, 50, 100, 150, 200 and 250 kg N ha−1, respectively. The same explanation applies to the TLA and LAD.
Determination of CNDCs
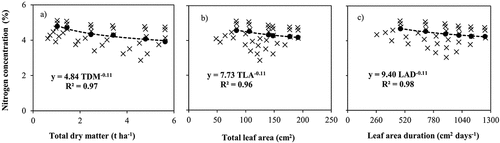
Based on the data from the first experiment, lettuce Nc values were determined for each sampling date. Then the CNDCs were described by negative power functions (). The R2 values of the fitted curves were 0.97, 0.96 and 0.98 for TDM, TLA and LAD, respectively. The constant Nc values for low TDM, TLA and LAD were determined as the average value of the minimum N concentration under non-N-limiting conditions and the maximum N concentration under N-limiting conditions. The proposed Nc values for TDM, LA and LAD-based curves were 4.84, 7.73 and 9.40% respectively.
Validation of CNDCs
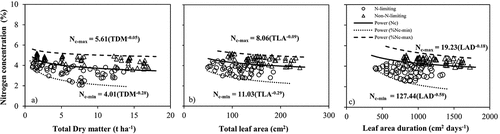
The obtained CNDCs were examined using independent datasets (n = 126) of the second experiment (). The results demonstrated that the developed curves discriminate well between non-N-limiting and N-limiting treatments. Most of the data points from non-N-limiting condition were close to or higher than the Nc curve, while the data points from N-limiting condition were close to or lower than the Nc curve. Also, maximum and minimum CNDCs were generated as a function of TDM, TLA and LAD, which are presented in .
Dynamic changes of NNI/NNIint under different N treatments
Dynamic changes of NNI/NNIint values for different N treatments are represented in . Based on the TDM-based CNDC, NNI values varied from 0.70 to 1.19 and from 0.60 to 1.23 for the first and the second experiments, respectively. The TLA-based NNI ranged from 0.65 to 1.15 for the first and 0.54 to 1.14 for the second experiment. Also, the LAD-based NNIint values varied from 0.69 to 1.14 and from 0.53 to 1.15 for the first and the second experiments, respectively. In all CNDCs, NNI/NNIint increased by increasing the N application rate at each sampling date. In contrast, NNI/NNIint decreased over time in N deficient treatments.
Figure 4. Changes in the nitrogen nutrition index (NNI) and integrated NNI (NNIint) under different nitrogen application rates for a) total dry matter-first experiment, b) total dry matter-second experiment, a) Total leaf area-first experiment, a) Total leaf area- second experiment, a) lLeaf area duration-first experiment and a) Leaf area duration-second experiment.
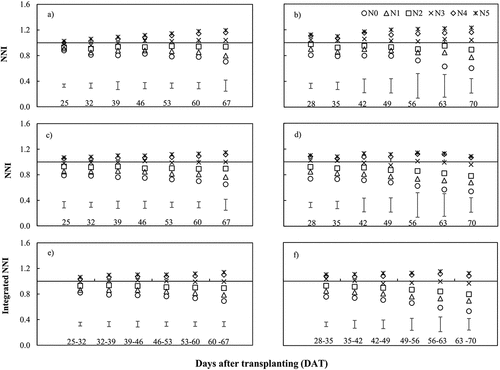
According to , the NNI/NNIint values in both experiments were approximately one for the N3 treatment, indicating an optimal crop N status for sustained growth of lettuce. In contrast, the NNI/NNIint values of N4 and N5 treatments were greater than one, indicating luxury use of N fertilizer.
Relationship between NNI and relative dry matter
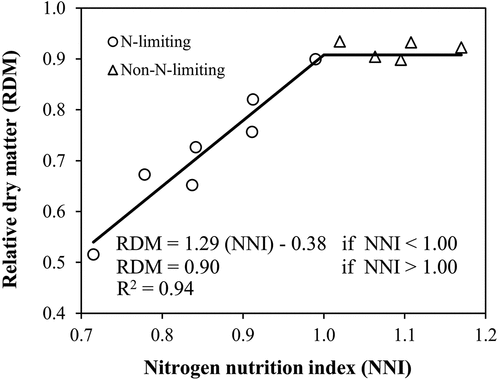
The relative dry matter (RDM) was defined as the ratio of the final DM at harvest obtained at a given N application rate to the maximum DM among other N fertilizer treatments. The NNI values used for the model extraction were taken from the TDM-based CNDC. Based on the data from both experiments, the RDM-NNI relationship was determined by the linear-plateau function and programming in RStudio software version 4.0.1 (RStudio, Inc., Boston, MA, U.S.A.). The linear-plateau model was defined by the following equation:
where Y represents relative dry matter (RDM), X is NNI, a is the intercept, b is the linear coefficient, C is the critical NNI value, determined from the intersection of the regression and horizontal line and P is the plateau value. As shown in , RDM was 0.90 (plateau value) when NNI was higher than 1.00, and RDM decreased when NNI was lower than 1.00.
Discussion
TDM-based CNDC
In the present study, a robust allometric relationship between lettuce TDM and N concentration was established. According to the developed equation (%Nc = 4.84 TDM−0.11), there is a declining trend of N concentration with dry matter accumulation, which is defined as the crop N dilution process. A comparison between the lettuce CNDC and previously developed ones, is presented in . As illustrated in , the developed TDM-based CNDC was above the previously developed ones. The parameter value of the coefficient ‘a’ was quite identical to the value of reference curve developed by Lemaire and Gastal (Citation1997) for C3 crop species. However, the value obtained for parameter ‘b’ in the current study was lower than the value reported for the generic Nc dilution curve of C3 crop species. Different values for parameter ‘b’ could be the result of shoot DM distribution differences between crop species and genetic variations (Zhao Citation2014; Ata-Ul-Karim et al. Citation2017).
Figure 6. Comparison of lettuce critical nitrogen dilution curve developed in the present study with previously developed ones.
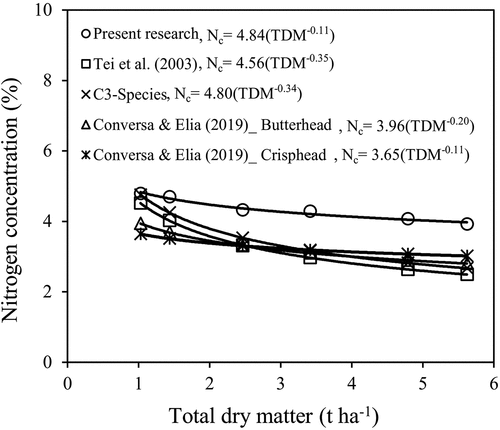
According to , slight differences were observed between the coefficients of the curve developed by Tei et al. (Citation2003) and current study particularly for the ‘b’ coefficient. There was a faster reduction of N concentration in response to crop growth in Tei et al. (Citation2003) equation compared to the current research. The ‘b’ coefficient values between the developed CNDCs in Conversa and Elia (Citation2019) and the present study were quite similar, however, there was a notable difference between the values of ‘a’ coefficient. The differences might be attributed to varied local climatic conditions and the crop growth duration that affect the range of TDM accumulation (Rahimikhoob et al. Citation2020b). As, the research of Conversa and Elia (Citation2019) was carried out under open field conditions and 2.94 to 3.62 t ha−1 TDM was produced, in contrast the TDM production in the present study ranged from 0.36 to 5.62 t ha−1 under greenhouse conditions for the first experiment. Differences between lettuce TDM-based CNDCs confirm the need for local calibration before implementing the relationships in a new environment.
TLA-based CNDC
The lettuce CNDC was described empirically by a negative power function relating crop N concentration to TLA (). The gradual decline of N concentration as a result of LA enhancement was in agreement with previous studies (Zhao et al. Citation2014, Citation2018; Ata-Ul-Karim et al. Citation2014). The TDM-based and TLA-based CNDCs can theoretically be linked by an allometric relationship (TLA = k TDMp) shown in . Higher LA results in higher radiation interception and thus a greater biomass and DM accumulation. Leaf photosynthetic response to radiation is highly dependent on N availability (Sadras and Calderini Citation2009). It has been reported that DM accumulation and LA expansion interact to uptake N from the soil and that they represent two perspectives on the same N regulation process (Lemaire et al. Citation2008). Since leaf area measurement can be performed more rapidly using non-destructive methods, the TLA-based CNDC could be a more practical alternative to the TDM-based CNDC. In addition, LA measurements can be repeated in time and space, so that high-precision information on the spatio-temporal dynamics of the LA can be collected, which is useful for in-season N management of crops.
LAD-based CNDC
The critical N concentration in lettuce could be estimated based on the values of LAD, which integrates the size and duration of the photosynthetic area. The results () showed that LAD was affected by different N fertilizer levels, mainly due to the accelerated LA expansion process. Similar results have been reported for winter wheat cultivated in China (Wang et al. Citation2017). There is a positive correlation between TLA, LAD and agricultural production, i.e. high crop LA indicates an increased ability to capture photosynthetically active radiation and is therefore one of the major reasons for achieving higher production. It has been stated that at high N application rates, leaf senescence is delayed, allowing plants to sustain high LA for longer period of time, thus increasing photosynthesis rate and TDM accumulation (Fan et al. Citation2023). In general, LAD-based CNDCs appear to be more appropriate for integration into the crop simulation models. Although, it should be noted that the LAD is not an instantaneous parameter and therefore it cannot be used for the rapid detection of N deficiency conditions.
Analysis of lettuce CNDCs with different bases
The newly developed lettuce CNDCs on three different bases are illustrated in . According to , in all lettuce CNDCs, the coefficient ‘b’ is in the lower range of the reported values of different C3 crop species such as basil (0.38, Rahimikhoob et al. Citation2020b), tomato (0.33, Tei et al. Citation2002), winter barley (0.39, Zhao Citation2014), rice (0.28, Ata-Ul-Karim et al. Citation2013), wheat (0.44, Justes et al. Citation1994), cotton (0.13, Xiaoping et al. Citation2007), and soybean (0.08, Divito et al. Citation2016). The attenuated lettuce CNDCs could be attributed to the cultivation density. In this research, our experiment was not a dense planting cultivation. Therefore, compared to a high planting density, there was no mutual shading effect of the plants. A comparison of the curves developed in this study () indicates that the coefficient ‘b’ is exactly the same in all three dilution curves. Accordingly, it can be concluded that the process of nitrogen dilution in lettuce is the same in different bases if cropped isolated.
It has been reported that the N dilution process is significantly affected by cultivation density (Seginer Citation2004). High planting densities lead to mutual shading of the leaves between individual plants and result in a larger negative exponent (coefficient ‘b’). Xiaoping et al. (Citation2007) and Lemaire et al. (Citation2005), highlighted that the coefficient ‘b’ varies at different crop densities. Lemaire et al. (Citation2005), reported the value of the coefficient ‘b’ for lucerne crop to be 0.12–0.15 when cropped isolated and 0.28 for those from dense stands. Therefore, our findings support the idea of a low ‘b’ coefficient for plants when cropped isolated (Lemaire et al. Citation2007). In addition, low values of the coefficient ‘b’ indicate the most efficient physiological N utilization during the crop growth period (Ata-Ul-Karim et al. Citation2017). Overall, when using the CNDCs for nitrogen fertilizer management, special attention should be paid to the cultivation/planting density. Planting density is a determinant factor affecting the coefficients of the N dilution curve. Therefore, it is suggested to investigate the impact of different plant densities on the coefficients of the lettuce critical N dilution curve in future studies. In addition, as agriculture evolves with innovations such as vertical farming gaining prominence, it becomes imperative to consider the unique challenges posed by this cultivation method. In vertical farming setups, where plants are grown in stacked layers, leaves from upper layers may cast shadows on those below, affecting light distribution and potentially altering the N status of shaded leaves. Therefore, for future research endeavors, it is essential to explore the implications of vertical farming on the coefficients of the lettuce CNDCs. Furthermore, it is recommended to analyze the uncertainties in the coefficients of the lettuce Nc dilution curves and to specify the source of variations between different management and environments.
NNI/NNIint variations
The NNI and NNIint during the lettuce growth period was calculated using the TDM, TLA and LAD-based CNDCs. The results demonstrated that the NNI/NNIint values were significantly affected by the N fertilization rates (). In each sampling date, the NNI/NNIint values decreased with decreasing N application level. In addition, a temporal dynamic change was observed during the growth period of lettuce. However, the reduction rate of NNI/NNIint was higher under N0 treatment. Since the value of NNI equal to 1 indicates sufficient N fertilizer application, it can be concluded that the optimal N application rate for lettuce under greenhouse conditions is approximately 150 kg ha−1, which is consistent with (Tei et al. Citation2003; Barickman et al. Citation2018) studies. NNI could be used as a reliable indicator for lettuce N status monitoring under greenhouse conditions. Particularly, if it could be determined by portable instruments such as SPAD-502 (Soil Plant Analysis Development), Yara sensor, etc. These portable devices can estimate crop N nutrition status indirectly and non-destructively also; their prediction accuracy has been proven in many researches (Zhao et al. Citation2016; Ravier et al. Citation2017; Jiang et al. Citation2021). Based on the NNI values obtained during the growth period, the crops’ response to different N fertilization frequencies and intensities could be analyzed effectively. Furthermore, diagnosing crop N status based on the concept of critical N concentration allows the identification of situations where physiological processes are not restricted by N deficiency, thus helping to establish crop growth models as a result of other growth limiting factors. Overall, more investigations are required to further evaluate these CNDCs for diagnosing lettuce N status in different regions of the world.
Lettuce RDM and NNI relationship
According to , the response of TDM production to NNI was expressed using the linear-plateau regression analysis. Below the critical NNI value (NNIc = 1.00), RDM decreased and above it there was no additional increase in RDM. The developed RDM-NNI relationship could guide in in-season lettuce DM estimation and contribute to precision agriculture. Moreover, the obtained RDM-NNI relationship could be used for developing growth prediction models and analyzing different management options such as the impact of split N fertilizer application on lettuce production. Although the parameters of the RDM-NNI relationship appear to be measured destructively, they could be estimated by indirect methods. For example, TDM could be calculated from the previously mentioned allometric relationship of TLA = k TDMp from LA data derived from non-destructive instruments. Also, NNI could be estimated based on the indirect methods already discussed.
Conclusions
Previously developed lettuce critical nitrogen dilution curves were only based on total dry matter. The total leaf area and leaf area duration could be used as a novel approach for determining lettuce critical nitrogen concentration without collecting the crop. In the present research, leaf area and leaf area duration-based critical nitrogen dilution curves were determined in addition to the total dry matter-based relationship for lettuce cultivated under greenhouse conditions. The total dry matter, total leaf area and leaf area duration-based critical nitrogen dilution curves were described by %Nc = 4.84 TDM−0.11, %Nc = 7.73 TLA−0.11 and %Nc = 9.40 LAD−0.11 equations, respectively. Results showed that, lettuce nitrogen concentration decreased as the crop total dry matter, leaf area and leaf area duration increased. Obtained allometric relationships were validated by the data of second experiment. Results indicated that the developed critical nitrogen dilution curves have the ability to distinguish non-N-limiting from N-limiting treatments accurately. Since plant leaf area can be rapidly and non-destructively estimated by several instruments or approaches, total leaf area and leaf area duration-based critical nitrogen dilution curves could be considered as a potential alternative to overcome the difficulties associated with total dry matter-based approach for assessing lettuce nitrogen status.
A gradual decline in lettuce nitrogen nutrition index was observed for treatment with no nitrogen fertilizer application. In contrast, the value of nitrogen nutrition index was approximately 1 for the optimal application of nitrogen fertilizer. Lettuce dry matter production was expressed as a function of the nitrogen nutrition index by fitting a linear-plateau function. The established relationship could provide useful information for decision making in lettuce production. Also, the critical nitrogen dilution curves developed in the present study together with the nitrogen nutrition index provides a guideline for in-season lettuce nitrogen fertilizer management under greenhouse conditions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ata-Ul-Karim S.T, Yao X, Liu X, Cao W, Zhu Y. 2013. Development of critical nitrogen dilution curve of Japonica rice in Yangtze River reaches. Field Crops Res. 149:149–158. doi: 10.1016/j.fcr.2013.03.012.
- Ata-Ul-Karim S, Zhu Y, Yao X, Cao W. 2014. Determination of critical nitrogen dilution curve based on leaf area index in rice. Field Crops Res. 167:76–85. doi: 10.1016/j.fcr.2014.07.010.
- Ata-Ul-Karim S.T, Zhu Y, Liu X, Cao Q, Tian Y, Cao W. 2017. Comparison of different critical nitrogen dilution curves for nitrogen diagnosis in rice. Sci Rep. 7(1):42679. doi: 10.1038/srep42679.
- Barickman T, Sublett W, Miles C, Crow D, Scheenstra E. 2018. Lettuce biomass accumulation and phytonutrient concentrations are influenced by genotype, N application rate and location. Horticulturae. 4(3):12. doi: 10.3390/horticulturae4030012.
- Baudoin W, Nersisyan A, Shamilov A, Hodder A, Gutierrez D, De Pascale S, Nicola S, Gruda N, Urban L, Tanny J. 2017. Good agricultural practices for greenhouse vegetable production in the south east European countries. Rome: Food and Agriculture Organization of the United Nations (FAO).
- Conversa G, Elia A. 2019. Growth, critical N concentration and crop N demand in butterhead and crisphead lettuce grown under Mediterranean conditions. Agronomy. 9(11):681. doi: 10.3390/agronomy9110681.
- Divito G.A, Echeverría H.E, Andrade F.H, Sadras V.O. 2016. Soybean shows an attenuated nitrogen dilution curve irrespective of maturity group and sowing date. Field Crops Res. 186:1–9. doi: 10.1016/j.fcr.2015.11.004.
- Dong S, Zhang J, Zha T, Dai X, He M. 2020. Increased plant density with reduced nitrogen input can improve nitrogen use efficiency in winter wheat while maintaining grain yield. Arch Agron Soil Sci. 66(12):1707–1720. doi: 10.1080/03650340.2019.1690139.
- Fan P, Ming B, Evers J.B, Li Y, Li S, Xie R, Anten N.P. 2023. Nitrogen availability determines the vertical patterns of accumulation, partitioning, and reallocation of dry matter and nitrogen in maize. Field Crops Res. 297:108927. doi: 10.1016/j.fcr.2023.108927.
- Huang S, Yuxin M.I, Qiang C.A, Yinkun Y.A, Guangming Z.H, Weifeng Y.U, Jianning S.H, Kang Y.U, Bareth G. 2018. A new critical nitrogen dilution curve for rice nitrogen status diagnosis in Northeast China. Pedosphere. 28(5):814–822. doi: 10.1016/S1002-0160(17)60392-8.
- Hunt R. 1978. Plant growth analysis. The inst bio studies bio Edward Arnold (Pub.) Ltd. 96:8–38.
- Jiang J, Wang C, Wang H, Fu Z, Cao Q, Tian Y, Zhu Y, Cao W, Liu X. 2021. Evaluation of three portable optical sensors for non-destructive diagnosis of nitrogen status in Winter wheat. Sensors. 21(16):5579. doi: 10.3390/s21165579.
- Justes E, Mary B, Meynard J.M, Machet J.M, Thelier-Huch´e L. 1994. Determination of a critical nitrogen dilution curve for winter wheat crops. Ann Bot. 74(4):397–407. doi: 10.1006/anbo.1994.1133.
- Kjeldahl J. 1883. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Fresenius J Anal Chem. 22(1):366–382. doi: 10.1007/BF01338151.
- Kumar M.K, Kumar R.S, Sankar V, Sakthivel T, Karunakaran G, Tripathi P.C. 2017. Non-destructive estimation of leaf area of durian (Durio zibethinus) – an artificial neural network approach. Sci Hortic (Amsterdam). 219:319–325. doi: 10.1016/j.scienta.2017.03.028.
- Lemaire G, Avice J.C, Kim T.H, Ourry A. 2005. Developmental changes in shoot N dynamics of lucerne (medicago sativa L.) in relation to leaf growth dynamics as a function of plant density and hierarchical position within the canopy. J Exp Bot. 56(413):935–943. doi: 10.1093/jxb/eri084.
- Lemaire G, Gastal F. 1997. N uptake and distribution in plant canopies. In: Lemaire G, editor. Diagnosis of the nitrogen status in crops. Heidelberg: Springer-Verlag; pp. 3–43.
- Lemaire G, Jeuffroy M.H, Gastal F. 2008. Diagnosis tool for plant and crop N status invegetative stage: theory and practices for crop N management. Eur J Agron. 28(4):614–624. doi: 10.1016/j.eja.2008.01.005.
- Lemaire G, van Oosterom E, Sheehy J, Jeuffroy M.H, Massignam A, Rossato L. 2007. Is crop N demand more closely related to dry matter accumulation or leaf area expansion during vegetative growth? Field Crops Res. 100(1):91–106. doi: 10.1016/j.fcr.2006.05.009.
- Paim B, Crizel R, Tatiane S, Rodrigues V, Rombaldi C, Galli V. 2020. Mild drought stress has potential to improve lettuce yield and quality. Sci Hortic (Amsterdam). 272:109578. doi: 10.1016/j.scienta.2020.109578.
- Peltonen-Sainio P. 1997. Leaf area duration of oat at high latitudes. J Agron Crop Sci. 178(3):149–155. doi: 10.1111/j.1439-037x.1997.tb00483.x.
- Plénet D, Lemaire G. 2000. Relationships between dynamics of nitrogen uptake and dry matter accumulation in maize crops. Determination of critical N concentration. Plant Soil. 216(1/2):65–82. doi: 10.1023/A:1004783431055.
- Qiang S, Zhang F, Dyck M, Zhang Y, Xiang Y, Fan J. 2019. Determination of critical nitrogen dilution curve based on leaf area index for winter wheat in the guanzhong plain, Northwest China. J Integr Agric. 18(10):2369–2380. doi: 10.1016/s2095-3119(19)62688-2.
- Rahimikhoob H, Sohrabi T, Delshad M. 2020a. Assessment of reference evapotranspiration estimation methods in controlled greenhouse conditions. Irrig Sci. 38(4):389–400. doi: 10.1007/s00271-020-00680-5.
- Rahimikhoob H, Sohrabi T, Delshad M. 2020b. Development of a critical nitrogen dilution curve for basil (Ocimum basilicum L.) under greenhouse conditions. J Soil Sci Plant Nutr. 20(3):881–891. doi: 10.1007/s42729-020-00174-5.
- Ravier C, Quemada M, Jeuffroy M. 2017. Use of a chlorophyll meter to assess nitrogen nutrition index during the growth cycle in winter wheat. Field Crops Res. 214:73–82. doi: 10.1016/j.fcr.2017.08.023.
- Renseigné N, Umar S.H, Qbal M. 2007. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron Sustain Dev Springer. 27(1):45–57. doi: 10.1051/agro:2006021.
- Rodríguez A, Peña-Fleitas M.T, Gallardo M, de Souza R, Padilla F.M, Thompson R.B. 2020. Sweet pepper and nitrogen supply in greenhouse production: critical nitrogen curve, agronomic responses and risk of nitrogen loss. Eur J Agron. 117:126046. doi: 10.1016/j.eja.2020.126046.
- Sadras V.O, Calderini D. 2009. Crop physiology: applications for genetic improvement and agronomy. London: Elsevier Academic Press.
- Seginer I. 2004. Plant spacing effect on the nitrogen concentration of a crop. Eur J Agron. 21:369–377. doi: 10.1016/j.eja.2003.10.007.
- Tei F, Benincasa P, Guiducci M. 2002. Critical nitrogen concentration in processing tomato. Eur J Agron. 18(1–2):45–55. doi: 10.1016/S1161-0301(02)00096-5.
- Tei F, Benincasa P, Guiducci M. 2003. Critical nitrogen concentration in lettuce. Acta Hortic. 627(627):187–194. doi: 10.17660/ActaHortic.2003.627.24.
- Ulrich A. 1952. Physiological bases for assessing the nutritional requirements of plants. Annu Rev Plant Physiol. 3(1):207–228. doi: 10.1146/annurev.pp.03.060152.001231.
- Wang X, Ye T, Ata-Ul-Karim S, Zhu Y, Liu L, Cao W, Tang L. 2017. Development of a critical nitrogen dilution curve based on leaf area duration in wheat. Front Plant Sci. 8. doi: 10.3389/fpls.2017.01517.
- Woodgate W, Disney M, Armston J, Jones S, Suarez L, Hill M, Wilkes P, Soto-Berelov M, Haywood A, Mellor A. 2015. An improved theoretical model of canopy gap probability for leaf area index estimation in woody ecosystems. For Ecol Mana. 358:303–320. doi: 10.1016/j.foreco.2015.09.030.
- Xiaoping X, Jianguo W, Zhiwei W, Wenqi G, Zhiguo Z. 2007. Determination of a critical dilution curve for nitrogen concentration in cotton. J Plant Nutr Soil Sci. 170(6):811–817. doi: 10.1002/jpln.200620627.
- Zhang K, Jifeng M.A, Yu W.A, Weixing C.A, Yan Z.H, Qiang C.A, Xiaojun L.I, Yongchao T.I. 2022. Key variable for simulating critical nitrogen dilution curve of wheat: leaf area ratio-driven approach. Pedosphere. 32(3):463–474. doi: 10.1016/S1002-0160(21)60086-3.
- Zhao B. 2014. Determining of a critical dilution curve for plant nitrogen concentration in winter barley. Field Crops Res. 160:64–72. doi: 10.1016/j.fcr.2014.02.016.
- Zhao B., Ata-Ul-Karim S.T, Duan A, Liu Z, Wang X, Xiao J, Qin A, Ning D, Zhang W, Lian Y. 2018. Determination of critical nitrogen concentration and dilution curve based on leaf area index for summer maize. Field Crops Res. 228:195–203. doi: 10.1016/j.fcr.2018.09.005.
- Zhao B, Liu Z, Ata-Ul-Karim S.T, Xiao J, Liu Z, Qi A, Ning D, Nan J, Duan A. 2016. Rapid and nondestructive estimation of the nitrogen nutrition index in winter barley using chlorophyll measurements. Field Crops Res. 185:59–68. doi: 10.1016/j.fcr.2015.10.021.
- Zhao B, Yao X, Tian Y, Liu X, Ata-Ul-Karim S.T, Ni J, Cao W, Zhu Y. 2014. New critical nitrogen curve based on leaf area index for winter wheat. Agron J. 106(2):379–389. doi: 10.2134/agronj2013.0213.