Abstract
Desiccation exposes tadpoles to a decrease in habitat size due to a reduction in water depth and surface area. We tested the effect of surface area and water depth on growth and development of tadpoles of Physalaemus albonotatus and whether habitat size effects are constant over the larval period. We performed a 2 × 2 factorial design: two water depths and two surface areas. We measured tadpoles at 15 and 30 days after starting the experiment, and recorded weight and time to metamorphosis. Our results indicate that habitat size influences the morphology, growth, and development of tadpoles and that the response changes during development. At 15 days, tadpoles reared in shallow water had reduced their morphological variables and developmental stage, whereas tadpoles reared in enclosures with different surface areas showed no differences. At 30 days, tadpoles reared in enclosures with small surface areas had increased their body length, body height, and developmental stage, whereas tadpoles reared in different water depths showed no differences. Tadpoles reared in small surface areas reached metamorphosis earlier than tadpoles reared in large surface areas. The results suggest that during ontogeny the surface area and water depth had different influence in the phenotypic plasticity of tadpoles.
Introduction
Many ecological factors affect the growth and development of tadpoles. Influential factors include variability in the aquatic environment (Van Buskirk Citation2009; Marquez-Garcia et al. Citation2010), predator presence (Relyea Citation2002, Citation2004; Relyea and Auld Citation2005; Eterovick et al. Citation2010), predator chemical cues (Relyea Citation2002; Gómez and Kehr Citation2011, Citation2012), and food availability (Bridges Citation2002; Eterovick et al. Citation2010). Hydroperiod is one of the most important abiotic factors that influences growth and development of tadpoles. The effect of pond desiccation on larval growth, development, and time to metamorphosis has been extensively studied. Most of the works agree that tadpole exposed to a risk of desiccation accelerate development and time to metamorphosis, entering the terrestrial habitat with a smaller body size (Semlitsch and Wilbur Citation1988; Crump Citation1989; Brady and Griffiths Citation2000; Maciel and Juncá Citation2009; Marquez-Garcia et al. Citation2010; Mogali et al. Citation2011; Perotti et al. Citation2011; Richter-Boix et al. Citation2011). However, most authors evaluated the risk of desiccation by exposing the larvae to a reduction in water volume, measuring only the impact of change in water depth. When a natural body of water dries out, it not only reduces the depth of the water, but also its surface area. Desiccation begins on the banks of natural ponds, changing both the volume of water and its surface area. Such changes in ponds lead to a crowded condition with two fundamental changes: less physical wall space and increased density with other tadpoles (John and Fusaro Citation1981).
Thus far, it is not clear how a reduction in habitat size by loss of surface area (physical walls) can influence the development and growth of tadpoles. Although some authors have also studied the effects of size and shape of aquatic habitat on amphibians (Yung Citation1885; Adolph Citation1931; John and Fenster Citation1975; Pearman Citation1993, Citation1995), until now it is unclear how habitat size affects traits correlated with individual fitness. Yung (Citation1885) and Adolph (Citation1931) studied the effects of surface area and water depth on tadpoles but reached contradictory conclusions. Yung (Citation1885) found that surface area was the dominant influence on tadpole growth and development whereas Adolph (Citation1931) found that water depth was the more important factor. More recently, Calich and Wassersug (Citation2012) studied how the size of the air–water interface (i.e. surface area), water depth, and the partitioning of the aquatic space affect the growth and development of tadpoles of Xenopus laevis (Daudin, 1802). These authors found that shallow water seems to physically impede the animal’s ability to breath. They suggest that shallow water, independent of other variables, may negatively impact air-breathing of X. leavis larvae.
In spite of the common use of amphibian larvae in research under micro- and mesocosm conditions, the effect of aquatic habitat size on the growth and development of tadpoles has received little consideration. Such studies are warranted because habitat size may affect the fitness of individuals and not just overall population size. Variation in aquatic habitat size can affect population and community process (Pearman and Wilbur Citation1990). The population density of some species decreased when aquatic habitats are reduced in size. For other species fitness can decline if the habitat is enlarged (Petrusewicz Citation1963; Petrusewicz and Trojan Citation1963).
We tested the effects of water depth and surface area on growth and development of tadpoles of the Menwig frog, Physalaemus albonotatus (Steindachner, 1864). This leptodactylid species breeds in small puddles formed after heavy rains, which often dry up within a few weeks in the absence of additional showers; therefore, these tadpoles provide a convenient model to studies phenotypic plasticity to habitat size. Our main questions were as follows:
How does variation in surface area and water depth influence growth and the development of tadpoles?
and
Are habitat size effects constant over the larval period?
We hypothesized that increasing the surface area while keeping the water volume constant (i.e. changing water depth) would induce morphological changes in tadpoles.
Materials and methods
Tadpoles were obtained from six foam nests collected on 20 November 2013, from an artificial semi-permanent pond (4 × 4 × 0.5 m) located on the grounds of the Centro de Ecología Aplicada del Litoral (CECOAL-CONICET), 10 km east from Corrientes (27°30’S 58°45’W), Argentina. The nests were placed in a shallow plastic wading pool (47 × 33 × 13 cm) filled with well water up to 10 cm deep. Three days after hatching, the larvae were randomly assigned to one of the four different treatments. Tadpoles were fed 0.150 g of boiled lettuce twice a week. The three tadpoles that died during the first 3 days of the experiment were replaced, so that the results of the experiment were not influenced by the individual deaths caused by initial stress.
Experimental design
The air temperature ranged between 26 and 29 °C and the photoperiod was 13 h:11 h light: darkness. The experiment consisted of a 2 × 2 factorial design: two depths of water (deep and shallow) and two surface areas (large and small). The tests were conducted in two cylindrical plastic containers with a surface area of 602 cm2 (12 cm diameter and 10 cm high) and 245 cm2 (6 cm diameter and 10 cm high) filled with water up to 9 and 4.5 cm. One-third of the water was replaced weekly to maintain healthy water conditions. Although there are no references of larval density for P. albonotatus under natural conditions, it is known than in other species the density is 1 larva/500 cm2 (Kehr et al. Citation2009). We used 1 larva/600 cm2 and 1 larva/245 cm2 to simulate an extreme condition of reduction in habitat size.
The experiment began on November 25 (day 0), when tadpoles at stage 26 (Gosner Citation1960) were randomly assigned to the treatments. It finished when the last tadpoles metamorphosed (four legs developed and tail totally reabsorbed). Each container held a single larva, and each treatment was replicated 25 times, resulting in a total of 100 experimental units.
At 15 and 30 days after starting the experiment (December 10 and December 26, respectively), tadpoles were measured and staged according to Gosner (Citation1960). To quantify morphological variables, we photographed the tadpoles in lateral view using a stereomicroscope (Leica DFC 295) equipped with an image capture system (Leica Application Suite, LAS V3.7). Four linear measurements were taken: body length, body height, tail-fin length, and tail-fin height. At metamorphosis, we recorded the date, along with the weight of the individual.
Water temperature, pH, conductivity, and dissolved oxygen concentration were recorded three times a week at approximately the same time each day (three replicates per container were recorded).
Statistical analysis
Morphology data were log-transformed to obtain a normal distribution. The data at 15 days, at 30 days and at metamorphosis were analyzed independently. Data at 30 days were calculated as the difference between measured value at 30 and 15 days, to make the data independent, and avoid the effect of repeated measure. Morphological traits were analyzed first through a multivariate analysis of variance (MANOVA) using surface area and water depth, as independent variables with the four morphological variables and developmental stage as dependent variables. Subsequently, if Wilk’s lambda indicated significance, we performed a one-way analysis of variance (ANOVA) for each dependent variable. For significant cases, a posteriori pairwise comparison was made using Tukey’s comparison test. Metamorphic data were analyzed first with a MANOVA using surface area and water depth, as independent variables and weight and time to metamorphosis as dependent variables. Subsequently if Wilk’s lambda indicated significance, we performed an ANOVA for each dependent variable. Environmental variables were analyzed with the Kruskal–Wallis test.
All statistical tests were carried out using SYSTAT 7.0 (SPSS 1997) and XLSTAT 7.5 (Addinsof Citation2006).
Results
Effect of water volume and surface area at Day 15
At 15 days, the morphology of tadpoles was significantly affected by the water depth (Wilks' Lambda = 0.800, F7, 65= 2.319, p = 0.036), but not by the surface area (Wilks' Lambda = 0.934, F7, 65= 0.660, p = 0.705) or by the interaction of both factors (Wilks' Lambda = 0.923, F7, 65= 0.777, p = 0.609). All morphological variables (body length, body height, tail-fin length, tail-fin height) reduced for the tadpoles reared in shallow water more than for those raised in deeper water. Tadpoles in shallow water, and small surface area had shorter bodies and tails. Their developmental stage was reduced as well relative to tadpoles reared in deeper water. Although the MANOVA showed no differences in the interaction of water depth and surface area, the ANOVA showed that body length and development rate were significantly influenced by the interaction of both factors, and also similar effect occurred in body height, which data were nearly significant. The body length, body height, and development stage of tadpoles were higher in tadpoles reared in deeper water with smaller surface area (, and ).
Figure 1 Differences observed at 15 days after the start of the experiment in the morphological variables of Physalaemus albonotatus tadpoles exposed to different water depth and surface areas. The values are means (± SE). The four morphological variables reduce significantly for tadpoles raised in containers with shallow water compared to one with deeper water. Interaction between deeper water and small surface area increased significantly body length.
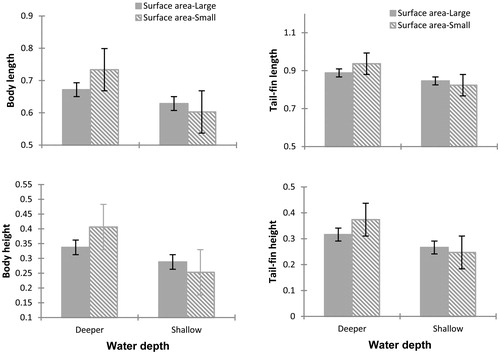
Figure 2 Differences observed at 15 days after the start of the experiment in the developmental stage of Physalaemus albonotatus tadpoles exposed to different water depths and surface areas. The values are means (± SE). The development stage increases significantly for tadpoles raised in containers with deeper water compared to one with shallow water. Interaction between deeper water and small surface area is increased significantly the development stage.
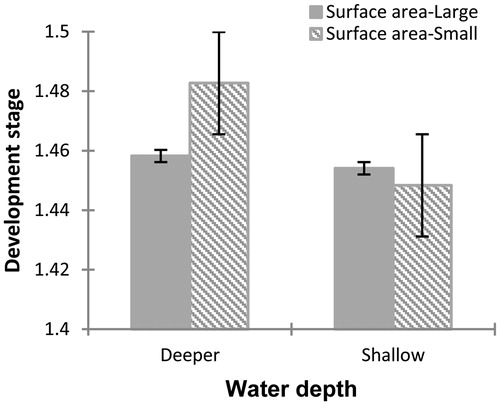
Table 1 Results of ANOVA test exploring water depth and surface area as factors that could influence four morphological variables and developmental stage of Physalaemus albonotatus tadpoles. Data registered at 15 days after the start of the experiment.
Effects of water volume and surface area at Day 30
At 30 days, tadpole morphology was affected by the surface area (Wilks' Lambda = 0.491, F7, 65= 4.294, p = 0.002), but not by water depth (Wilks' Lambda = 0.842, F7, 65= 0.775, p = 0.613) or the interaction of both factors (Wilks' Lambda = 0.834, F7, 65= 0.825, p = 0.575). Tadpoles exposed to a small surface area increased their body length, body height and developmental stage relatively more than tadpoles reared in containers with large surface area. Tadpoles in small surface area and deeper water had longer and taller bodies. No difference was observed in tail-fin length and tail-fin height between tadpoles reared in different treatments (, and ). Tadpoles in enclosures with small surface area showed an investment in the growth of the body instead of the tail, which may facilitate reaching metamorphosis sooner.
Figure 3 Differences observed at 30 days after the start of the experiment in the morphological variables of Physalaemus albonotatus tadpoles exposed to different water depths and surface areas. The values are means (± SE). The body length and body height are increased significantly for tadpoles raised in containers with small surface area compared to one with large surface area. No significant difference was observed in the interaction of both factors.
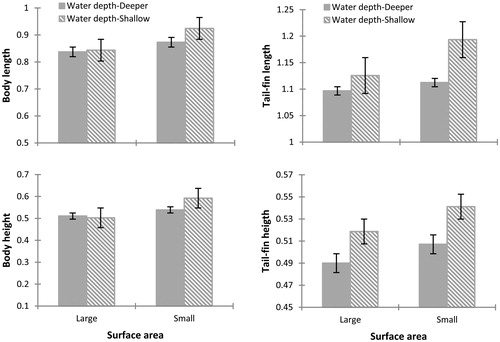
Figure 4 Differences observed at 30 days after the start of the experiment in the developmental stage of Physalaemus albonotatus tadpoles exposed to different water depths and surface areas. The values are means (± SE). The development stage is increased significantly for tadpoles raised in containers with small surface area compared to one with large surface area. No significant difference was observed in the interaction of both factors.
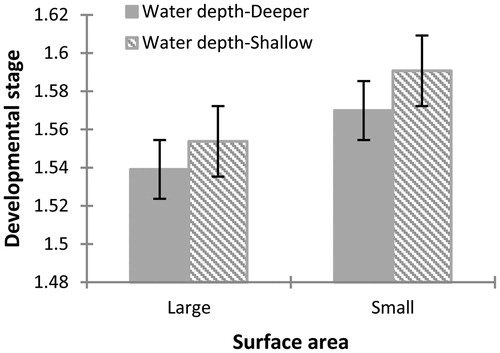
Table 2 Results of ANOVA test exploring water depth and surface area as factors that could influence four morphological variables and developmental stage of Physalaemus albonotatus tadpoles. Data registered at 30 days after the start of the experiment.
Effects of water volume and surface area on the weight and time to metamorphosis
Time to metamorphosis was influenced by the surface area (Wilks' Lambda = 0.498, F7, 65= 8.077, p = 0.004), but not water depth (MANOVA Wilks' Lambda = 0.776, F7, 65= 2.315, p = 0.131) or interaction of both factors (Wilks' Lambda = 0.852, F7, 65= 1.395, p = 0.276). Tadpoles reared in enclosures with small surface reached metamorphosis earlier than those reared in a large surface. This is consistent with the results obtained at 30 days for larvae reared in small surface area containers, whose bodies were larger and more developed; they also metamorphosed earlier than tadpoles reared in containers with large surface area. There were no significant differences in the weight of metamorphic between treatments (, ).
Figure 5 Differences observed in days to metamorphosis for Physalaemus albonotatus tadpoles exposed to different water depth and surface areas. The values are means (± SE). Tadpoles reach metamorphosis earlier in containers with small surface area than in one with large surface area. No significant difference was observed in the interaction of both factors.
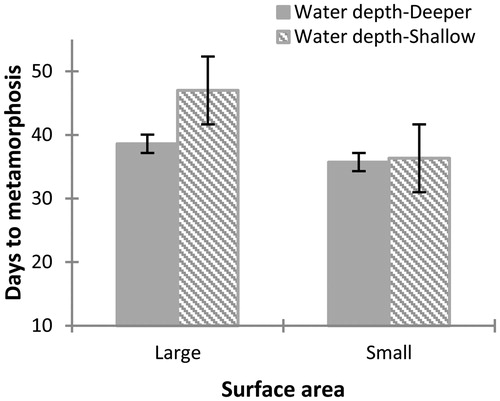
Table 3 Results of ANOVA test exploring water depth and surface area as factors that could influence two metamorphic variables of Physalaemus albonotatus.
Effects on water volume and surface area on environmental variables
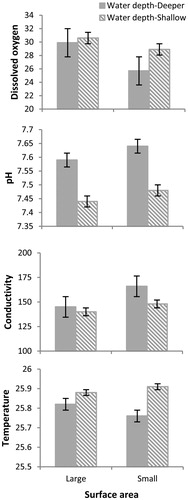
Although there was no significant difference on environmental variables between treatments (temperature: Kruskal–Wallis = 0.441, gl = 3, p = 0.932; dissolved oxygen: Kruskal–Wallis = 4.515, gl = 3, p = 0.211; conductivity: Kruskal–Wallis = 7.512, gl = 3, p = 0.057; pH: Kruskal–Wallis = 6.583, gl = 3, p = 0.086), the conductivity and pH differences were nearly significant. Results showed that in containers with small surface area and deeper water the pH and conductivity were a little bit higher than in containers with large surface area and shallow water. On the contrary, dissolved oxygen and temperature were a little bit higher in containers with shallow water ().
Discussion
Our results suggest that water depth and surface area influence the morphology, growth, and development of P. albonotatus tadpoles and that the response to the habitat size can change during development. In our study, the variations of habitat size did not alter the physical properties of the water, such as temperature, pH, conductivity, and dissolved oxygen concentration, indicating that it is habitat size that induces changes in morphology and development of P. albonotatus tadpoles. However, differences in conductivity and pH were nearly significantly, the results show that in deeper water and small surface area, the conductivity and pH were higher and the dissolved oxygen shows the lowest values this could be explained by the concentration of food remains and larvae waste, which would be greater in small surface area containers.
We found that during the first 15 days, P. albonotatus tadpoles were significantly influenced by water depth but not surface area, showing greater growth and development in deeper water relative to shallow water. Our observations contrast with most of work performed with other species, where tadpoles accelerate the development rate when ponds dry up (Semlitsch and Wilbur Citation1988; Crump Citation1989; Marquez-Garcia et al. Citation2010; Mogali et al. Citation2011; Perotti et al. Citation2011). However Maciel and Juncá (Citation2009) found that tadpoles of Pleurodema diplolister (Peters, 1870) raised in shallow water delay their development compared to tadpoles raised in deeper water.
The responses observed in P. albonotatus could be related to tadpole activity, and the space available for them to move about. It is know that increased intraspecific competition reduce significantly the size of tadpoles. Some studies had reported that tadpoles exposed to simulated high density (by the use of mirrors) show alteration in developmental stage, overall size, activities, and oral structures (Rot-Nikcevic et al. Citation2006; Sutherland et al. Citation2009). John and Fusaro (Citation1981) found that tadpole growth is inhibited by shallow depths only when the physical space is so limited that the mobility is impaired. In this case, the reduction in physical space could be perceived by tadpoles as a proxy for increased density, inducing tadpoles to reduce morphological variables, we observed that larvae exposed to shallow water and small surface area showed the lowest values on this variables.
The result also shows significant difference in the interaction between water deep and surface area on body length and developmental stage and is nearly statistically significant for body height. This could indicate that the effect of surface area becomes important earlier in development of P. albonotatus. This can be explained by the type of habitat where P. albonotatus lives. They are benthic, spending most of their time in the bottoms of ponds and relatively little time in the water column. In contrast, X. laevis tadpoles spend more of their time feeding while suspended in the water column in a head-down position (Wells Citation2007). This could explain why P. albonotatus were more influenced by changes in surface area and X. laevis were influenced by changes in water depth (Calich and Wassersug Citation2012).
At 30 days, tadpoles maintained in enclosures with reduced surface area showed higher growth in body than tail, which may facilitate reaching metamorphosis sooner. Tadpoles reared in smaller surface area containers increased their body length, body height, and developmental stage.
When tadpoles are larger, the available space is more important because they need more space to move (John and Fusaro Citation1981), and so the surface area becomes more important for P. albonotatus tadpole performance. Furthermore, in the most common pools inhabited by P. albonotatus, when a puddle is drying, there is first a reduction in depth produced by evaporation and then progressively reduces surface area. This could explain why tadpoles in early developmental stages respond to water depth while later in development the most important factor changes to surface area.
A reduction in habitat size induced in P. albonotatus tadpoles increased body size as well as developmental stage consistent with their reaching metamorphosis sooner and escaping from increasingly restrictive living space (John and Fusaro Citation1981). At 30 days, the average developmental stages were 36–39 (Gosner Citation1960) and, since tadpoles were close to metamorphosis, it was probably advantageous at that point for the tadpoles to metamorphose earlier rather than to delay their differentiation toward metamorphic morphology. Data recorded at metamorphosis support this idea because tadpoles metamorphosed earlier in enclosures with small surface areas, suggesting that surface area influenced the morphology of the tadpoles. Also, larvae, whose bodies were larger and more developed at 30 days (i.e. those reared in small surface areas), metamorphosed earlier than those in container with large surface areas. We found that changes in the volume of water and surface area did not induce differences in body size at metamorphosis.
A key finding was that the effect of habitat size for P. albonotatus changed during their ontogeny. Conditions that are favorable for change in growth at early stages may not produce the best-fit individuals at later stages (Pearman Citation1993). Semlitsch and Gibbons (Citation1990) found that earlier in the development the egg sized is the more important factor that determines differences in body size of Ambystoma talpoideum (Holbrook, 1838). Later in development that factor was overtaken by pond drying.
Our results suggest that the water depth and surface area affect the growth and development of tadpoles and that this impact changes during ontogeny.
Acknowledgments
The English language was improved by Ingles Científico (http://www.inglescientifico.com.ar). The authors considered all animal care and ethic manipulations suggested by the National Council of Scientific Researching from Argentina (CONICET).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Addinsoft. 2006. XLSTAT version 7.5 para Excel interface. Addinsoft, UK.
- Adolph EF. 1931. The size of the body and the size of the environment in the growth of tadpoles. Biological Bulletin. 61:350–375.
- Brady L, Griffiths R. 2000. Developmental response to pond desiccation in tadpoles of the British anuran amphibians. Journal of Zoology. 252:6–69.
- Bridges CM. 2002. Tadpoles balance foraging and predator avoidance: effects of predation, pond drying, and hunger. Journal of Herpetology. 36:627–634.
- Calich HJ, Wassersug RJ. 2012. The architecture of the aquatic environment and its influence on the growth and development of tadpoles (Xenopus laevis). Copeia. 4:690–697.
- Crump ML. 1989. Effect of habitat drying on developmental time and size at metamorphosis in Hyla pseudopuma. Copeia. 1988:794–797.
- Eterovick PC, Lazarotti I, Franco BP, Dias CJ. 2010. Seasonal variation of tadpole spatial niches in permanent streams: the roles of predation risk and microhabitat availability. Austral Ecology. 35:879–887.
- Gómez VI, Kehr AI. 2011. Morphological and developmental responses of anuran larvae (Physalaemus albonotatus) to chemical cues from the predators Moenkhausia dichoroura (Characiformes: Characidae) and Belostoma elongatum (Hemiptera: Belostomatidae). Zoological Studies. 50:203–210.
- Gómez VI, Kehr AI. 2012. The effect of chemical signal of predatory fish and water bug on the morphology and development of Elachistocleis bicolor tadpoles (Anura: Microhylidae). Biologia. 67:1–6.
- Gosner KL. 1960. A simplified table for staging anurans embryos and larvae with notes of identification. Herpetologica. 16:183–190.
- John KR, Fenster D. 1975. The effects of partitions on the growth rates of crowded Rana pipiens tadpoles. American Midland Naturalist. 93:123–130.
- John KR, Fusaro JM. 1981. Growth and metamorphosis of solitary Rana pipiens tadpoles in confined space. Copeia. 1981:737–741.
- Kehr AI, Duré MI, Schaefer EF. 2009. Utilización de un modelo simple de remoción para estimar el tamaño poblacional en larvas de anfibios. Boletín de la Asociación Herpetológica Española. 20:103–105.
- Maciel TA, Juncá FA. 2009. Effects of temperature and volume of water on the growth and development of tadpoles of Pleuroderma diplolister and Rhinella granulosa (Amphibia: Anura). Zoologia. 26:413–418.
- Marquez-Garcia M, Correa-Solís M, Mendez MA. 2010. Life-history trait variation in tadpoles of the warty toad in response to pond drying. Journal of Zoology. 281:105–111.
- Mogali SM, Saidapur SK, Shanbhag BA. 2011. Receding water levels hasten metamorhosis in the frog, Sphaerotheca breviceps (Schneider, 1799): a laboratory study. Current Science. 101:1219–1222.
- Pearman PB. 1993. Effects of habitat size on tadpoles populations. Ecology. 74:1982–1991.
- Pearman PB. 1995. Effects of pond size and consequent predator density on two species of tadpoles. Oecologia. 102:1–8.
- Pearman PB, Wilbur HM. 1990. Changes in pop population dynamics resulting from oviposition in a subdivided habitat. American Naturalist. 135:708–723.
- Perotti MG, Jara FG, Ubeda CA. 2011. Adaptive plasticity of life-history traits to pond drying in three species of Patagonian anurans. Evolutionary Ecology Research. 13:415–429.
- Petrusewicz K. 1963. General remarks on the productivity of confined populations. Ekologia Polska-Seria A. 11:617–624.
- Petrusewicz K, Trojan P. 1963. The influence of the size of the cage on the numbers and density of a self-ranging population of white mice. Ekologia Polska-Seria A. 11:611–614.
- Relyea RA. 2002. Competitor-induced plasticity in tadpoles: consequences, cues, and connections to predator induced plasticity. Ecological Monograph. 72:523–540.
- Relyea RA. 2004. Fine-tuned phenotypes: tadpole plasticity under 16 combinations of predators and competitors. Ecology. 85:172–179.
- Relyea RA, Auld JR. 2005. Predator- and competitor-induced plasticity: how changes in foraging morphology affect phenotypic trade-offs. Ecology. 86:1723–1729.
- Richter-Boix A, Tejedo M, Rezende E. 2011. Evolution and plasticity of anuran larval development in response to desiccation. A comparative analysis. Ecology and Evolution. 1:15–25.
- Rot-Nikcevic I, Taylor CN, Wassersug RJ. 2006. The role of images of conspecifics as visual cues in the development and behavior of larval anuran. Behavioral Ecology and Sociobiology. 60:19–25.
- Semlitsch RD, Gibbons JW. 1990. Effects of egg size on success of larval salamanders in complex aquatic environments. Ecology. 71:1789–1795.
- Semlitsch RD, Wilbur HM. 1988. Effect of pond drying time on metamorphosis and survival in the salamander Ambystoma talpoideum. Copeia. 1988:978–983.
- Sutherland MA, Gouchie GM, Wassersug RJ. 2009. Visual stimulation alone induce phenotypically plastic responses in Rana sylvatica tadpoles oral structure? Journal of Herpetology. 43:165–168.
- Van Buskirk J. 2009. Natural variation in morphology of larval amphibians: phenotypic plasticity in nature? Ecological Monographs. 79:681–705.
- Wells KD. 2007. The ecology and behavior of amphibians. Chicago: The University of Chicago Press. XI + 1148 p.
- Yung E. 1885. De l’influence des variations de milieu physico-chimique sur le développement des animaux. Archives des Sciences physiques et naturelles. 14:502–522.