Abstract
This study aims at studying the reproductive aspects of Mugil curema in two coastal systems in southeastern Brazil. Samplings were carried out from March 2009 to February 2010 in Santos estuary and from May 2008 to April 2009 in the Cananéia-Iguape coastal system, using gillnet, cast net, and fish trap. Weight and total length were measured and, subsequently, gonads were removed and weighed. Permanent preparations on the ovaries were carried out. The sexual proportion, first gonadal maturation length (L 50), and the quantitative indicators (gonadosomatic index and ΔK) were estimated for all seasons of the year. There was a significant predominance of females (1:2.4) in both areas, and L 50 was estimated at 248.6 mm. The species showed a synchronous oocyte development and the highest values of quantitative indicators were observed at the start and end of the rainy season. The mean potential and relative fecundity of the species was estimated at 350,430 oocytes and 926.46 oocytes/g, respectively, for individuals ranging from 300 to 402 mm. In conclusion, M. curema from the Cananéia-Iguape coastal system and from Santos estuary showed the start of gonadal maturation in October, which extended to April, with two annual spawning events (April and November), possibly in more offshore areas.
Introduction
Variations in length at first gonadal maturation (L 50), spawning season, sexual proportion, oocyte development, and fecundity have been described as reproductive tactics with a high level of variability and are important for the success of a generation or cohort resulting from spawning (Frank and Leggett Citation1994; Vazzoler Citation1996; Morita and Morita Citation2002). These tactics have spatial and temporal intraspecific variations related mainly to abiotic and biotic conditions prevailing in the area occupied or in the period when the population was subject to those conditions (Vazzoler Citation1996).
Knowing such tactics is of utmost importance to manage and evaluate fish stocks, because those tactics are used to set the minimum lengths for capture, to determine the mesh size for the nets used, as parameters to estimate the spawning biomass, in projection models, and to estimate the maximum sustainable yield (Roff Citation1981; Dillinger et al. Citation1987; Sparre and Venema Citation1998).
Mugil curema Valenciennes, 1836, known as white mullet, occurs predominantly in the American continent, from Cape Code (USA) to Southern Brazil in the western Atlantic and from the Gulf of California to Northern Chile in the eastern Pacific (Thompson Citation1997). However, its presence has been reported in Argentinean waters (37°46′ S; 57°27′ W), which are in more southern latitudes than previously described (González-Castro et al. Citation2006; Heras et al. Citation2006). Adult specimens inhabit mainly mud-bottom estuarine areas with turbid waters but, when they are juveniles, they are found on sandy beaches, near the mouth of the rivers (Ferreira Citation1989; Vieira Citation1991).
White mullet fishing is traditional throughout the Brazilian coast, and is mainly exploited by small-scale fishing, with capture peaks in spring months (Menezes and Figueiredo Citation1985). In São Paulo (SP) state, M. curema fishing has increased significantly. According to the Fishing Institute of SP state, landings in 1988 were approximately 34 tons, and, in 2010, landings were higher than 127 tons, showing a 373.52% increase over the past 12 years. However, it is worth emphasizing that the production of M. curema landed in SP state from January to May 2011 (66 tons) was the highest registered over the past 12 years, when compared to the same period of the year.
Studies on the aspects of the reproductive biology of M. curema were carried out in temperate (Jacot Citation1920; Anderson Citation1957; Moore Citation1974) and tropical waters (Angell Citation1973; Moore Citation1974; Alvarez-Lajonchere Citation1976; Yáñez-Arancibia Citation1976; Alvarez-Lajonchere Citation1980; Couto and Nascimento Citation1980; Garcia and Bustamante Citation1981; Blanco Citation1985; Ibañez-Aguirre Citation1993; Marin et al. Citation2003; Ibañez-Aguirre and Gallardo Citation2004; Solomon and Ramnarine Citation2007). Mugil curema is the most abundant Mugilidae on the Brazilian coast (Couto and Nascimento Citation1980; Cergole Citation1986; Ferreira Citation1989; Santana Citation2007; Albieri, Araújo and Ribeiro Citation2010; Albieri, Araújo and Uehara 2010).
Generally, individuals of M. curema from the Brazilian coast have a prolonged reproductive period, with one or two spawning peaks throughout the year. Cergole (Citation1986) and Ferreira (Citation1989) observed that, in São Vicente estuary, in the southeastern area of Brazil, the reproductive period of M. curema ranged from September to January, and spawning events occurred at the end of spring – start of summer. In the coast of Pernambuco state, Couto and Nascimento (Citation1980) observed that the spawning events of the species occurred in March and August. However, Santana (Citation2007) observed, in the same area, that the spawning events take place in November and January. Albieri, Araújo and Ribeiro (Citation2010) and Albieri, Araújo and Uehara (2010) observed, in the coast of Rio de Janeiro state, a reproductive period that ranged from August to January, with one single spawning peak in October.
The main objective of this study was to elucidate the aspects of the reproductive biology of M. curema in the Cananéia-Iguape coastal system and Santos estuary, mainly the species spawning strategies and its environmental context. The data made available by this study provide a better understanding of the species population dynamics, in view of improving management.
Material and methods
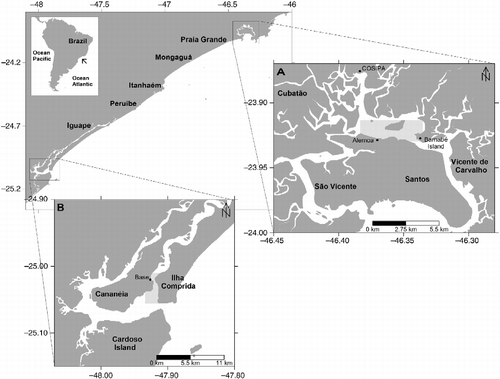
The sampling of M. curema specimens in Santos estuary (Figure ) was carried out monthly from March 2009 to February 2010 using a cast net with a 20-mm size mesh between consecutive knots and a gillnet with a 60-mm mesh between consecutive knots. In the Cananéia-Iguape coastal system (Figure ), samplings were carried out on a monthly basis, from May 2008 to May 2009, using a cast net with a 12-mm mesh between consecutive knots and fish-trap with a 30-mm distance between bamboos.
Figure 1 Representation of (A) Santos estuary, (B) the Cananéia-Iguape coastal system, in the coast of SP state, with the locations of fishing areas for individuals of Mugil curema.
Data on sea surface temperature were obtained through the ANTARES network (http://www.antares.ws), whose data come from coastal stations (in situ) and satellite data (remote sensing), and precipitation, through the pluviometric database of SP state (http://www.sigrh.sp.gov.br).
Total length (TL in mm) and total weight (TW in g) were measured in the individuals of M. curema. All gonads were removed, weighed [gonad weight (GW) in g], identified by sex, and fixed in 10% neutralized formalin. Permanent preparations of female gonads were mounted according to Vazzoler (Citation1996). To identify the gonadal maturation stages, the description of development phases of female germ cells proposed by Mcmillan (Citation2007) was used as the basis for gonadal classification (Dias et al. Citation1998) and description.
For the study of sexual proportion in the speciemens from the two areas, calculations focusing on two important aspects were carried out: population structure for the whole period of study and temporal variation of proportion between adult males and females. Sex ratio in each area was compared to the expected 1:1 sex ratio using χ2 test (Zar Citation1999).
To estimate the first gonadal maturation length (L 50), immature individuals (Stage A) were considered juveniles and those classified in the other stages (Stages B, C, D, and E) were considered adults, regardless of the sampling area and sex. Subsequently, the proportion between juveniles and adults by TL was calculated. A logistic regression was adjusted to the data on TL and adult individual percentage, through the iterative method of square minimums with Microsoft Excel Solver tool, and the value of L 50 was obtained from the formula proposed by King (Citation2007).
For a better interpretation of the reproductive processes, some quantitative indicators were estimated, such as the difference between the alometric condition factors (ΔK), calculated with the TW [K = (TW/TL b ) × 104] and the body weight [K′ = (TW–TL b ) × 104], where b is the angular coefficient obtained from the weight–length relationship. This difference expresses, relatively, the share of reserves transferred to the gonads and provides an additional indicator of the reproductive period (Scott 1979; Vazzoler Citation1996). The gonadosomatic index (GSI) was calculated using the formula GSI = GW/TW × 102, which expresses the ratio, as a percentage, between the GW and the individual weight. Its temporal variation can indicate the period with the highest reproductive activity of the species (Wootton Citation1990).
Estimations of potential fecundity (i.e. the number of oocytes which can be eliminated in the spawning period) and relative fecundity [i.e. the number of oocytes by the body weight (BW)] were carried out by removing two aliquots (anterior and posterior) of 0.3 g from 10 ovaries in the mature development stage (five from each area studied) to count the oocytes in Phase V (oocytes with complete vitellogenesis). After the count, the student t-test was carried out between the aliquots with a 0.05 significance level (Collins et al. Citation1996) to check whether the oocyte maturation is homogeneous within the ovary. Subsequently, the potential fecundity for each specimen was calculated using the formula F p = N o × TW/W am, where N o is the number of oocytes in the sample and W am is the sample weight. Relative fecundity for each individual was calculated using the formula F r = F p/BW. To compare the potential fecundity and the relative fecundity between the two areas of study, the student t-test at a significance level of 0.05 was applied to the data.
Results
A total of 867 individuals of M. curema were collected during the period of study; 526 individuals were from the Cananéia-Iguape coastal system (137 females, 76 males, and 313 juveniles) ranging from 65 to 409 mm of TL, and 341 individuals were from Santos estuary (205 females, 67 males, and 71 juveniles) ranging from 148 to 405 mm of TL.
In the Cananéia-Iquape coastal system, sex ratio differed significantly (p < 0.05) from the expected equal sex ratio (1:1) in 6 of the 12 months (August, September 2008 and January, February, March, April 2009; Figure ). In Santos estuary, sex ratio also differed significantly from the expected 1:1 sex ratio in 6 of the 12 months (March, May, June, August, October 2009 and February 2010; Figure ).
Figure 2 Frequency of the occurrence of males and females of Mugil curema by season of the year in (A) the Cananéia-Iguape coastal system and (B) in Santos estuary, coast of SP state. The months that had a significant difference (p < 0.05) are highlighted with asterisks.
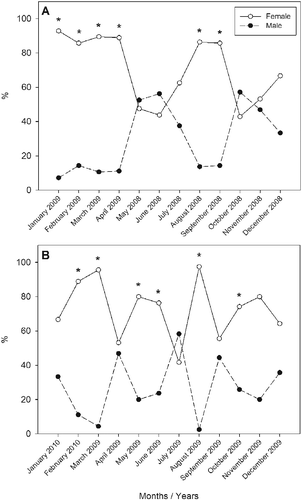
The predominance of females in the Cananéia-Iguape coastal system occurred in almost all the months studied, except for May and October 2008. In Santos estuary, the only month that had a higher frequency of males was July 2009. The overall sex ratio for the two areas was 1:2.4 (M:F), with 1:1.8 and 1:3.1 in the Cananéia-Iguape coastal system and Santos estuary, respectively.
The microscopic gonadal maturation scale, assessed for females of M. curema in Santos estuary and the Cananéia-Iguape coastal system, is comprised of five gonadal maturation stages: immature (A), maturing (B), mature (C), hydrated (D), and spawned (E). This scale considered the presence and quantity of oocytes in different development phases (Table ; Figure ).
Table 1 Description of the phases of female germ cell development and deriving structures, and oocyte development stages in the ovaries of Mugil curema from the Cananéia-Iguape coastal system and Santos estuary, coast of SP state, and their microscopic characteristics.
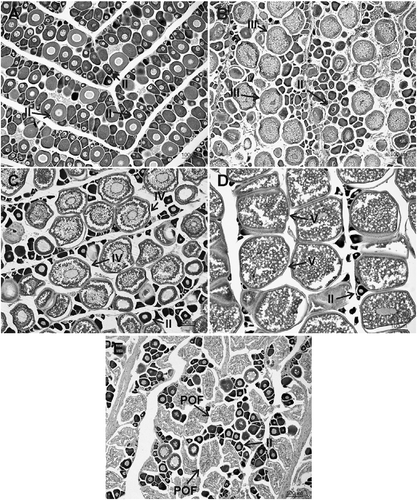
Figure 3 Phases of female germ cell development and deriving structures of Mugil curema from the Cananéia-Iguape coastal system and Santos estuary, coast of SP state. (A) Oogonia; (B) oocyte with primary growth (Phase II); (C) oocyte with cortical alveoli (Phase III); (D) oocyte with early vitellogenesis (Phase IV); (E) oocyte with complete vitellogenesis (Phase V); (F) oocyte with initial hyalinization, (G) POF, and (H) atresic oocyte. N, nucleus; n, nucleolus; PZ, pellucid zone; LV, lipid vacuole; YG, yolk granules; IH, oocytes with initial hyalinization; TC, techa cells; FC, follicle cells; ATR, atresic oocyte.
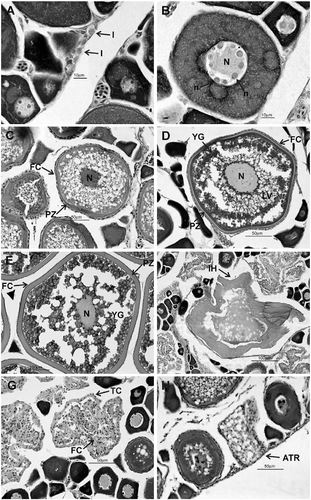
The species showed synchronous oocyte development, in which two groups of oocytes can be distinguished: the oocytes of a reserve stock and maturing oocytes which synchronously mature in the ovaries. During the period studied, most of the oocyte development phases occurred in both areas (except for oocytes in Phase VI), as did postovulatory follicles (POFs) and, consequently, at all stages of gonadal maturation (Figure ). There were no individuals in Stage D (hydrated) in the areas, in any period of the year, and no individuals in Stage E occurred in Santos estuary. However, it is worth emphasizing that the females classified as spawned (Stage E) showed the same conditions of structural disorganization in all POFs, i.e. all mature oocytes were spawned during reproduction with no evidence of successive batches of oocytes spawned sequentially.
Figure 4 Slides of female gonads of Mugil curema from the Cananéia-Iguape coastal system and Santos estuary, coast of SP state. (A) Early maturation, (B) advanced maturation, (C) early mature, (D) advanced mature, and (E) spawned. II, oocyte with primary growth (Phase II); III, oocytes with cortical alveoli (Phase III); IV, oocytes with early vitellogenesis (Phase IV); V, oocytes with complete vitellogenesis (Phase V); and POFs, postovulatory follicles.
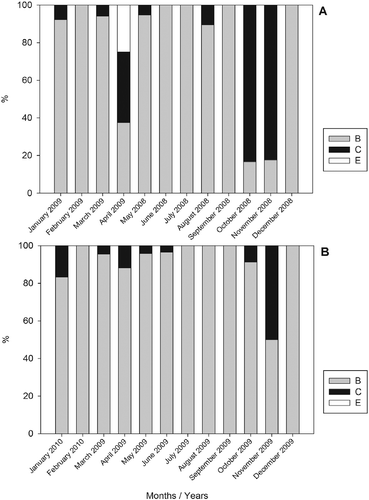
The frequency of stages of gonadal maturation by sampling period for females in both areas was evidenced by a higher frequency of immature individuals in almost all sampling months. There was a high frequency of individuals in Stage B, which had already started the gonadal maturation process, in all seasons of the year in both areas. However, individuals in Stage C in the Cananéia-Iguape coastal system occurred more frequently in October and November 2008 and April 2009. Spawned individuals only occurred in April 2009 (Figure ). In Santos estuary, individuals in Stage C showed a higher frequency in November 2009 and January 2010, whereas spawned individuals were not found in the area (Figure ).
Figure 5 Monthly variation in female proportion in different stages of gonadal maturation of Mugil curema from (A) the Cananéia-Iguape coastal system and from (B) Santos estuary, coast of SP state.
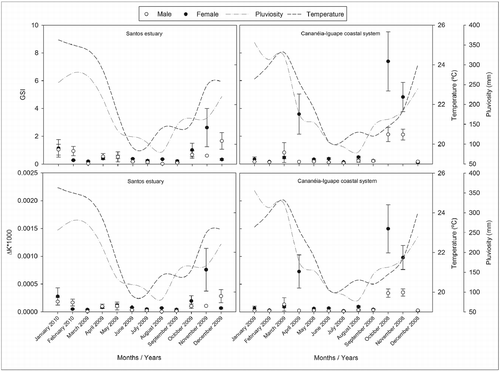
The mean values of GSI per sampling period in the Cananéia-Iguape coastal system were higher in October and November 2008 for females and males, in April 2009 for females, and in March 2009 for males, with a sharp fall in subsequent months. A similar tendency was found for mean values of ΔK also for the sampling period for males and females. In Santos estuary, the mean values of GSI were higher in April and May 2009 for males and females, in November 2009 and January 2010 for females, and in December 2009 for males, and, from this moment on, there was a fall in the subsequent months. This tendency was observed for the mean values of ΔK. Such results indicate a higher GW, and consequently, a higher reproductive activity in these seasons of the year, and a possible spawning event after such periods, due to the sharp fall in the mean values of GSI and in the ΔK. The highest values, both for the GSI and for the difference in the condition factor, were found in the start and end of the rainy season, which lasts from October to March, when sea surface temperatures are not so high and rainfall indexes are medium (Figure ). The mean length at first gonadal maturation (L 50) of M. curema in the two areas of study was estimated at 248.60 mm of TL for the grouped sexes.
Figure 6 Monthly variation of the mean values of GSI and of the condition factor (ΔK) for males and females of Mugil curema and the annual mean ocean surface temperature (°C) and rainfall index (mm) of the Cananéia-Iguape coastal system and Santos estuary, coast of SP state.
The oocyte counts in each gonad portion (anterior and posterior) did not show significant differences (p < 0.05). The quantity of oocytes per gonad in Santos estuary and the Cananéia-Iguape coastal system did not differ significantly. Therefore, the ovarian aliquots in both areas of study were grouped to estimate the potential fecundity of the species.
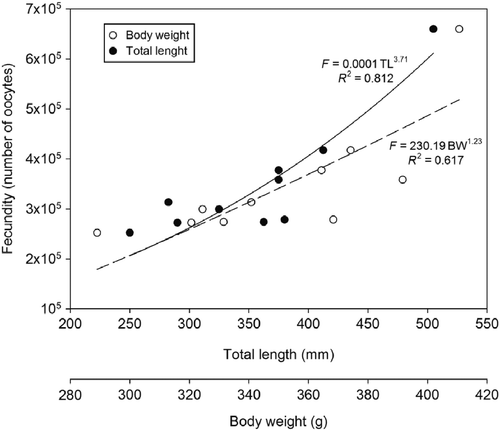
The individual value of potential fecundity varied between 252,578 and 659,801 oocytes per gonad [with an average ± standard deviation (SD) of 350,430 ± 120,920 oocytes], for the individuals with TL between 300 and 402 mm (with an average ± SD of 342.30 ± 29.42 mm) and body weight ranging from 222.57 to 526.65 g (with an average ± SD of 378.98 ± 91.95 g). The potential fecundity for M. curema increased gradually due to the TL and the body weight, as shown in Figure . The relative fecundity calculated for M. curema ranged from 662.30 to 1252.82 oocytes/g, with a mean value ± SD of 926.46 ± 171.44 oocytes/g.
Discussion
Reproductive strategy is a group of reproductive traits (age at maturity, fecundity, parity, etc.) that maximize the reproductive success of a species (Wootton Citation1989; Stearns Citation1992). As species are distributed over environmental gradients, variations in reproductive traits are expected because of variation in physiology and behavior. These variations constitute reproductive tactics. Thus, reproductive strategy is the general reproductive pattern shown by a species while tactics are variations on the strategy caused by environmental variation. Both strategies and tactics are considered as adaptive (Wootton Citation1989).
Sexual proportions in populations of fish might vary during their life cycle due to successive events, which act differently on the individuals of each sex (Vazzoler Citation1996). This study has shown a significant predominance of females in almost all periods of the year in both areas of study. The predominance of females might be a common phenomenon for M. curema because the same tendency was observed by Cergole (Citation1986) in São Vicente estuary; Ibañez-Aguirre and Gallardo-Cabello (Citation2004) in the Gulf of Mexico, with a proportion of males and females of 1:1.4; Couto and Nascimento (Citation1980) in Pernambuco, with the proportion estimated at 1:1.7; and finally Santana (Citation2007), also in Pernambuco, with a proportion of 0.74:1. Differences in mortality, growth, longevity, sexual activity, and migration rates for reproductive areas might be factors responsible for the dominance of one sex (Wootton Citation1992), in this case females, and all of them are applicable to the present species.
However, in both areas of study, there was a tendency toward equality between males and females in the months when the GSI and ΔK values were higher. These results might be an indication of a potential grouping of mature individuals before migration. According to Angell (Citation1973), the grouping in even bigger shoals is common in Restinga Lagoon (Venezuela) just before the migration to further areas.
Histological analyses of ovaries in white mullet in Santos estuary and the Cananéia-Iguape coastal system showed, according to Marza (1938; apud Wallace and Sellman Citation1981), a synchronous development, where two groups of oocytes are evident in each cycle: oocyte with primary growth and oocytes that mature synchronously and be spawned in the reproductive period. This oocyte development mechanism, together with the presence of POFs showing the same conditions of structural disorganization, or same age (Figure ), made it evident that one event of spawning took place. According to Murua and Saborido-Rey (Citation2003), individuals of M. curema could be classified as total spawners with group synchronous oocyte development. This pattern spawn for the species was also observed by Santana (Citation2007) in Pernambuco (Brazil), Solomon and Ramnarine (Citation2007) in the Gulf of Paria (Trinidad), and Ferreira (Citation1989) in São Vicente estuary.
The spawning of M. curema happens in areas farther from the coast, mainly on the continental shelf (Jacot Citation1920; Anderson Citation1957; Cergole Citation1986; Santana Citation2007). This is corroborated by the presence of Mugilidae larvae in Santos region (SP), in waters up to 380 m deep (Matsuura et al. Citation1978) in December and January. These months coincide with the spawning season of M. curema estimated by Cergole (Citation1986) and Ferreira (Citation1989) in Santos region.
Another species of Mugilidae, Mugil platanus ( = Mugil liza, Menezes et al. Citation2010), is also abundant in Santos region (Menezes and Figueiredo Citation1985). However, its spawning takes place in a different period, according to the studies carried out farther south in Brazil: from September to November in Paranaguá Bay – Paraná (Esper et al. Citation2001); from June to October in Cananéia – SP (Andrade-Talmelli et al. Citation1996); and in May and June (autumn) in Patos Lagoon – Rio Grande do Sul (Vieira and Scalabrin Citation1991). Therefore, although Matsuura et al. (Citation1978) did not identify the larvae in Santos region at a species level, it is very likely that they belong to the species M. curema because of the period in which they were found.
Therefore, the small frequency of females in stage E and their absence in stage D derive from the migration of these individuals to more offshore areas due to the spawning season. Thus, spawning is not indicated by the increase in frequency of females in advanced gonadal development stages (stages D and E), but by the significant and sudden decrease in individuals in the advanced mature stage in coastal areas. This significant decrease of M. curema in advance gonadal development stages when the spawning season approaches was also reported by Angell (Citation1973) and Marin et al. (Citation2003).
In this study, a significant decrease in mature females of white mullet was observed, mainly after November 2008 and April 2009 in the Cananéia-Iguape coastal system, and in November 2009 and January 2010 in Santos estuary. Thus, the species, at these times, migrates out of both estuaries to spawn offshore. Cergole (Citation1986), Ferreira (Citation1989), and Santana (Citation2007) emphasize in their studies that the species migrates to more offshore areas for spawning.
Maximum values of the difference between condition factor (ΔK) coincide with the higher occurrence of individuals with mature gonads (at the start and end of the rainy season). However, the lowest values of ΔK were found in dry season, which coincides with the higher frequency of maturing individuals. These higher values of ΔK found in advanced maturing periods for M. curema were previously observed by Cergole (Citation1986), Ferreira (Citation1989), Santana (Citation2007), Albieri, Araújo and Ribeiro (Citation2010), and Albieri, Araújo and Uehara (2010).
Maximum and minimum GSI values were equal to those pattern obtained for ΔK, for both males and females from both areas of study. The maximum values were obtained in the beginning and end of the rainy season, whereas the lowest were obtained in the dry season. Therefore, the results obtained from these two variables corroborate the other indicators of the reproductive process, indicating that the start of maturation gonadal of M. curema in Santos estuary and the Cananéia-Iguape coastal system takes place in the rainy season, which ranges from October to April. The spawning occurs in April and November, during the end and start of the rainy season, respectively.
The reproductive period and the spawning seasons of M. curema show a great variation throughout its geographical distribution (Table ). Many authors report a prolonged reproductive period for the species, generally with two spawning peaks throughout the year (Jacot Citation1920; Anderson Citation1957; Angell Citation1973; Moore Citation1974; Alvarez-Lajonchere Citation1976, Citation1980; Yáñez-Arancibia Citation1976; Rodriguez and Nascimento Citation1980; Garcia and Bustamante Citation1981; Ibañez-Aguirre Citation1993; Marin et al. Citation2003; Santana Citation2007). Angell (Citation1973) suggests that M. curema forms large shoals just before migrating offshore for spawning, provoking a decrease in the mean GSI in more coastal areas. Moore (Citation1974) also reported that mature individuals are rarely found in these areas during the spawning periods of the species.
Table 2 Spawning period for Mugil curema in tropical waters.
The start of the reproductive period for various species of fish is stimulated mainly by changes in environmental variables, which act on the individuals (Redding and Patiño Citation1993; Vazzoler Citation1996). The species M. curema possibly uses temperature to start the reproductive period and to synchronize spawning in a season that is more favorable to offspring survival and growth. Such conditions, for instance, might be represented indirectly by the increase in primary productivity (Ferraz-Reyes Citation1983; Müller-Karger et al. Citation1989) or hydrographic mechanisms that might enable the transport of larvae to coastal areas used as nursery areas by the species (Blaber Citation1987), so that the survival rate is significantly higher. Nevertheless, it is worth emphasizing that different populations of M. curema have adapted to existing environmental conditions in each particular area because local hydrographic patterns develop over geological time scales (Bakun et al. Citation1974; Sinclair Citation1988; Heath Citation1992).
The results obtained for mean length at the start of the first gonadal maturation (L 50) are similar to those obtained in other studies. It is important to emphasize that, in this study, only the calculation for grouped sexes was performed, as a precaution, due to the difficulty in identifying the sex of immature individuals. Santana (Citation2007) estimated the mean length of the first gonadal maturation for grouped sexes of M. curema in Recife, Pernambuco, at 255 mm of TL. However, Ibañez-Aguirre and Gallardo-Cabello (Citation2004) estimated, in the Gulf of Mexico, 274 mm of TL, which is a little higher than the value in this study (248.60 mm of TL). The differences between the values of length at first gonadal maturation for one species in different seasons might be mainly associated with the geographical location of the studied populations. This phenomenon has already been observed in other species such as Micropogonias furnieri (Vazzoler Citation1991), Salvelinus leucomaenis (Morita and Morita Citation2002) and Microstomus pacificus (Abookire and Macewicz Citation2003).
The mean potential fecundity found in this study for M. curema was 350,430 oocytes for individuals with mean TL of 342.30 mm. These values were relatively close to the values found by Alvarez-Lajonchere (Citation1982), Marin and Dodson (Citation2000), Ibañez-Aguirre and Gallardo-Cabello (Citation2004), Santana (Citation2007), Albieri, Araújo and Ribeiro (Citation2010), Albieri, Araújo and Uehara (2010), and Cabral-Solís et al. (Citation2010). Although these fecundity values are relatively low when compared to other species of Mugilidae, such as Mugil cephalus and M. liza estimated by Alvarez-Lajonchere (Citation1982), Ibañez-Aguirre and Gallardo-Cabello (Citation2004), Ribeiro (Citation2010Araújo and Uehara (Citation2010), the mean relative fecundity estimate for M. curema was 926.46 oocytes/g. This value was very similar to the values estimated by Ibañez-Aguirre and Gallardo-Cabello (Citation2004), Santana (Citation2007), Ribeiro (Citation2010Araújo and Uehara (Citation2010), and Cabral-Solís et al. (Citation2010) (Table ).
Table 3 Potential and relative fecundity of Mugil curema in tropical waters.
The increase in mean potential fecundity, proportional to the body growth, observed in this study, might be related to the increase in the size of the abdominal cavity of individuals throughout their growth, with possible growth in the gonadal size. This increase in fecundity peaks when gonadal maturity reaches its peak and tends to decrease from the moment the individual starts to reach senility. This occurs mainly for species with longer life span, such as Argentine hake (Vaz-Dos-Santos et al. Citation2009).
In conclusion, based on the results of this study, the individuals of M. curema from the Cananéia-Iguape coastal system and Santos estuary, coast of SP state, Brazil, start to mature in October extending to April, which occurs at two yearly spawning events, one in April and the other, with higher intensity, in November, marking the start and end of the rainy season, respectively. M. curema is a total spawner with group synchronous oocyte development. It migrates out of these estuaries, possibly to more offshore areas during the spawning season.
Acknowledgements
We are grateful to the team of the Reproductive Ecology and Marine Organism Recruitment Laboratory from the Department of Biological Oceanography of the University of São Paulo (IOUSP) for their support and incentive while this paper was carried out and to the Coordination for the Improvement of Higher Level Personnel (CAPES) for granting a scholarship.
References
- Abookire , AA and Macewicz , BJ . 2003 . Latitudinal variation in reproductive biology and growth of female Dover sole (Microstomus pacificus) in the North Pacific, with emphasis on the Gulf of Alaska stock . Journal of Sea Research , 50 : 187 – 197 .
- Albieri , RJ , Araújo , FG and Ribeiro , TP . 2010 . Gonadal development and spawning season of white mullet Mugil curema (Mugilidae) in a tropical bay . Journal of Applied Ichthyology , 26 : 105 – 109 .
- Albieri , RJ , Araújo , FG and Uehara , W . 2010 . Differences in reproductive strategies between two co-occurring mullets Mugil curema Valenciennes 1836 and Mugil liza Valenciennes 1836 (Mugilidae) in a tropical bay . Tropical Zoology , 23 ( 1 ) : 51 – 62 .
- Alvarez-Lajonchere , LS . 1976 . Contribucion al estudio del ciclo de vida de Mugil curema Valenciennes in Cuvier et Valenciennes, 1836 (Pisces: Mugilidae) . Revista Investigaciones Marinas , 28 : 1 – 130 .
- Alvarez-Lajonchere , LS . 1980 . Composicion pore specie y distribuicion de las post-larvas y juveniles de Lisas (Pisces, Mugilidae) en tunas de Zaza, Cuba . Revista Investigaciones Marinas , 1 ( 2/3 ) : 28 – 60 .
- Alvarez-Lajonchere , LS . 1982 . The fecundity of mullet (Pisces, Mugilidae) from Cuban waters . Journal of Fish Biology , 21 : 607 – 613 .
- Anderson , WW . 1957 . Early development, spawning, growth, and occurrence of the silver mullet (Mugil curema) along the south Atlantic coast of the United States . Fishery Bulletin of the Fish and Wildlife Service , 57 ( 119 ) : 397 – 414 .
- Andrade-Talmelli , EF , Romagosa , E , Narahara , MY and Godinho , HM . 1996 . Características reprodutivas de tainha Mugil platanus (Teleostei, Peciformes, Mugilidae), da região Estuarino-Lagunar de Cananéia, São Paulo . Revista Ceres , 43 : 165 – 185 .
- Angell , C . 1973 . Algunos aspectos de la biologia de la lisa, Mugil curema Valenciennes, en aguas hipersalinas del noriente de Venezuela . Memorias de la Sociedad de Ciencias Naturales “La Salle” , 51 : 223 – 238 .
- Bakun , A , Mclain , DR and Mayo , FV . 1974 . The mean annual cycle of coastal upwelling off western North America as observed from surface measurements . Fishery Bulletin , 72 : 843 – 844 .
- Blaber , SJM . 1987 . Factors affecting recruitment and survival of mugilids in estuaries and coastal waters of southeastern Africa . American Fisheries Society Symposium Series , 1 : 507 – 518 .
- Blanco R. 1985. Estudios Biológicos de las Lisas (Teleostei-Mugilidae) de la Laguna de Patanemo. Edo. Carabobo. Tesis de grado. Caracas, Venezuela: Universidad Central de Venezuela, 93 p
- Cabral-Solís , EG , Gallardo-Cabello , M , Espino-Barr , E and Ibáñez , AL . 2010 . Reproduction of Mugil curema (Pisces: Mugilidae) from the Cuyutlán lagoon, in the Pacific coast of México . Avances en Investigación Agropecuaria , 14 : 19 – 32 .
- Cergole MC. 1986. Aspectos sobre a biologia de M. curema Valenciennes, 1836 (Pisces, Mugilidae) no estuário de São Vicente. Vicente, SP [MSc dissertation]. São Paulo: University of São Paulo, 272 p
- Collins , LA , Johnson , AG and Keim , CP . 1996 . “ Spawning and annual fecundity of the red snapper (Lutjanus campechanus) from the northestern Gulf of Mexico ” . In Biology, fisheries and culture of the tropical groupers and snappers Edited by: Arregin-Sanchez , F. , Munro , JL , Balgos , MC and Pauly , D. Vol. 48 , 174 – 188 . ICLARM Conference Proceedings
- Couto , IMMR and Nascimento , IV . 1980 . microscópico dos ovários de Mugil curema Valenciennes, 1836, em Águas estuarinas de Pernambuco Brasil . Academia Brasileira de Ciências , 1 : 213 – 219 .
- Dias , JF , Peres-Rios , E , Chaves , P T. C and Rossi-Wongtschowski , C L. D. B . 1998 . Análise macroscópica dos ovários de teleósteos: problemas de classificação e recomendações de procedimentos . Revista Brasileira de Biologia , 58 ( 1 ) : 55 – 69 .
- Dillinger , RE Jr , Green , JM and Birt , TP . Preliminary analysis of life history strategies in northern fishes with special reference to three species of ciscoes . In Final Proceedings of the International Conference on Common Strategies of Anadromous and Catadromous Fishes. Bethesda, MD: American Fisheries Society, 557 p
- Esper , MLP , Menezes , MS and Esper , W . 2001 . Época reprodutiva de Mugil platanus (Günther, 1880), Pisces Mugilidae da Baia de Paranaguá (Paraná, Brasil) . Acta Biológica Paranaense , 30 : 5 – 17 .
- Ferraz-Reyes , E . 1983 . Estudio del fitoplancton de la Cuenca Tuy-Cariaco, Venezuela . Boletín del Instituto Oceanográfico de Oriente , 22 ( 1/2 ) : 111 – 124 .
- Ferreira LI. Estudo de aspectos da reprodução de Mugil curema Valenciennes, 1836 (Pisces, Mugilidae) no estuário de São Vicente. SP [MSc dissertation]. São Paulo: University of São Paulo, 90 p
- Frank , KT and Leggett , WC . 1994 . Fisheries ecology in the context of ecological and evolutionary theory . Annual Review of Ecology, Evolution, and Systematics , 25 : 401 – 422 .
- Garcia , A and Bustamante , G . 1981 . Resultados preliminares del desove in inducido de lisa (Mugil curema Valenciennes) en Cuba . Informe Cientifico-Tecnico Academia de Ciencias de Cuba , 158 : 7 – 26 .
- González-Castro , M , Díaz De Astarloa , JM and Cousseau , MB . 2006 . First record of a tropical affinity mullet, Mugil curema (Mugilidae), in a temperate southwestern Atlantic coastal lagoon . Cybium , 30 : 90 – 91 .
- Heath , MR . 1992 . Field investigations of the early life stages of marine fish . Advances in Marine Biology , 28 : 1 – 173 .
- Heras , S , Castro , MG and Roldán , MI . 2006 . Mugil curema in Argentinean waters: combined morphological and molecular approach . Aquaculture , 261 : 473 – 478 .
- Ibañez-Aguirre , AL . 1993 . Coexistence of Mugil cephalus and M. curema in a coastal lagoon in the Gulf of Mexico . Marine Biology , 42 : 959 – 961 .
- Ibañez-Aguirre , AL and Gallardo-Cabello , M . 2004 . Reproduction of Mugil cephalus and Mugil curema (Pisces: Mugilidae) from a coastal lagoon in the Gulf of Mexico . Bulletin of Marine Science , 75 : 37 – 49 .
- Jacot , AP . 1920 . Age, growth and scale characters of the mullets, Mugil chephalus and Mugil curema . Transactions of the American Microscopical Society , 39 : 119 – 229 .
- King , M . 2007 . Fisheries biology, assessment and management , 2nd ed. , Oxford : Blackwell Science . 382 p
- Marin , BJE and Dodson , JJ . 2000 . Age, growth and fecundity of the silver mullet, Mugil curema (Pisces: Mugilidae), in coastal areas of Northeastern Venezuela . Revista de Biologia Tropical , 48 : 389 – 398 .
- Marin , BJE , Quintero , A , Bussière , D and Dodson , JJ . 2003 . Reproduction and recruitment of white mullet (Mugil curema) to a tropical lagoon (Margarita Island, Venezuela) as revealed by otolith microstructure . Fishery Bulletin , 101 ( 4 ) : 809 – 821 .
- Marza , VD . 1938 . Histophysiologie de I'ovogenese , Paris: Hermann . 81 p
- Matsuura , YK , Nakatani , G , Sato , Y and Tamassia , STJ . 1978 . Exploração e avaliação de estoque de peixes pelágicos no sul do Brasil , Relatório técnico de atividades convênio, São Paulo, Brazil: FINEP/IOUSP . 46 p
- Mcmillan , DB . 2007 . “ Fish histology – female reproductive system ” . 598 p Okkelberg, The Netherlands : Springer .
- Menezes , NA and Figueiredo , JL . 1985 . Manual de peixes marinhos do sudeste do Brasil. V Teleostei (4) , 105 p São Paulo : Museu de Zoologia da Universidade de São Paulo .
- Menezes , NA , Oliveira , C and Nirchio , M . 2010 . An old taxonomic dilemma: the identity of the western south Atlantic lebranche mullet (Teleostei: Perciformes: Mugilidae) . Zootaxa , 2519 : 59 – 68 .
- Moore , RH . 1974 . General ecology, distribution and relative abundance of Mugil cephalus and Mugil curema on the south Texas coast . Contributions in Marine Science , 18 : 241 – 245 .
- Morita , K and Morita , SH . 2002 . Rule of age and size at maturity: individual variation in the maturation history of resident white-spotted charr . Journal of Fish Biology , 61 : 1230 – 1238 .
- Müller-Karger , FE , Mcclain , CR , Fisher , TR , Esaias , WE and Varela , R . 1989 . Pigment distribution in the Caribbean Sea: observations from space . Progress in Oceanography , 23 : 23 – 64 .
- Murua , H and Saborido-Rey , F . 2003 . Female reproductive strategies of marine fish species of the North Atlantic . Journal of Northwest Atlantic Fisheries Science , 33 : 23 – 31 .
- Redding , M and Patiño , R . 1993 . “ Reproductive physiology ” . In Fish physiology , Edited by: Evans , E. 503 – 534 . Boca Raton, FL : CRC Press .
- Rodriguez , CL and Nascimento , IV . 1980 . “ Estudio microscópico dos ovários de Mugil curema Valenciennes, Brasil ” . In I Simpósio Brasileiro de Aquicultura, Recife , 213 – 219 . Rio de Janeiro : Academia Brasileira de Ciências .
- Roff , DA . 1981 . Reproductive uncertainty and the evolution of iteroparity: why don't flatfish put all their eggs in one basket? . Canadian Journal of Fisheries and Aquatic Sciences , 38 : 968 – 977 .
- Santana , FMS . 2007 . Biologie, Pêche et Dynamique de la Population de Mulet Blanc (Mugil curema, Valenciennes, 1836) de Pernambuco – Brésil [dissertation] Quimper: University of Bretagne Occidentale, 260 pp.
- Scott , DBC . 1979 . Environmental timing and the control of reproduction in teleost fish . Symposium of the Zoological Society of London , 44 : 105 – 132 .
- Sinclair , M . 1988 . Marine populations: an essay on population regulation and speciation , 252 p Seattle : University Washington Press .
- Solomon , FN and Ramnarine , IW . 2007 . Reproductive biology of white mullet, Mugil curema (Valenciennes) in the Southern Caribbean . Fisheries Research , 88 : 133 – 138 .
- Sparre P, Venema SC. 1998. Introdução à avaliação de mananciais de peixes tropicais. Parte 1: manual. FAO Documento Técnico sobre as Pescas. No. 306/1, Ver.2. Roma. 404 p
- Stearns , SC . 1992 . The evolution of life histories , 249 p London : Oxford University Press .
- Thompson , JM . 1997 . The Mugilidae of the world . Memoirs of the Queensland Museum , 41 : 457 – 562 .
- Vaz-Dos-Santos , AM , Rossi-Wongtschowski , CLDB and Figueiredo , JL . 2009 . Parâmetros da reprodução e relação comprimento-peso da merluza Merluccius hubbsi (teleostei: merlucciidae), estoque sudeste brasileiro (21°S-29°S), ano 2004 . Boletim do Instituto de Pesca , 35 ( 1 ) : 1 – 16 .
- Vazzoler , AEAM . 1991 . Síntese de conhecimento sobre a biologia da corvina Micropogonias furnieri (Desmarest, 1823), da costa do Brasil . Atlântica , 13 ( 1 ) : 55 – 74 .
- Vazzoler , AEAM . 1996 . Biologia da reprodução de peixes teleósteos: teoria e pratica , 169 p Maringá : EDUEM .
- Vieira , JP . 1991 . Juvenile mullets (Pisces: Mugilidae) in the estuary of Lagoa dos Patos, Rio Grande do Sul, Brazil . Copeia , 2 : 409 – 418 .
- Vieira , JP and Scalabrin , C . 1991 . Migração reprodutiva da tainha (Mugil platanus, Günter, 1880) no litoral sul do Brasil . Atlântica , 13 : 131 – 141 .
- Wallace , RA and Selman , K . 1981 . Cellular and dynamic aspects of oocyte growth in teleost . American Zoologist , 21 : 325 – 343 .
- Wootton , RJ . 1989 . “ Introduction: strategies and tatics in fish reproduction ” . In Fish reproduction: strategies and tatics , Edited by: Potts , GW and Wootton , MN . 410 p London : Academic Press .
- Wootton , RJ . 1990 . Ecology of Teleost fishes , 404 p London : Chapman and Hall .
- Wootton , RJ . 1992 . Fish ecology 212 p New York, NY: Chapman and Hall
- Yáñez-Arancibia , LA . 1976 . Observaciones sobre Mugil curema Valenciennes en áreas naturales de crianza en México. Alimentación, crecimiento, madurez y relaciones ecológicas . Anales del Instituto Ciencias del Mar y Limnologia, Universidad Nacional Autonoma de Mexico , 3 : 211 – 243 .
- Zar , JH . 1999 . Biostatistical analysis , 4th ed. , 663 p New Jersey, NJ : Prentice Hall .