Abstract
Drawing on global value chain analysis, this article discusses the possibilities for upgrading in a domestically oriented import-dependent industry. The pharmaceutical industry in Uganda consists of a large number of importers, nine of which have upgraded into assembly and four a step further into manufacturing. The industry upgrades by a process of ‘learning by importing’. Although not engaged with global buyers, pharmaceutical producers in Uganda are tied into the global pharmaceutical value chain by international linkages with their suppliers, mainly in India, from whom they access technology and intermediates for production. Hence, this industry is based on South–South networks for production of low-value pharmaceutical products. With the globalisation of the pharmaceutical industry, an increasing number of global lead firms are ceasing to manufacture these products. This study illustrates an alternative form of industrialisation and upgrading that has not been adequately considered in the development of the African pharmaceutical industry.
1. INTRODUCTION
There are growing incentives for African countries to manufacture products that are necessary to ensure public health, such as pharmaceuticals. First, following the establishment of the World Trade Organisation and the Agreement on Trade Related Aspects of Intellectual Property Rights (the TRIPs Agreement), intellectual property rights such as patents on pharmaceuticals have become increasingly globalised. The option of importing generic versions of patented products has diminished, and hence access to low-cost copies of new products has decreased, mainly because of the implementation of TRIPs in the producing countries such as India and China. Although World Trade Organisation member states have the legal option to issue compulsory licences, this has so far been applied by few developing countries, and primarily to products aimed at domestic markets. So far, Rwanda is the only country that has issued a compulsory licence for import. The products were anti-retrovirals from Canada and they reached Rwanda in September 2008 (International Centre for Trade and Sustainable Development, Citation2008). Second, the sources of off-patent, low-cost pharmaceuticals–such as India, Brazil and China–are redirecting their production to high-value markets (see Haakonsson, Citation2009, for the case of India). Accounting for only 1 per cent of the global pharmaceutical market in 2003, the market in Africa is not attractive to global pharmaceutical companies (Africa Research Bulletin, Citation2003). Moreover, the global pharmaceutical companies tend increasingly to focus on cardiovascular diseases, cancer and diabetes, and are abandoning tropical diseases such as sleeping sickness, river blindness and malaria (Cohen-Kohler et al., Citation2008).
Little research has been done on the possibilities of and constraints to establishing and upgrading pharmaceutical industries in sub-Saharan Africa (Abdelgafar, Citation2003; Kaplan & Laing, Citation2005; Cohen et al., Citation2005; Nkrumah & Osawa, Citation2007). Generally, production of pharmaceuticals in Africa is limited. Domestic production accounts for 10 per cent of demand in Africa, on average, and it is supplied by relatively few companies: 15 in Kenya, 20 in Zimbabwe, nine in Uganda and four each in Cameroon, Senegal and Côte d'Ivoire (Export Processing Zones Authority, Citation2005; Africa Research Bulletin, 2003). In South Africa and Nigeria, subsidiaries of foreign companies are engaged in manufacturing pharmaceuticals for regional markets. But generally the African pharmaceutical industry is only linked to Northern companies to a limited extent. On paper, building a national pharmaceutical industry is a high priority in most African countries (African Union, Citation2007). In practice, however, the potential for establishing and upgrading pharmaceutical industries in Africa has remained unexplored.
Based on fieldwork carried out in Uganda in late 2003, this article analyses the circumstances under which a pharmaceutical industry has upgraded through upstream linkages with suppliers, here called ‘learning by importing’. Despite the constraints of being landlocked, dependent on imports and servicing a small market, a small domestic pharmaceutical industry has developed in Uganda over the past 20 years. This development has been driven by a process of learning through imports of intermediates, technology and know-how from suppliers in the global pharmaceutical value chain. The producing part of the industry consists of nine players manufacturing low-technology, off-patent pharmaceuticals (i.e. generics). All of the products manufactured in Uganda are simple formulations for domestic consumption, mainly treatments for the most common ailments and diseases (antimalarial drugs, painkillers, cough syrups, rehydration salts). Since the 1970s, pharmaceutical companies in Uganda have upgraded from their original activities as importers of finished pharmaceuticals (i.e. formulations) to repackaging formulations imported in bulk, assembling ready-mixed packages of intermediates and, for four of the companies, manufacturing their own equipment. Today, the domestic industry supplies 10 per cent of the national pharmaceutical market (National Drug Authority [NDA], Citationn.d.).
Of the nine producing companies, four have annual turnovers above US$1 million and can be categorised as large-scale manufacturers in the Ugandan context. They have strong international linkages to suppliers in India and China. Hence, they rely on South–South networks in pharmaceutical-producing developing countries rather than on the classic global value chain linkages with northern companies that are research-intensive and transnational. The literature on upgrading in global value chains (GVCs) has focused merely on developing countries linking up with northern lead firms as suppliers. It has also so far ignored the role of the importing industries. Furthermore, in analysing linkages between developing-country suppliers and developed-country buyers, the GVC literature has focused overwhelmingly on the concept of governance. This study shows that it is the institutional framework, policies of liberalisation, privatisation and government capacity-building programmes rather than GVC governance that have played a major role in determining the prospects for the Ugandan pharmaceutical industry.
The analysis is based on formal and informal interviews with the managing directors of all nine pharmaceutical companies, combined with interviews with 11 importers and retailers of generics and branded products, and the government importer. The interviews have been coded for reasons of confidentiality. The codes refer to the interviews presented in and . Likewise, the identity of other informants has been concealed to ensure confidentiality.
Table 1: Pharmaceutical producers in Uganda
Table 2: Importing companies interviewed during fieldwork
The following section presents the theoretical framework used in this article: GVC analysis. Section 3 presents the salient features of the Ugandan pharmaceutical industry. This industry has developed on the basis of a combination of linkages upstream (international) and downstream (national), and the institutional framework within which the industry exists. In Section 4, the forms of upgrading that have taken place in the industry are discussed in the context of these linkages. The process of upgrading is discussed in terms of ‘South–South relations’ and ‘learning by importing’. The concluding section uses the experience of the Ugandan pharmaceutical industry to reflect on GVC analysis as a tool for analysing importing industries in developing countries.
2. UPGRADING IN GLOBAL VALUE CHAINS
GVC analysis examines the social and economic processes involved in production and trade by examining four dimensions: input–output structure, territoriality, governance and the institutional framework. These are typically used for understanding processes in, and the consequences of, the recent global reorganisation of production for actors in various positions in a value chain. GVC analysis concerns ‘the full range of activities, including coordination, that are required to bring a specific product from its conception to its end use and beyond’ (Gibbon & Ponte, Citation2005:77). For research in developing countries, this has resulted in a number of analyses of how producers are linked into GVCs as suppliers for buyers in developed-country end-markets (Gereffi et al., Citation1994; Fold, Citation2001; Gibbon & Ponte, Citation2005).
Moreover, GVC analysis has primarily been used to analyse the consequences of export-oriented development strategies at the firm level as well as the national level, whether for industrial production (i.e. in the newly industrialised countries) or agricultural production (in least developed countries). This article shows that GVC analysis can also be applied to analyses of domestically oriented import-dependent industries, such as the pharmaceutical industry in Uganda. Moreover, GVC analysis can significantly benefit the analysis of marginalised industries, their prospects for upgrading and the obstacles they face.
Upgrading has become a central concept for understanding how industrial development can be achieved and measured. The concept has its origins in development research concerning the shift in industrial development from Fordism to post-Fordism. In this research, producers are said to upgrade when they move from assembly to own-equipment manufacture (OEM) to own-design manufacture, and further to own-brand manufacture. This development represents the industrial ‘high road’ to industrialisation that has been observed in newly industrialised Asian countries (Gereffi, Citation1999; Dicken, Citation2007). Gereffi defines upgrading as ‘a process of improving the ability of a firm or an economy to move to more profitable and/or technologically sophisticated capital- and skill-intensive economic niches’ (Gereffi, Citation1999:51–2). Upgrading is said to operate at the factory, firm, local, national and regional levels.
Humphrey and Schmitz Citation(2000) have developed a typology of upgrading, which includes:
-
product upgrading; that is, moving to more value-added products – in the case of pharmaceuticals, this means branded and/or patented products;
-
process upgrading; that is, producing more efficiently; and
-
functional upgrading; that is, occupying more value-adding positions in the chain–for example, engaging in design or research and development.
Schmitz and Knorringa Citation(2001) have assessed under what circumstances developing country producers upgrade by ‘learning from global buyers’. In their study of the world footwear industry, they observe three sets of circumstances that have an impact on producer potentials for upgrading through ‘learning from global buyers’:
-
the current state of the producer; for example, newly established producers need more assistance from their buyers–the involvement of the buyer decreases with more experienced producers;
-
whether the chain is quality-driven or price-driven; for example, if the products are of high quality, the buyers are interested in upgrading their suppliers and developing mutual relationships; and
-
the type of upgrading taking place, as it is unlikely that buyers facilitate functional upgrading to the level of their own core activities, such as design and marketing in the footwear sector (Schmitz & Knorringa, Citation2001).
The concept of technological capabilities complements the GVC institutional framework by incorporating conditions for industrial development at the national level (trade policy, domestic industrial policies, physical infrastructure, human capital, banks, property rights, commercial law), as well as at the firm level (experience, technology transfer, research, investment capabilities). All of these factors influence an industry's performance. At the firm level, technological capabilities can take the form of investment capabilities (the skills needed to identify, prepare and obtain technology); production capabilities (quality control, operation and maintenance, and the ability to absorb technologies, imitated from elsewhere); and linkage capabilities (the skills required to receive and transmit information from suppliers or consultants) (Lall, Citation1987, Citation1992). In the case study examined in the following sections, the firms gained their technological capabilities by learning from suppliers. This unleashes only limited possibilities for upgrading. As Lall (Citation1992:181) points out: ‘Easy capabilities may be acquired by brief training combined with learning-by-doing (i.e. repetition without technical search, investment or experimentation) … the developing country affiliate receives the results of innovation, not the innovative process itself’.
In this article, upgrading through ‘learning by importing’ is used to describe a process in which industries in downstream (e.g. marketing and retail) positions in a GVC may upgrade within activities and markets that are not core for lead firms. Hence, learning by importing involves adopting technology and knowledge from suppliers, which is possible only to the extent that suppliers are willing to share the relevant knowledge. This process may result in process upgrading as a result of technology transfer, for example by suppliers of machinery; product upgrading, as suppliers of intermediates or knowledge have the capabilities needed to produce new products; and functional upgrading, where a supplier improves firm-level technological capabilities. Thus, ‘learning by importing’ is a process that involves different suppliers and leads to different upgrading outcomes.
In order to upgrade, pharmaceutical industries in developing countries need access to reliable sources of knowledge and technology from either their global buyers or their international suppliers. This links the companies to what can be identified as global production (or information) networks. Although not producing for the pharmaceutical GVC, domestically oriented import-dependent developing-country pharmaceutical industries can learn from it as importers of technology. In such situations, the suppliers become the determinants of what technology is available for overcoming the entry barriers facing the developing-country producers and furthermore their possibilities for upgrading. In the following section, GVC analysis is used to examine the Ugandan pharmaceutical industry.
3. THE DEVELOPMENT OF THE UGANDAN PHARMACEUTICAL INDUSTRY
Over the past two decades, the pharmaceutical industry in Uganda has developed almost from scratch into an industry covering 10 per cent of the domestic market. Political instability caused it to collapse in the period from 1975 to 1985. According to the Information Officer at the NDA, ‘All the factories stopped. Some facilities were closed down and some were not cared for. There was no organised body to inspect the products’ (interview, 14 October 2003). A report from the United Nations Industrial Development Organisation Citation(1972) concluded that pharmaceutical production in East Africa was at the best substandard, and at worst non-existent.
Since the late 1980s, the Ugandan political economy has shifted from economic decline, conflict and a repressive government to increasing macroeconomic stability and higher growth levels. This process was initiated when President Museveni came into power in 1986 with an ambitious programme of economic liberalisation and post-conflict recovery (Hansen & Twaddle, Citation1991; Reinikka & Collier, Citation2001). The Economic Recovery Programme of 1987, developed with assistance of the World Bank, the International Monetary Fund and other international donor agencies, was intended among other things to improve the macroeconomic environment by creating a favourable business climate and to encourage private entrepreneurs investing in the country. From 1985 to 1999, the average annual growth rate of the Gross Domestic Product was 6.4 per cent (Basu & Srinivasan, Citation2002). Inflation declined from a level of more than 360 per cent in the early 1980s to −2 per cent in 1993 (Zake, Citation1995). At the microeconomic level, the private sector grew with increasing individual firm size and investments in the manufacturing sector (Reinikka & Collier, Citation2001). However, poor delivery of public services seems to be one of the most serious obstacles to investment and growth (Obwona, Citation1999; Obwona & Mugumbe, Citation2001; Reinikka & Collier, Citation2001).
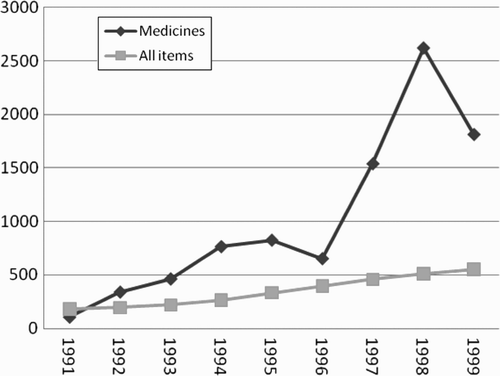
Liberalisation and privatisation have constituted the policy environment of the Ugandan pharmaceutical industry since the early 1990s. Manufacturing of pharmaceuticals in Uganda only emerged in the private sector in the 1990s. Prior to this there were three state-owned production units, whose main activities were the repackaging of formulations imported in large quantities. These three units were sold to private investors. The pharmaceutical industry grew dramatically during the 1990s, at almost four times the average rate for total industrial production (see ), but it has remained relatively small. The highest growth occurred with the privatisation of two state-owned pharmaceutical plants. The industry depends entirely on foreign suppliers for all its inputs, and manufacturers face severe competition from imported low-cost formulations. India and China in particular have price advantages in the market, with their subsidised exports, larger production sites and markets, cheap and qualified labour, and locally produced bulk drugs and raw materials. The market for pharmaceuticals in Uganda is growing: imports of pharmaceuticals and other medical products accounted for over 5 per cent of Uganda's total imports in 1999, ranking third after petroleum (8.9 per cent) and road vehicles (7.1 per cent) (Uganda Bureau of Statistics, Citation2003). The value of medicine imports more than tripled from US$19.6 million in 1993 to US$73.7 million in 1999 (the value of domestic production was US$7.4 million in 1999).
Figure 1: Growth in production of medicines and total industrial production in Uganda 1991–1999, annual summary (Index 1987=100)
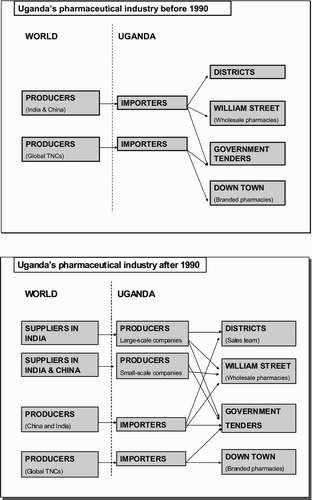
The Ugandan pharmaceutical industry consists of two sectors: the formal sector, monitored by the NDA, which includes manufacturing companies and pharmacies that produce and distribute pharmaceutical products; and the informal sector, which includes ‘traditional’ medicine operators and self-taught pharmacists, whose activities are directed towards their local markets. Informal production is not covered here. The formal manufacturing sector has nine players, as presented in . Four of them (large-scale companies, UP1–UP4) have manufacturing plants producing tablets, syrups and lotions. Of these, two companies (UP2 and UP3) account for 50 per cent of total pharmaceutical production in Uganda. The other five (small-scale companies, UP5–;UP9) have limited production facilities and capacities, mostly with small manufacturing sites or production facilities connected to pharmacies or retail outlets in Kampala.
All of the pharmaceutical companies have a history as importers of medicine, some of them since the early 1970s. They developed into packaging companies when they started importing large quantities that they could repackage into smaller dosage packs at their facilities. During the 1980s, some of these turned into pharmaceutical manufacturers importing less processed packages (pharmaceutical ready-mix) of intermediates they could ‘mix-and-bottle’, primarily for cough or quinine syrups, anti-malarial drugs and painkillers – the products most commonly used in Uganda. During this period the pharmacies upgraded functionally from repackaging to simple (assembly) production. Three of the small-scale companies are still engaged in the repackaging and assembly of ready-mix, hence their functional upgrading consisted of one step: from importing to assembly. With one exception, all of the companies are still working as importing agents.
When the Ugandan Government sold two of its pharmaceutical production facilities in the mid-1990s they were bought by families already engaged as pharmaceutical wholesalers in Uganda. These two companies eventually became large-scale pharmaceutical manufacturers. Later, two other companies expanded their production facilities, financed through joint ventures with foreign investors from India and the United Kingdom. These four large-scale companies have functionally upgraded to manufacturing their own equipment (OEM). illustrates the overall shift in activities carried out by the industry during the 1990s, from importing medicines to producing simple formulations.
Pharmaceutical production has increased steadily since the early 1990s and, according to managing directors in the industry, is still growing. The following sections examine the switch that some companies made from importing to manufacturing, through an analysis of their upstream linkages to actors in the global pharmaceutical value chain, their downstream linkages, and their institutional framework. As the pharmaceutical products manufactured in Uganda are relatively simple, the entry barriers in this industry are poor access to information, low level of technology, inadequate access to reliable sources of intermediates and limited market reach.
3.1 International upstream linkages
The Ugandan industry, manufacturers as well as importers, depends entirely on accessing formulations (final pharmaceutical products) and bulk drugs (intermediates) from the global pharmaceutical and chemical value chains. Contrary to what has been found in most of the GVC literature–namely that developing countries rely on vertical linkages downstream in the GVCs (i.e. international buyers)–international linkages with actors upstream in this chain determine the companies’ ability to access the knowledge, information and technology necessary for engaging in and maintaining pharmaceutical production. Such linkages vary from arm's-length market relations to personal networks with suppliers and to vertical integration (with foreign ownership).
The upstream linkages are particularly strong in large-scale companies, which may also be the reason why these companies are large; that is, they have better access to inputs. All of the large-scale companies have direct relationships with foreign companies: three are joint ventures, co-owned by either Indian companies or in one case an English company, and one has family ties with a pharmaceutical producer in India. In this part of the industry, the foreign suppliers of inputs are key actors in helping local manufacturers to overcome the entry barriers and to upgrade.
To upgrade functionally into manufacturing and to improve efficiency further (i.e. process upgrading), the Ugandan companies need imported and specialised technology and machinery. At the time of the fieldwork, one of the large-scale companies (UP2) had ordered a new machine from India that would increase the production of tablet strips from 50 to 240 per minute while using the same level of labour. This machine was bought through their contacts and investors in India. A lack of linkages upstream is a massive barrier to upgrading for others. To illustrate the case, one small-scale company (UP7) with weak international linkages ordered second-hand machinery from India, but received it with spare parts missing, the wrong specifications and defective functioning. Without direct upstream linkages, the companies’ prospects for process upgrading are restricted to second-hand machinery spillovers from India, China or Brazil.
Three of the small-scale companies do not have linkages with foreign suppliers but link up with them when they have the opportunity. These companies typically import bulk drugs and low technology from a single supplier as a ‘sample’ they can mix, pack and sell. Functionally, therefore, they can be seen as assembly and in some cases (re-)packaging companies. Access to suppliers of chemicals is much easier for those with established personal networks in India that can facilitate the delivery of safe products at fair prices. Buying bulk drugs in the chemical spot-market is akin to gambling. Thus, it is a common experience for the companies to receive low-standard bulk drugs, which further limits their prospects for upgrading in the industry.
When it was taken over from the state in 1995, UP3 had a product range of 10 products. By 2003 this had increased to 42 different products, and the company employed 160 people. The information about new products and processes comes from abroad. Besides the direct vertical linkages, access to information depends on the individual company's ability to access the channels available through international trade fairs, the Internet and agents in India. The companies recognise the importance of accessing different sources. The managing director of one of the larger companies (UP1) explained:
We keep a good and wide network and buy from many places, mainly in India, China, the EU and US, and sometimes find new suppliers via the Internet, trade fairs, or by using intermediate companies who can pack a container and ship it from India … We have a key person in India who develops new ideas and technology.
I went to India to learn how to manufacture and I started small-scale production. I bought the machinery and materials from the same people … it is still them I get the materials from. Our relationship goes back 12 years.
The importing companies, retailers and wholesalers of pharmaceuticals are linked to specific suppliers abroad. Approximately 70 per cent of the pharmaceuticals sold in Uganda are produced in India. According to the importers, this is because ‘We have good connections in India and we know whom to trust and how to deal with them’ (UI2; see ). This explains why many of those who have upgraded into manufacturing direct their upstream linkages primarily towards India. Only two wholesalers in Uganda import from global transnational corporations, but a large number of them import from producers in India, China, and the Middle East (see ). The importing companies obtain marketing materials from the producers and are also trained in selling the products, again by the producing companies.
3.2 Downstream national linkages
In Uganda, domestic networks exist both among pharmaceutical producers and between producers and the importers and retailers. The first kind of networks exhibit a very limited degree of cluster dynamics. Horizontal linkages among producers are predominantly ad hoc relationships among the large-scale companies. According to their managers, these are limited to lending bulk drugs to each other when there are shortages, or sharing a large order from the government by licensing parts of the production to others. This was the case for a local antimalarial product, where the producer did not have sufficient production capacity (in terms of scale) and shared an order with two other companies. Only the large-scale companies cooperate horizontally, since their products are equivalent and well established as generics in the domestic market. Hence there is no division of labour between the companies and no specialisation, or, as the managing director of a large-scale company (UP2) put it: ‘We choose our product range according to the demand. The market is big enough for all of us.’
Domestic networks of the second kind, between producers and the importers and retailers, vary as well. Most companies have distribution systems for reaching rural areas, often through contacts with doctors, pharmacists and drug shop owners in the districts. Teams of medical representatives for each company travel into the districts to provide information about their products, deliver them, and establish credit arrangements with their buyers. This market reach, grounded in their downstream networks, is one of the main comparative advantages held by local manufacturers. One company (UP3) explained that their sales teams visit customers in the districts monthly, delivering the previous month's order and collecting payment for the products they delivered on their previous visit. With this arrangement, the drug shops do not need access to capital and may buy what they think they can sell, only paying for the products after they have been sold.
Another service, provided only by the large-scale companies, is taking back date-expired products. However costly, this ensures that their products are on the shelves (because the companies take back expired drugs, more pharmacies are willing to sell their products, since they will not lose money) and helps establish a good corporate image, as they risk acquiring a bad reputation if a patient takes the medicine and it is ineffective. Unfortunately this service was not provided by all suppliers of medicines, and many date-expired products were found in the rural drug shops. Finally, all of the companies have outlets in the pharmaceutical wholesale market area in William Street in Kampala, from where they sell primarily to traders buying for pharmacies and drug shops in Uganda, but occasionally also to traders from Rwanda, Burundi and Sudan. However, regional exports are small and occasional.
The fact that small-scale companies have developed, despite weak upstream linkages in the value chain, indicates that downstream linkages have an impact on their possibilities for upgrading. Moreover, they have substituted imported products with own-produced ones. These companies are owned by local pharmacists who, on their own, have functionally upgraded into production in order to increase their competitiveness in the market (UP5, UP8). The strategies of both small-scale and large-scale companies are generally driven by the idea of substituting imports of the most commonly used products; as one of them (UP1) said, ‘we can distribute the essential medicine better and produce it at the same quality as our competitors in India and China’. The domestic companies have experience and good insights into the market, as evidenced by the following remark: ‘We never go into a brand new product for the market. Our products replace imports, and we know the market well’ (UP3).
The companies aim to make their products look ‘professional’ and to distinguish them from each other's by establishing domestic brands. The managing director of UP4 put it this way: ‘Past performance determines your position in the market’. Although the industry sells in the local market, there is a strong need for their products to look like the imported ones, so the industry is constantly improving its packaging materials. Some materials are available locally, but the flashy ones that make the products ‘look like real medicine’ are imported from India (UP1).
The pharmaceutical industry also relies on some domestic inputs. Until recently, plastic bottles were produced locally. According to the manufacturers, this production closed down and plastic bottles are now imported from India and Kenya (glass bottles available from India are preferred for the more expensive products as they ‘make the products look like imported products’; UP2). Cardboard boxes are still used domestically.
3.3 Institutional framework
In its function as a producer of essential medicines, the pharmaceutical industry in Uganda is highly regulated. Regulation is twofold: by the government (the Ministry of Health and NDA), and by the international donor community, which, according to the Head of Pharmacy, finances 57 per cent of the health budget. Sometimes these two complement each other, sometimes they are in conflict, and sometimes internal conflicts occur within each one (Haakonsson & Richey, Citation2007). However, there is a political will to support the development of this industry. As an official from the Ministry of Tourism, Trade and Industry said: ‘We should be able to make a solid pharmaceutical industry. Because it is needed … and we are well located to supply Rwanda, Congo and Sudan. They have large markets.’ Furthermore, during the process of liberalisation and privatisation the Government of Uganda asked the Commonwealth Secretariat to help develop a pharmaceutical industry in Uganda, which was initiated in 1995. The aim was to establish production of medicine for the local market and save foreign exchange while introducing new technology. The result was a programme developed and carried out in partnership with a Ugandan pharmacy that is now operating as a small-scale manufacturing company.
Currently, the industry is being supported by the government as well as by the donors. For example, the domestic industry is allowed 10 per cent higher prices in bids on public tenders than international bidders. Most of the drugs used by government institutions are bought, distributed and managed by the government import and wholesale institution, National Medical Stores, which accounts for 25 per cent of the Ugandan pharmaceutical market and has the most developed distribution system in Uganda, covering all districts, regional hospitals and local clinics. National Medical Stores influences overall price levels, as their operations are not-for-profit and prices are kept as low as possible. The government also runs a quality control unit under the NDA, whose task is to guide domestic companies in reaching higher levels of quality. Its main function is to supervise the production of pharmaceuticals and approve new products for the market. However, the NDA is also empowered to temporarily close production facilities that do not meet the national quality requirements.
Few donors pay attention to local industrialisation when dealing with public health programmes. When they have done so, the outcome has varied. One large-scale company (UP4) had a partnership with a donor for supplying rehydration salts to the government. During fieldwork, their products covered almost the entire market for rehydration salts in Uganda. They were sold in two different packages: blue bags for sale, and red bags for free distribution by government and donors. According to the manager, they faced a problem as products labelled ‘not for sale’ were occasionally resold by health personnel at lower prices than the actual production costs. Hence, although they managed to establish a strong domestic brand, the company faced severe competition from its own products. Besides this, donors sometimes sponsor a product for a special health programme, for example against tuberculosis or malaria. The market for the treatment of these diseases may disappear or decrease drastically immediately after the donation, and whatever medicine the local industry has in stock may expire before the market is re-established. Alternatively, if the programmes buy from local manufacturers, the demand increases dramatically.
In summary, the Ugandan pharmaceutical industry operates only in the downstream activities in the South–South strand of the global pharmaceutical value chain; namely, in the local importing, assembly, production and marketing segments. The products are simple, and this strand of the pharmaceutical value chain is price-driven. Inputs for production are obtained from abroad, mainly from industry contacts in India. Likewise, information, technology and machinery for functional, product and process upgrading are facilitated by their upstream linkages. Companies with strong international linkages therefore have better access to upgrading than locally based ones. As the main strategy of this industry is to substitute imports with locally manufactured goods, one could argue that product upgrading does not take place simply because the companies are already engaged in marketing the products they have started producing. However, there is in fact continuous product upgrading; for example, when companies improve product quality or change their packaging materials to make them look more like ‘real’ medicines. Downstream linkages give the Ugandan companies advantages in terms of demand knowledge in the end-markets.
4. DISCUSSION: UPGRADING BY SOUTH–SOUTH NETWORKS AND LEARNING BY IMPORTING
As shown above, the pharmaceutical industry in Uganda has functionally upgraded during the past 20 years. It did so firstly from imports of finished pharmaceuticals to packaging and assembly and secondly, in the case of the large-scale companies, to OEM. This upgrading took place even though the companies were never engaged as suppliers to lead firms in the pharmaceutical GVC. Moreover, the upgrading was facilitated by a combination of their upstream vertical linkages to suppliers, their existing linkages downstream in the chain as importers and retailers of pharmaceuticals for the domestic market, and by the institutional framework (the process of liberalisation, industrialisation and privatisation led by the national government and donor programmes). This section discusses the types of upgrading (functional, process and product upgrading) that have occurred in the industry and the possibilities for further upgrading along this path within the new institutional framework of the global pharmaceutical value chain.
Functional upgrading into OEM was made possible for the large-scale companies by their capacity to access international networks of knowledge and to obtain investments from abroad, mainly India. Hence, ‘learning by importing’ has enabled these companies to initiate production by taking in inputs from their partners in joint ventures or from their foreign investors. As the industry consisted of a large number of importers, only nine of them managed to upgrade into assembly, and only four functionally upgraded further into manufacturing. This path is likely to continue as an option for importers with strong upstream vertical networks, although at the company level. The recent joint venture setting up an ARV production site illustrates this possibility.
However, further functional upgrading in the industry (into own-design manufacture or own-brand manufacture) is unlikely to happen as there are neither R&D facilities nor plans to build them. The companies face the entry barriers of limited access to skilled personnel and a limited market size (UP1, UP2, UP4). They are already having problems finding personnel for their quality control departments, and the domestic and regional markets are price-driven, so there is no demand for patented high-value products. Besides, in the process of upgrading via ‘learning by importing’, the companies have functionally upgraded by importing finished technologies and knowledge, not by learning production methods as such. Although the companies have functionally upgraded into OEM, this has been done parasitically, by copying their suppliers. Hence production is at a low level technologically and has not increased the companies’ technological capabilities in terms of higher prospects for further functional upgrading.
Process upgrading in the Ugandan pharmaceutical industry has been facilitated by South–South linkages, such as trading networks. The machinery and technology for producing more efficiently are those dominating the small and medium-sized companies in developing countries, primarily India, as there is a huge technology gap between the Ugandan industry and the Northern companies. The companies have upgraded by taking over second-hand technology from their suppliers in developing countries.
The Ugandan pharmaceuticals market is characterised by high demand for low-value generics. Hence, process upgrading (to improve economies of scale) is the main strategy for all companies. However, access to more efficient equipment depends on the reliability of their individual access to machinery from outside. Their vertical upstream linkages determine the risks involved in how they access machinery: whether from their parent company, from long-term relations with suppliers of technology, or from the spot market. As a result, large-scale companies with strong vertical linkages upstream have greater possibilities for process upgrading than small-scale ones with weak linkages.
Product upgrading remains a limited strategy for this industry. The market consists of relatively poor people in need of basic medicine, and there is no scope for producing high-value products in Uganda. Likewise, the producers are not introducing new products into the market, but are replacing imports with in-house production. However, the companies do upgrade their products; for example, by improving packaging materials and product quality. One way to encourage product upgrading further could be for donors and the government to tie up with domestic industries while developing new health projects and treatment programmes. This would require the government to engage in new types of public–private partnership by taking a two-sided development agenda of trade and health into account. Treating these two as one interconnected nexus would potentially benefit the domestic industry.
5. CONCLUSION
This article has shown that GVC analysis, although so far primarily used in analysing export-oriented industries linked to North-bound GVCs as suppliers, is relevant for studies of upgrading prospects in import-dependent, domestically oriented developing-country industries. Moreover, there is scope for extending the concept of functional upgrading from an understanding of ‘learning by exporting’ or ‘learning from global buyers’ to encompass industries that have upgraded functionally through imports, which is what the author has called ‘learning by importing’.
The case study of Uganda has shown that developing pharmaceutical production in a sub-Saharan African country is a rational idea and has possibilities (although perhaps limited ones) for upgrading and consolidation. So far, upgrading in the Ugandan pharmaceutical industry has come about entirely through imports of knowledge, technology and machinery, aimed at increasing product value (product upgrading) and improving efficiency (process upgrading). The functional upgrading was based on the companies’ linkages upstream in the chain and resulted in a shift in activities from importing to manufacturing pharmaceuticals. The companies upgraded through technology transfers from their suppliers in a process of ‘learning by importing’, which was facilitated by South–South networks among producers and their suppliers in the low-value segment of the global pharmaceutical value chain.
Although the overall prospects for further upgrading into the global pharmaceutical value chain seem extremely limited, this may in fact be a fortunate situation for the industry. The domestic market is growing and, given the global reorganisation of the industry, other sources of low-cost pharmaceuticals are drying up. The producers in India and China who were earlier known as the ‘producers for the poor’ are increasingly focusing their production on developed markets as a result of their new TRIPs-compliant patent regimes; or, alternatively, on taking in more contract production from lead firms in developed countries. The growing market for low-value pharmaceuticals in Africa heightens the potential for establishing and upgrading domestic pharmaceutical industries. These markets are not as profitable as developed country markets, and are thus less interesting for northern-based companies. Process and product upgrading through ‘learning by importing’ and functional upgrading through import substitution remain viable strategies for African pharmaceutical industries–whether domestically oriented as in Uganda, or oriented to regional markets as in South Africa and Nigeria.
Notes
REFERENCES
- ABDELGAFAR , B I . 2003 . Implications of the WTO: TRIPs Agreement from a National Innovations Systems perspective. The pharmaceutical industry in Egypt . PhD thesis, Department of Public Policy and Administration, Carleton University, Ottawa
- AFRICA RESEARCH BULLETIN, 2003. Pharmaceutical industry, Africa's market. Africa Research Bulletin, Economic, Financial and Technical Series, 40(1), 16 April–15 May.
- AFRICAN UNION . Pharmaceutical manufacturing plan for Africa . Paper presented at the Third Session of the African Union Conference of Ministers of Health . April 9–13 , Johannesburg.
- BASU , A and SRINIVASAN , K . 2002 . Foreign Direct Investment in Africa: some case studies , Washington, DC : IMF . International Monetary Fund Working Paper No. 02/61
- COHEN , J C , GYANSA-LUTTERODT , M , TORPEY , K , ESMAIL , L C and KUROKAWA , G . 2005 . TRIPs, the Doha Declaration and increasing access to medicines: policy options for Ghana . Globalisation and Health , 1 : 17
- COHEN-KOHLER , J C , FORMAN , L and LIPCUS , N . 2008 . Addressing legal and political barriers to global pharmaceutical access: options for remedying the impact of the Agreement on Trade Related Aspects of Intellectual Property Rights (TRIPs) and the imposition of TRIPs-plus . Health Economics, Policy and Law , 3 : 229 – 56 .
- DICKEN , P . 2007 . Global shift: mapping the changing contours of the world economy , 5 , London : Sage .
- EXPORT PROCESSING ZONES AUTHORITY . 2005 . Kenya's pharmaceutical industry 2005 , Nairobi : EPZA .
- FOLD , N . 2001 . Restructuring of the European chocolate industry and its impact on cocoa production in West Africa . Journal of Economic Geography , 1 ( 4 ) : 405 – 20 .
- GEREFFI , G . 1999 . International trade and industrial upgrading in the apparel commodity chain . Journal of International Economics , 48 : 37 – 70 .
- GEREFFI , G , KORZENIEWICZ , M and KORZENIEWICZ , R P . 1994 . “ Introduction: global commodity chains ” . In Commodity chains and global capitalism , Edited by: Gereffi , G and Korzeniewicz , M . London : Praeger .
- GIBBON , P and PONTE , S . 2005 . Trading down: Africa, value chains, and the global economy , Philadelphia : Temple University Press .
- GIULIANI , E , PIETROBELLI , C and RABELLIOTTI , R . 2005 . Upgrading in global value chains: lessons from Latin American clusters . World Development , 33 ( 4 ) : 549 – 73 .
- GOVERNMENT OF UGANDA . 1991 . The Patents Act , Kampala : Ministry of Justice and Constitutional Affairs .
- HAAKONSSON , S J . 2009 . “ How does ‘linking up with global buyers’ impact the prospects for upgrading in pharmaceuticals? The case of India ” . In Transnational corporations and development policy: critical perspectives , Edited by: Rugraf , A , Sanchez-Ancochea , D and Sumner , A . Basingstoke : Palgrave .
- HAAKONSSON , S J and RICHEY , L A . 2007 . TRIPs and public health: the Doha Declaration and Africa . Development Policy Review , 25 ( 1 ) : 71 – 90 .
- HANSEN , H B and TWADDLE , M . 1991 . Changing Uganda: the dilemmas of structural adjustment and revolutionary change , London : James Curry .
- HUMPHREY , J and SCHMITZ , H . 2000 . Governance and upgrading: linking industrial cluster and global value chain research , University of Sussex, Brighton . IDS Working Paper 120, IDS
- HUMPHREY , J and SCHMITZ , H . 2002 . How does insertion in global value chains affect upgrading in industrial clusters? . Regional Studies , 36 ( 9 ) : 1017 – 27 .
- INTERNATIONAL CENTRE FOR TRADE AND SUSTAINABLE DEVELOPMENT . 2008 . “ First batch of generic drugs en route to Africa under 5-year-old WTO deal ” . In Bridges Weekly Trade Review , Geneva : ICTSD . 12(31), September
- KAPLAN , W and LAING , R . 2005 . Local production of pharmaceuticals: industrial policy and access to medicines. An overview of key concepts, issues and opportunities in future research , Washington, DC : World Bank . World Bank Health Nutrition and Population Discussion Paper
- LALL , S . 1987 . Learning to industrialize: the acquisition of technological capability in India , London : Macmillan .
- LALL , S . 1992 . Technological capabilities and industrialization . World Development , 20 ( 2 ) : 165 – 86 .
- LUNDVALL , B Å . 1998 . Why study national systems and national styles of innovation? . Technology Analysis and Strategic Management , 10 ( 4 ) : 407 – 22 .
- NATIONAL DRUG AUTHORITY . n.d . National Drug Authority , www.nda.or.ug/index.php Accessed 31 August 2007
- NKRUMAH , Y K and OSAWA , P L . 2007 . TRIPs compliance and public health: opportunities and obstacles for African countries . International Journal of Biotechnology , 9 ( 2 ) : 138 – 55 .
- OBWONA , M . 1999 . Foreign Direct Investments growth linkages and institutional constraints in sub-Saharan Africa: a case of Uganda . African Review of Money Finance and Banking , 1999 : 99 – 125 .
- OBWONA , M and MUGUME , A . 2001 . Credit accessibility and investment decisions in Uganda's manufacturing sector: an empirical investigation . African Review of Money Finance and Banking , 1 January : 75 – 101 .
- REINIKKA , R and COLLIER , P . 2001 . “ Reconstruction and liberalization: an overview ” . In Uganda's recovery: the role of farms, firms and government , Edited by: Reinikka , R and Colliers , P . Kampala : Fountain Publishers .
- SCHMITZ , H and KNORRINGA , P . 2001 . Learning from global buyers . Journal of Development Studies , 37 ( 2 ) : 177 – 205 .
- SCHMITZ , H and NADVI , K . 1999 . Clustering and industrialization: introduction . World Development , 27 ( 9 ) : 1503 – 14 .
- UGANDA BUREAU OF STATISTICS . 2003 . Index of Industrial Production Annual Summary: Report on the Uganda Business Register 2001/2002 , www.ubos.org/industry Accessed 12 August 2003
- UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANISATION . 1972 . Establishment of a pharmaceutical sector in the East African community , UNIDO Interim Report. Paris: UNIDO
- WHITLEY , R . 1996 . Business systems and global commodity chains: competing or complementary forms of economic organisation? . Competition & Change , 1 ( 4 ) : 411 – 25 .
- ZAKE , J . 1995 . “ creating an enabling environment for the development of small-scale enterprises through tax reforms: the case of Uganda, 1986–93 ” . In Agents of change: studies of the policy environment for small enterprises in Africa , Edited by: English , P and Hénault , G . Ottawa : International Development Research Centre .