Abstract
Understanding the genetic resistance to wheat leaf rust, caused by Puccinia triticina, in Canadian wheat cultivars is critical for maximizing resistance in future-bred cultivars. This knowledge also helps to predict the impact of changes in virulence to specific resistance genes within the P. triticina population. ‘Superb’ and ‘McKenzie’ are two of the most popular high-yielding wheat cultivars in Canada. ‘HY644’ has moderate resistance to Fusarium head blight (caused primarily by Fusarium graminearum). All three of these cultivars have been used extensively as parents in Canadian wheat breeding programmes. To analyze the nature of resistance in these cultivars, they were crossed and then backcrossed to the susceptible cultivar ‘Thatcher’. The BC1F3 populations were inoculated at the seedling and adult plant stages with various P. triticina races to determine the number and identity of the resistance genes in each cultivar. Allelism tests, to confirm the postulated genes, were performed by crossing each cultivar to the ‘Thatcher’ isolines containing the postulated genes and analyzing the F2 progeny for rust resistance. ‘Superb’ was demonstrated to have genes Lr2a and Lr10, ‘McKenzie’ had Lr10, Lr13, Lr16 and Lr21, and ‘HY644’ had Lr1, Lr17, Lr34 and an unknown resistance gene.
Résumé
Afin de maximiser la résistance à la rouille brune des futurs cultivars, il est essentiel de comprendre les mécanismes de la résistance génétique à cette maladie causée par Puccinia triticina chez les cultivars canadiens de blé. Ces connaissances aideront également à prédire les effets des changements sur le plan de la virulence quant aux gènes spécifiques de résistance au sein de la population de P. triticina . ‘Superb’ et ‘McKenzie’ sont deux des cultivars de blé à haut rendement les plus populaires au Canada. ‘HY644’ affiche une résistance moyenne à la fusariose de l’épi (causée principalement par Fusarium graminearum). Ces trois cultivars ont été utilisés extensivement comme plantes mères dans les programmes canadiens de sélection du blé. Afin d'analyser la nature de la résistance chez ces cultivars, ils ont été croisés puis rétrocroisés avec le cultivar réceptif ‘Thatcher’. Les populations BC 1 F 3 ont été inoculées aux stades jeune et adulte avec différentes races de P. triticina afin de déterminer le nombre de gènes de résistance dans chaque cultivar et de les identifier. Des tests d'allélisme ont été effectués afin de confirmer l'identité des gènes hypothétiques en croisant chaque cultivar avec les lignées isogéniques du cultivar ‘Thatcher’ contenant les gènes hypothétiques et en analysant la descendance F 2 pour en évaluer la résistance à la rouille. Cela a servi à démontrer que ‘Superb’ possède les gènes Lr2a et Lr10 ; ‘McKenzie’, les gènes Lr10 , Lr13 , Lr16 et Lr21 ; et ‘HY644’, les gènes Lr1 , Lr17 , Lr34 ainsi qu'un gène de résistance inconnu.
Introduction
Wheat leaf rust, caused by Puccinia triticina Eriks., is a major disease of wheat in Canada. It occurs annually and causes losses in the range of 1–20% (McCallum et al., Citation2007). The severity of leaf rust epidemics are determined by three main factors: environmental conditions favourable for infection and spread of the disease, presence of P. triticina inoculum, and genetic resistance of the wheat cultivars in use. Knowledge of the genes present in the predominant wheat cultivars helps to assess the vulnerability of the crop to leaf rust. This information can also be combined with detailed virulence information on the P. triticina population in Canada to make informed decisions for improving the leaf rust resistance in Canadian wheat cultivars. The genetics of leaf rust resistance in many key Canadian cultivars have been determined previously (Kolmer, Citation1996; McCallum et al., Citation2007). Resistance genes commonly found in Canadian wheat cultivars include Lr10, Lr13, Lr14a, Lr16 and Lr34. Recently, Lr21 and Lr22a have been incorporated into many of the newer cultivars (McCallum et al., Citation2007; McCallum & DePauw, Citation2008). The P. triticina population in Canada has evolved virulence against Lr10, Lr13, Lr14a and to some extent to Lr16. However, Lr21 and Lr22a remain completely effective against all isolates tested and Lr34 conditions partial resistance to all isolates (McCallum & Seto-Goh, Citation2008). The three cultivars that were analyzed in this study were ‘Superb’, ‘McKenzie’ and ‘HY644’.
‘Superb’ is a high-yielding Canadian Western Red Spring (CWRS) cultivar. It yielded on average 24% higher than ‘Neepawa’ and 14.5% more than ‘AC Barrie’ in cooperative yield tests (Townley-Smith et al., Citation2010). It was the highest ranked wheat cultivar in Canada, in terms of seeded area in 2006 (McCallum & DePauw, Citation2008) and remained a dominant cultivar through 2008 (Anonymous, Citation2008) (). It was derived from doubled haploid lines from the cross Grandin*2/AC Domain. ‘Superb’ was moderately resistant to leaf rust during co-operative testing prior to its registration in 2001 but it has subsequently become susceptible to leaf rust in the field (B. McCallum, unpublished data). ‘AC Domain’ was reported to have Lr10 and Lr16 which are effective at the seedling stage in addition to the adult plant resistance genes Lr12 and Lr34 (Liu & Kolmer, Citation1997a ). ‘Grandin’ was reported to have Lr2a, Lr3, and Lr10 and was heterogeneous for Lr16 which are all effective at the seedling stage, in addition to the adult plant resistance genes Lr13 and Lr34 (Liu & Kolmer, Citation1997b ).
Fig. 1. Proportion of CWRS area seeded to ‘AC Barrie’, ‘McKenzie’ and ‘Superb’ from 1999 to 2008. Data from Canadian Wheat Board variety surveys 1999–2008 (Anonymous, 2008).
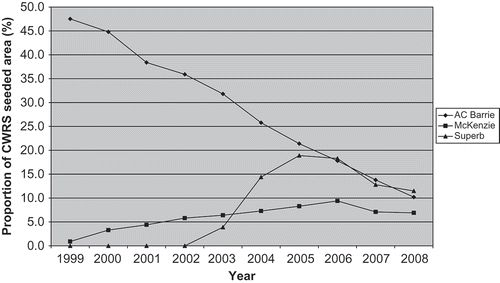
‘McKenzie’, registered in 1998, was the first doubled haploid wheat cultivar registered in Canada (Graf et al., Citation2003) and was derived from the cross ‘Columbus’/ ‘Amidon’. It is a high yielding CWRS cultivar with early maturity. The mean grain yield of ‘McKenzie’ was 15.0% higher than ‘Neepawa’ and 12.8% higher than ‘Katepwa’ during three years of yield trial testing (Graf et al., Citation2003). ‘McKenzie’ demonstrated a high level of leaf rust resistance in the field during registration tests which has continued in widely distributed field scale production in western Canada since its release. ‘McKenzie’ has also been a very popular wheat cultivar in western Canada, due primarily to its high yield potential and good leaf rust resistance (Anonymous, Citation2008; McCallum & DePauw, Citation2008) (). ‘Columbus’ was reported to have Lr13 and was heterogeneous for Lr16 (Samborski & Dyck, Citation1982). ‘Amidon’ is a Dark Northern Spring wheat cultivar developed and released by North Dakota State University in 1988. It was thought to carry the Lr21 gene (R. Graf, personal communication), originally transferred from Aegilops squarrosa L. (Rowland & Kerber, Citation1974). The Lr21 resistance gene has conditioned effective resistance to all North American isolates of P. triticina since it was discovered in the 1970s (McCallum et al., Citation2007).
The cultivar ‘HY644’ was granted plant breeders rights in the United States; however, it was not registered in Canada. It was the first cultivar developed in western Canada with a moderate level of resistance to Fusarium head blight (FHB). It was derived from the cross A16//Alpha*4/BgBSR/3/Sceptre/Ning 8331 where A16 is Alpha 16, a pure line reselected out of ‘Alpha’ that is highly resistant to leaf rust; ‘Alpha’ is HY612, a Canada Prairie Spring (CPS) breeding line, and BgBSR is a derivative of the CPS cultivar ‘Biggar’ to which bunt and smut resistance were added by backcrossing. Ning 8331 is the source of FHB resistance in ‘HY644’. ‘HY644’ was proposed for registration into the CPS wheat class but had a small proportion of kernels that were visually indistinguishable from Canada Western Red Spring (CWRS) wheat. It was therefore not registered as a bread wheat cultivar in western Canada due primarily to these problems of a lack of kernel visual distinguishability (KVD). ‘HY644’ has a moderate level of leaf rust resistance in the field in Canada.
The objective of this study was to determine the inheritance of leaf rust resistance in the wheat cultivars ‘Superb’, ‘McKenzie’ and ‘HY644’. Knowledge of the number and identity of the leaf rust resistance genes in these important cultivars will be useful in understanding their field reaction to changing P. triticina populations and in using these cultivars as parents for breeding future wheat cultivars.
Materials and methods
‘Superb’, ‘McKenzie’ and ‘HY644’ were used as pollen parents and crossed with ‘Thatcher’, a leaf rust susceptible cultivar. The F1 plants were used as pollen parents to backcross to ‘Thatcher’. Approximately 110 BC1F1 plants per population were selfed to create a single BC1F2 family for each BC1F1 plant. Each BC1F2 family was individually increased, using a minimum of 30 plants per family, to the BC1F3 family stage to obtain sufficient seed for testing. In total there were 109 BC1F3 families from Thatcher*2/Superb, 108 from Thatcher*2/McKenzie, and 103 from Thatcher*2/HY644. These families were seeded as rows with two replicates in the leaf rust nursery near Glenlea, MB, during the summer of 2003 and adjacent susceptible spreader rows were inoculated with a mixture of P. triticina virulence phenotypes representative of those collected across western Canada in 2002 (McCallum & Seto-Goh, Citation2005). Within this mixture of virulence phenotypes, the frequency of virulence to Lr1, Lr3, Lr10, Lr12, Lr13 and Lr14a was greater than 90%, whereas it was less than 10% for Lr9, Lr24, Lr26, Lr3ka, Lr11, Lr30 and Lr18. The frequency of virulence to Lr2a and Lr2c was 58.5%, to Lr16 it was 33.3%, and 53.4% of the isolates were virulent to Lr17 and LrB (McCallum & Seto-Goh, Citation2005). These lines were rated for leaf rust severity on the flag leaves, once the leaves of susceptible check cultivars were infected with high levels of rust, using a modified Cobb scale of 0–100% flag leaf infection (Peterson et al., Citation1948). Families were rated as either susceptible (no resistant plants) or segregating (a mixture of susceptible and resistant plants).
The BC1F3 families were also inoculated at the seedling or two-leaf stage with specific genetically homogeneous isolates of P. triticina. Each of these isolates had been collected during a previous virulence survey in Canada and was initiated from a single pustule collection. The isolates used were 1-1 BBBD, 96-12-3 MBDS and 95-77-2 TJBJ. Isolate 1-1 BBBD is avirulent to most resistance genes and therefore useful in identifying most or all of the resistance genes present in a given cultivar. Isolates 96-12-3, MBDS and 95-77-2 TJBJ represent common virulence phenotypes and differ in their virulence/avirulence spectra. Purity of each isolate was tested by inoculating a set of standard host differential lines as described previously (McCallum & Seto-Goh, Citation2005). To inoculate with each isolate, approximately 10 seeds from each of the BC1F3 families were planted in a clump and the clumps were evenly spaced in a fibre flat (25 × 15 cm). The plants were spray inoculated with urediniospores mixed with a light mineral oil (Bayol, Esso Canada, Toronto, ON) seven days after seeding, were dried for 1 h, then incubated in near 100% relative humidity for approximately 17 h before being transferred to a greenhouse at 20 ± 4 °C with supplemental high-pressure sodium lighting. The first and second leaves of the plants were rated for their reaction to leaf rust 12 to 14 days after inoculation. Seedling infection phenotypes were defined as follows: ‘;’ small hypersensitive flecks, ‘1’ small uredinia surrounded by necrosis, ‘2’ moderate size uredinia surrounded by chlorosis, ‘3’ medium-sized uredinia without chlorosis or necrosis, ‘4’ large uredinia without chlorosis, ‘+’ larger uredinia than typical for the infection type, ‘-’ smaller uredinia than typical for the infection type, ‘=’ much smaller uredinia than typical for the infection type. Infection types ‘;’, ‘1’, and ‘2’ were considered resistant, whereas ‘3’ and ‘4’ were considered susceptible. Progeny families were scored as susceptible if all plants were susceptible or segregating if the line contained a mixture of susceptible and resistant plants.
The BC1F3 families that were susceptible to 1-1 BBBD at the seedling stage were also tested at the adult plant stage with 1-1 BBBD to reveal the presence of any adult plant resistance genes. Adult plant genes are not effective at the seedling stage but they become effective as the plant reaches the heading stage (Dyck & Kerber, Citation1985). Six seeds from each BC1F3 family that was susceptible to 1-1 BBBD at the seedling stage were grown in 15 cm diameter pots in a growth cabinet set at 15 °C night and 18 °C day with supplemental fluorescent lighting. These plants were inoculated at the seedling stage with 1-1 BBBD as described previously to confirm that they were susceptible; any segregating families were removed. Then the plants were grown to the heading stage and inoculated with 1-1 BBBD, as described previously, when the flag leaves were fully exposed. The flag leaves on these plants were rated for their reaction to leaf rust 14–21 days after inoculation, using the infection type scale described above.
‘Superb’, ‘McKenzie’, ‘HY644’ and the 16 near-isogenic Thatcher lines, containing Lr1, Lr2a, Lr2c, Lr3, Lr9, Lr16, Lr24, Lr26, Lr3ka, Lr11, Lr17, Lr30, LrB, Lr10, Lr14a and Lr18, respectively, constituting the standard differential set for P. triticina (McCallum & Seto-Goh, Citation2008), were inoculated with 37 different P. triticina isolates. The procedure used is described above. The 37 isolates were chosen to represent a range in virulence phenotypes.
To confirm the identity of the postulated genes in these cultivars, tests of allelism were done by crossing the cultivar with the appropriate ‘Thatcher’ near-isogenic line that carried the gene in question. ‘Superb’ was crossed with the ‘Thatcher’ lines carrying Lr2a (RL6016), Lr10 (RL6004) and Lr16 (RL6005), ‘McKenzie’ was crossed with the ‘Thatcher’ lines carrying Lr10 (RL6004), Lr13 (RL4031), Lr16 (RL6005) and Lr21 (RL6043) and ‘HY644’ was crossed with the ‘Thatcher’ lines carrying Lr1 (RL6003a), Lr10 (RL6004), Lr17 (RL6008) and Lr34 (RL6058). In all crosses, the cultivar was used as the pollen parent and the F1 plants produced from the crosses were selfed to generate F2 populations. All F2 populations were tested for rust resistance at the seedling stage except for those crosses involving Lr13 or Lr34 that were tested at the adult plant stage as described above. The ratios of resistant to susceptible plant or families were compared to expected ratios using chi-squared tests.
Results
‘Superb’
In field tests during the summer of 2003, ‘Superb’ was moderately susceptible and all families from the Thatcher*2/Superb cross were susceptible to leaf rust (). These populations were then screened at the seedling stage with P. triticina virulence phenotype 1–1 BBBD (Long & Kolmer, Citation1989; McCallum & Seto-Goh, Citation2005). This virulence phenotype is avirulent to nearly all leaf rust resistance genes and is useful in detecting most putative genes which are present. The Thatcher*2/Superb BC1F3 families segregated into 83 segregating and 26 susceptible families, which fit a 3:1 ratio for two resistance genes (χ2 = 0.08, P = 0.78). When plants from these families were inoculated with 96-12-3 MBDS, there were 62 segregating families and 47 susceptible families which fit a 1:1 ratio for a single resistance gene (χ2 = 2.06, P = 0.15) but did not fit a 3:1 ratio for two resistance genes (χ2 = 19.09, P = 0.00). All these families were susceptible to 95-77-2 TJBJ. There were 26 families susceptible to 1-1 BBBD at the seedling stage which were also inoculated at the adult plant stage with 1-1 BBBD. None of these families had adult plant resistance to 1-1 BBBD, indicating that ‘Superb’ did not have any adult plant resistance genes.
Table 1. Segregation for reaction to virulence phenotypes of Puccinia triticina in BC1F3 families of ‘Thatcher’ crossed with ‘Superb’, ‘McKenzie’ and ‘HY644’
The parents of ‘Superb’ were reported to carry Lr10, Lr16, Lr12 and Lr34 (‘AC Domain’; Liu & Kolmer, Citation1997a ), and Lr2a, Lr3, Lr10, Lr16, Lr13 and Lr34 (‘Grandin’; Liu & Kolmer, Citation1997b ). Of these Lr2a, Lr3, Lr10 and Lr16 are seedling genes and Lr12, Lr13 and Lr34 are adult plant resistance genes. From these tests, ‘Superb’ appeared to have two seedling resistance genes that were effective against 1–1 BBBD. Only one of these genes was effective against 96-12-3 MDBS and neither of these genes was effective against 95-77-2 TJBJ.
Seedling tests with various P. triticina isolates on ‘Superb’ demonstrated that it did not have Lr16 since isolates such as 96-27-2 TDQF and 96-16-3 TDMJ were virulent on ‘Superb’ but avirulent on the TcLr16 line (). Similarly ‘Superb’ did not have Lr3 since 17 SBJQ, 00-149-2 SBDG, and 00-74-1 SGBJ were avirulent to Lr3 but virulent to ‘Superb’ (). All isolates tested that were avirulent to either Lr2a or Lr10 were also avirulent to ‘Superb’. To confirm the presence of these genes, the ‘Thatcher’ near-isogenic lines containing either Lr2a or Lr10 were each crossed with ‘Superb’. No F2 progeny susceptible to 1–1 BBBD were detected from 440 or 340 progeny tested from the crosses involving Lr2a and Lr10, respectively (). In contrast, there were 115 resistant and five susceptible F2 progeny from the cross of Superb/TcLr16 which fit a three gene ratio, and confirmed that ‘Superb’ did not have Lr16.
Table 2. Seedling infection types of ‘Superb’, ‘McKenzie’, ‘HY644’ and selected Thatcher near isogenic lines to 37 virulence phenotypes of Puccinia triticina
Table 3. Segregation for reaction to P. triticina isolates in allelism crosses between ‘Superb’, ‘McKenzie’ and ‘HY644’ and selected Thatcher near-isogenic lines
‘McKenzie’
In the field leaf rust nursery, the Thatcher*2/McKenzie cross consisted of 49 homozygous susceptible families and 59 families that segregated for resistance, which fit an expected 1:1 segregation ratio for a single resistance gene in a backcross population (χ2 = 0.93, P = 0.336) (). The resistant plants in these families had very low rust reactions which varied from ‘trace’ amounts of rust to 10% using the scale described by Peterson et al. (Citation1948) with resistant or moderately resistant pustules.
These families were differentiated into 97 segregating and 11 susceptible families for seedling reaction to 1-1 BBBD which fit a 7:1 ratio for three independent resistance genes (χ 2 = 0.19, P = 0.66) (). When these same families were inoculated with 96-12-3 MBDS, there were 76 segregating and 32 susceptible families which fit a 3:1 ratio for two independent resistance genes (χ2 = 1.23, P = 0.27) (). When inoculated with 95-77-2 TJBJ, there were 61 segregating families and 47 susceptible families which fit a 1:1 ratio for a single resistance gene (χ2 = 1.81, P = 0.18) (). The 11 families that were susceptible to 1-1 BBBD as seedlings were inoculated as adult plants and one of these families segregated for an adult plant resistance gene.
‘McKenzie’ was derived from the cross Columbus/Amidon (Graf et al., Citation2003). Based on the genes reported in these cultivars, ‘McKenzie’ could have the seedling resistance genes Lr16 and Lr21 and the adult plant resistance gene Lr13. ‘McKenzie’ was resistant to all isolates tested (). All isolates in , and all other Canadian P. triticina isolates tested to date, are avirulent to the Thatcher-Lr21 line (data not shown) and produce similar infection types.
Based on the reactions of the BC1F3 progeny of Thatcher*2/McKenzie to the P. triticina isolates 1-1 BBBD, 96-12-3 MBDS and 95-77-2 TJBJ (), and the resistance genes thought to be present in ‘Columbus’ and ‘Amidon’, ‘McKenzie’ was crossed to the ‘Thatcher’ isogenic lines with Lr10, Lr13, Lr16 and Lr21. The F2 generation from the cross McKenzie/TcLr10 was tested at the seedling stage with isolate 1-1 BBBD. There were no susceptible progeny detected among 874 plants tested. The Thatcher*2/McKenzie population segregated for three effective seedling genes to 1-1 BBBD, if Lr10 was not one of those genes then a four gene segregation ratio of 255 resistant to 1 susceptible would be expected. Therefore, ‘McKenzie’ was determined to have Lr10.
The F2 generation from the cross McKenzie/TcLr16 was tested at the seedling stage with isolate 96-12-2 MBDS. This isolate is virulent to Lr10 but avirulent to Lr16 and Lr21, and therefore if ‘McKenzie’ did not have Lr16, a three gene segregation ratio of 63 resistant plants to one susceptible, would be expected. All 188 progeny tested were resistant, demonstrating that ‘McKenzie’ has Lr16. The F2 generation from the cross McKenzie/TcLr21 was also tested at the seedling stage with isolate 74-2 MGBJ. This isolate was chosen since it was virulent to both Lr10 and Lr16, but avirulent to Lr21. If ‘McKenzie’ did not have Lr21 then a two gene segregation ratio would be expected. All 61 F2 progeny from this cross were resistant to 74-2 MGBJ, demonstrating that McKenzie also has Lr21.
The McKenzie/TcLr13 F2 progeny were inoculated at the adult plant stage with isolate 74-2 MGBJ. This isolate is virulent to Lr10 and Lr16 but avirulent to Lr13 and Lr21. With this isolate we expected a three gene segregation ratio, of 63 resistant plants to one susceptible plant, if Lr13 was not present in ‘McKenzie’. From 239 progeny tested from this cross, no susceptible plants were found, demonstrating that ‘McKenzie’ contains Lr13.
‘HY644’
In a leaf rust inoculated field nursery, the Thatcher*2/HY644 cross had 43 susceptible families and 58 families that segregated for resistance, which fit a 1:1 ratio for a single gene (χ2 = 2.23, P = 0.135) (). Resistant plants in these families varied from 5 to 35% on the Peterson et al. (Citation1948) scale with pustules ranging from moderately resistant to moderately susceptible, and pustules decreasing in size towards the tip of the leaf which resembled the reaction of the Thatcher near-isogenic line with Lr34 (RL6058).
These families were classified into 86 segregating and 17 susceptible families when inoculated with 1-1 BBBD at the seedling stage, which fit a three gene or 7:1 ratio (χ2 = 1.51, P = 0.22) (). All families were susceptible to 96-12-3 MBDS. When inoculated with 95-77-2 TJBJ, there were 55 segregating and 48 susceptible families, which fit a single gene or 1:1 ratio (χ2 = 0.52, P = 0.47). Of the 35 additional P. triticina isolates tested, all that were virulent to ‘HY644’ were also virulent to Lr1 and Lr17 and those that were avirulent to either gene were also avirulent to ‘HY644’ (). Other genes could be eliminated if an isolate that was virulent to ‘HY644’ was avirulent to any of the known resistance genes. Both 96-12-3 MBDS and 95-77-2 TJBJ are virulent to Lr1 but 95-77-2 TJBJ is avirulent to Lr17, whereas 96-12-3 MBDS is virulent to Lr17; therefore, the effective resistance gene against 95-77-2 TJBJ could be Lr17.
Allelism tests were done between ‘HY644’ and Lr1, Lr10, Lr17 and Lr34 by crossing ‘HY644’ with the Thatcher near-isogenic line carrying each of these resistance genes. The F2 progeny from the cross ‘HY644’/TcLr1 were tested for leaf rust resistance by inoculating with virulence phenotype 1-1 BBBD. Since this isolate is avirulent to Lr1, Lr10 and Lr17, this cross would be expected to segregate for four resistance genes if ‘HY644’ did not have Lr1. All 800 progeny tested were resistant. An additional 467 F2 progeny from this cross were inoculated with 96-14-1 CCDS which is avirulent to Lr1 but virulent to Lr17 and Lr10. All progeny were resistant, with a “0;” phenotype characteristic of Lr1, demonstrating that ‘HY644’ has Lr1. Similarly, the F2 progeny from the cross HY644/TcLr17 were tested for leaf rust resistance by inoculating with virulence phenotype 1-1 BBBD, and all 800 progeny tested were resistant. Additionally, a further 655 F2 progeny from this cross were inoculated with 95-77-2 TJBJ which is virulent to Lr1and Lr10 but avirulent to Lr17, and all progeny were resistant; therefore, ‘HY644’ was demonstrated to carry Lr17. Attempts were made to determine the identity of the third seedling resistance gene in ‘HY644’, effective against 1-1 BBBD but ineffective against 96-12-3 MBDS and 95-77-2 TJBJ at the seedling stage and ineffective in the field. Additional isolates were used to inoculate both ‘HY644’ and the Thatcher*2/’HY644’ families (data not shown). While many genes effective against 1-1 BBBD were ruled out, such as Lr15 and Lr23; no known resistance gene could be positively identified.
To determine if Lr34 was present in ‘HY644’, 190 F2 progeny from the cross HY644/TcLr34 were tested at the adult plant stage with 96-12-3 MBDS. This isolate was avirulent to Lr34 but virulent to Lr1 and Lr17. If ‘HY644’ did not have Lr34 then a two-gene segregation ratio of 15 resistant plants to one susceptible plant, would be expected. All 50 F2 progeny tested were resistant to 96-12-3 MBDS with pustule type characteristics of Lr34. Therefore, ‘HY644’ was demonstrated to have the Lr34 resistance gene.
Molecular marker analysis confirmed that both ‘McKenzie’ and ‘Superb’ have the susceptible allele at the Lr34 locus, whereas ‘HY644’ had the resistant allele (S. Cloutier, personal communication). Similarly, marker analysis confirmed that both ‘HY644’ and ‘Superb’ have the susceptible allele at the Lr21 locus, whereas ‘McKenzie’ had the resistant allele (C. Hiebert, personal communication).
Discussion
‘Superb’ was demonstrated to have resistance genes Lr2a and Lr10. ‘Superb’ was derived from the cross Grandin*2/AC Domain. ‘Grandin’ was reported to have Lr2a, Lr3, Lr10, Lr13 and Lr34 and was heterogeneous for Lr16 (Liu & Kolmer, Citation1997b ), whereas ‘AC Domain’ was reported to have Lr10, Lr16, Lr12 and Lr34 (Liu & Kolmer, Citation1997a ). Based on this information, ‘Superb’ should have the important leaf rust resistance gene Lr34. However, molecular marker analysis of ‘Grandin’ indicates that it is heterogeneous for Lr34, with some plants having the resistance allele and other plants having the susceptible allele (S. Cloutier, personal communication). Additionally, based on leaf rust evaluation in the field and molecular marker analysis, ‘AC Domain’ has the susceptible allele for Lr34 (McCallum et al., Citation2008).
The frequency of virulence to Lr10 has been very high (close to 100%) for collections of isolates from western Canada in recent years (McCallum & Seto-Goh, Citation2005, Citation2008) and it is relatively ineffective in controlling wheat leaf rust in this area. The Lr2a resistance gene was effective during the 1990s when the frequency of virulence to this gene was relatively low in the P. triticina population in western Canada but became ineffective in the 2000s when the frequency of isolates virulent to Lr2a increased dramatically (McCallum & Seto-Goh, Citation2004) (). ‘Superb’ was registered in 2000 and was initially rated as intermediate for leaf rust resistance, since Lr2a had provided some protection prior to this time, but became susceptible in the field due to a shift to Lr2a virulent pathotypes within the P. triticina population.
Fig. 2. Frequency of virulence to Lr2a, Lr16 and Lr17 in Manitoba and Saskatchewan 1998–2008. Data from annual virulence surveys of Puccinia triticina in Canada (including McCallum & Seto-Goh, Citation2004, Citation2005, Citation2008).
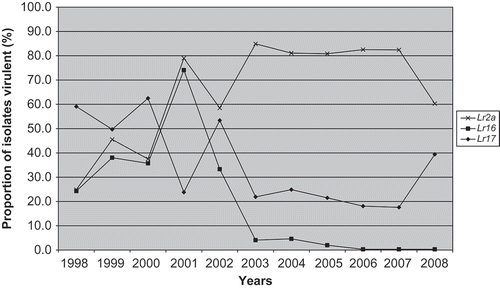
‘McKenzie’ was demonstrated to have Lr10, Lr13, Lr16 and Lr21. The frequency of virulence to Lr10 and Lr13 in the P. triticina population in western Canada is very high (McCallum & Seto-Goh, Citation2004, Citation2005, Citation2008). However, Lr21 has remained completely effective in controlling leaf rust in Canada since its initial release in the 1994 cultivar ‘AC Cora’ (McCallum & Seto-Goh, Citation2004, Citation2005, Citation2008; Townley-Smith & Czarnecki, Citation2008). ‘AC Cora’ was not a major Canadian wheat cultivar, in terms of seeded area over time (McCallum & DePauw, Citation2008) and no other Canadian cultivars were registered with Lr21 until ‘McKenzie’ was registered in 1998. Currently, Lr21 is thought to be deployed in a number of Canadian and US wheat cultivars (C. Hiebert, personal communication; Kolmer et al., Citation2008). Lr21 was initially discovered in Aegilops squarossa L. and transferred into bread wheat by Canadian researchers in 1974 (Rowland & Kerber, Citation1974). However, it was not deployed in a major Canadian cultivar until 1998 when ‘McKenzie’ was released. ‘McKenzie’ inherited Lr21 from ‘Amidon’, which is a USA cultivar, since the other parent of ‘McKenzie’ – ‘Columbus’ – was only demonstrated to carry Lr13 and Lr16 (Samborski & Dyck, Citation1982). Resistance gene Lr16 is still partially effective in controlling wheat leaf rust. The frequency of virulence to Lr16 in the P. triticina population has fluctuated in recent years (McCallum & Seto-Goh, Citation2004, Citation2005, Citation2008) but most isolates have an intermediate level of virulence to Lr16. In field leaf rust nurseries, lines with only Lr16 suffer considerable leaf rust damage, while lines with Lr16 in combination with other resistance genes, particularly Lr34, are very resistant (B. McCallum, unpublished work).
In the 10 seedling susceptible BC1F3 families Thatcher*2/McKenzie, only one family segregated for resistance at the adult plant stage, due to the presence of Lr13 (). We would expect half the families to be segregating for Lr13 if it was independent of the seedling resistance genes. The genes Lr13 and Lr16 are both located on chromosome 2BS, but based on molecular markers, these genes are located at considerable distance from one another and are essentially independent (Bansal et al., Citation2008). It is possible that the actual genetic distance between these genes may be smaller than previous estimates, in which case genetic linkage to Lr16 would eliminate Lr13 in those families that were selected to have the susceptible allele at the Lr16 locus. This hypothesis requires further investigation since the 10 families reported here represent a very small sample size.
‘HY644’ was demonstrated to have Lr1, Lr17, Lr34 and an unknown seedling resistance gene effective only against 1-1 BBBD. The frequency of virulence to Lr1 in western Canada is close to 100% in most years, but Lr17 is still relatively effective (). The frequency of virulence to Lr17 was very low in western Canada prior to 1996, increased in 1996 (Kolmer Citation1998) and subsequent years, then recently declined somewhat (McCallum and Seto-Goh Citation2004, Citation2005, Citation2008). The Lr34 resistance gene is one of the most effective and durable leaf rust resistance genes. It has never been overcome by a change in pathogen virulence during its long deployment in wheat cultivars throughout the world. In Canada, Lr34 has provided effective resistance since it was first deployed in the cultivar ‘Glenlea’ in 1972 (McCallum and DePauw, Citation2008).
Understanding the genetics of leaf rust resistance in these cultivars will help to inform producers, seed growers and others involved in wheat production on the nature of resistance in these cultivars and how changes in the P. triticina population will affect this resistance. This information is also vital to wheat breeding programmes that use these cultivars, and their derivatives, as parents in crosses for future cultivars.
References
- Anonymous . 2008 . Canadian Wheat Board. Annual wheat variety surveys. Available from the Canadian Wheat Board, 423 Main St., P.O. Box 816 Station Main Winnipeg MB, , Canada R3C 2P5
- Bansal , U.K. , Hayden , M.J. , Venkata , B.P. , Khanna , R. , Saini , R.G. and Bariana , H.S. 2008 . Genetic mapping of adult plant leaf rust resistance genes Lr48 and Lr49 in common wheat . Theor. Appl. Genet. , 117 : 307 – 312 .
- Dyck , P.L. and Kerber , E.R. 1985 . “ Resistance of the race specific type ” . In The cereal rusts. Vol. 2. Diseases, distribution, epidemiology, and control , Edited by: Roelfs , A.P. and Bushnell , W.R. 469 – 500 . Orlando, FL : Academic Press .
- Graf , R.J. , Hucl , P. , Orshinsky , B.R. and Kartha , K.K. 2003 . McKenzie hard red spring wheat . Can. J. Plant Sci. , 83 : 565 – 569 .
- Kolmer , J.A. 1996 . Genetics of resistance to wheat leaf rust . Annu. Rev. Phytopathol. , 34 : 435 – 455 .
- Kolmer , J.A. 1998 . Physiologic specialization of Puccinia recondita f.sp. tritici in Canada in 1996 . Can. J. Plant Pathol. , 20 : 176 – 181 .
- Kolmer , J.A. , Long , D.L. and Hughes , M.E. 2008 . Physiologic specialization of Puccinia triticina on wheat in the United States in 2006 . Plant Dis. , 92 : 1241 – 1246 .
- Liu , J.Q. and Kolmer , J.A. 1997a . Genetics of leaf rust resistance in Canadian spring wheat varieties AC Domain and AC Taber . Plant Dis. , 81 : 757 – 760 .
- Liu , J.Q. and Kolmer , J.A. 1997b . Inheritance of leaf rust resistance in wheat cultivars Grandin and CDC Teal . Plant Dis , 81 : 505 – 508 .
- Long , D.L. and Kolmer , J.A. 1989 . A North American system of nomenclature for Puccinia recondita f.sp tritici . Phytopathology , 79 : 525 – 529 .
- McCallum , B.D. , Fetch , T. and Chong , J. 2007 . Cereal rust control in Canada . Aust. J. Agric. Res. , 58 : 639 – 647 .
- McCallum , B.D. and DePauw , R.M. 2008 . A review of wheat cultivars grown in the Canadian prairies . Can. J. Plant Sci. , 88 : 649 – 677 .
- McCallum , B.D. and Seto-Goh , P. 2004 . Physiologic specialization of Puccinia triticina, the cause of wheat leaf rust, in Canada in 2001 . Can. J. Plant Pathol. , 27 : 90 – 99 .
- McCallum , B.D. and Seto-Goh , P. 2005 . Physiologic specialization of wheat leaf rust (Puccinia triticina) in Canada in 2002 . Can. J. Plant Pathol. , 27 : 90 – 99 .
- McCallum , B.D. and Seto-Goh , P. 2008 . Physiologic specialization in Puccinia triticina in Canada in 2005 . Can. J. Plant Path. , 30 : 124 – 132 .
- McCallum , B.D. , Somers , D.J. , Humphreys , D.G. and Cloutier , S. 2008 . “ Molecular marker analysis of Lr34 in Canada Western Red Spring wheat cultivars ” . In Proceedings of 11th International Wheat Genetics Symposium 137 Brisbane, , Australia
- Peterson , R.F. , Campbell , A.B. and Hannah , A.E. 1948 . A diagrammatic scale for estimating rust intensity on leaves and stems of cereals . Can. J. Res. , 26 : 496 – 500 .
- Rowland , G.G. and Kerber , E.R. 1974 . Telocentric mapping hexaploid wheat of genes for leaf rust resistance and other characters derived from Aegilops squarrosa . Can. J. Genet. Cytol. , 16 : 137 – 144 .
- Samborski , D.J. and Dyck , P.L. 1982 . Enhancement of resistance to Puccinia recondita by interactions of resistance genes in wheat . Can. J. Plant Pathol. , 4 : 152 – 156 .
- Townley-Smith , T.F. and Czarnecki , E.M. 2008 . AC Cora hard red spring wheat . Can. J. Plant Sci. , 88 : 157 – 160 .
- Townley-Smith , T.F. , Humphreys , D.G. , Czarnecki , E. , Lukow , O.M. , McCallum , B.D. , Fetch , T.G. , Gilbert , J.A. and Menzies , J.G. 2010 . Superb hard red spring wheat . Can. J. Pl. Sci. , 90 : 347 – 352 .