Abstract
The global movement of solid wood packaging material is an important pathway by which invasive organisms have increased their range. The International Standard for Phytosanitary Measures No. 15 (ISPM No. 15) was published by the Secretariat of the International Plant Protection Convention to provide guidelines for wood treatment to reduce the risk of accidental pest movement via solid wood packaging material. This study assessed the exposure temperature and time combinations necessary to kill a range of fungi and one species of Phytophthora colonizing wood. The ISPM No. 15 protocol (heat to 56 °C core temperature for 30 min) was included as one of the treatments in the experiment. The survival data collected in this experiment were utilized to develop a binomial generalized linear model that allowed statistical assessment of survival following treatment. The tolerance of the isolates to heat treatment was variable and it was found that the ISPM No. 15 protocol did not result in mortality of all species that were tested.
Résumé
La circulation mondiale des matériaux d'emballage en bois massif constitue une des voies par laquelle les organismes invasifs ont étendu leur habitat. La Norme internationale pour les mesures phytosanitaires No 15 (NIMP No 15) a été publiée par le Secrétariat de la Convention internationale pour la protection des végétaux afin de fournir des directives quant au traitement du bois, visant ainsi à réduire le risque de dissémination accidentelle des organismes nuisibles que pourrait contenir le bois massif servant à la fabrication des emballages. Cette étude évalue la combinaison du temps et de la durée d'exposition au traitement nécessaire pour tuer une variété de champignons et une espèce de Phytophthora qui colonise le bois. Le protocole de la NIMP No 15 (chauffer le cœur du matériau à 56 °C pendant 30 min) a été inclus dans les traitements à évaluer au cours de l'expérience. Les données sur la survie collectées durant l'expérience ont été utilisées pour développer un modèle binomial linéaire généralisé qui permet l'évaluation statistique du taux de survie à la suite du traitement. La tolérance des isolats au traitement à la chaleur a varié et l'on a observé que le protocole de la NIMP No 15 n'a pas provoqué la mort de toutes les espèces testées.
Introduction
International trade in wood and wood products, and the by-products of trade such as wooden pallets and dunnage, have resulted in the movement of pests around the world (Brockerhoff et al., Citation2006; Haack, Citation2006). The introduction of insects, such as the Asian longhorned beetle (Anoplophora glabripennis (Motchulsky)) to North America, via this pathway has resulted in catastrophic invasions (Haack et al., Citation2010). Solid wood packaging materials are often composed of relatively low-grade unprocessed wood of unknown origin and have been found to harbour a variety of insects (Allen & Humble, Citation2002). In order to reduce the probability of accidental movement of pests in this wood, the Secretariat of the International Plant Protection Convention published the International Standard for Phytosanitary Measures No. 15 (ISPM No. 15). This document describes phytosanitary measures to reduce the risk of the introduction and spread of quarantine pests via wood packaging material, one of which is exposure to a temperature of 56 °C to the core of the wood for 30 minutes (FAO, Citation2007). Many other countries have adopted ISPM No. 15 and New Zealand has included it in the Import Health Standard for Wood Packaging Material from All Countries (MAF, Citation2006). New Zealand requires a more extreme heat treatment protocol for imported sawn wood; the Import Health Standard for Sawn Wood requires that a core temperature of 70 °C be maintained for four hours (MAF, Citation2003).
The ISPM No. 15 temperature and time combination of 56 °C for 30 min was experimentally developed in Canada to kill the nematode Bursaphelenchus xylophilus (Steiner & Buhrer) Nickle and its insect vectors (Smith, Citation1992; Uzunovic et al., Citation2006). Since fungi may also be vectored on wood and have varying temperature thresholds (i.e. Chidester, Citation1937, 1939; Jones, Citation1973; Newbill & Morrell, Citation1991), an internationally standardized protocol was developed by Uzunovic et al. (Citation2006) to determine the lethal temperature and time combinations for fungi colonizing wood and to determine if the heat treatment protocol of ISPM No. 15 is robust enough to kill fungi colonizing wood.
The objective of this research was to use the protocol of Uzunovic et al. (Citation2006) to determine the lethal temperature and time combinations for a range of different fungi that colonize wood.
Materials and methods
Fungi
Species from nine fungal genera and one species of Phytophthora were selected for heat treatment. The fungi selected for the study represent a range of fungal types associated with wood. The fungi include decay organisms, pathogens and moulds that affect different parts of trees or wood and are considered to pose a biosecurity risk. Members of the Ascomycota included Cladosporium herbarum (Pers.) Link, Cladosporium tenuissimum Cooke, Fusarium circinatum Nirenberg & O'Donnell, Lasiodiplodia theobromae (Pat.) Griffon & Maubl., Neonectria fuckeliana (C. Booth) Castl. & Rossman, Ophiostoma novo-ulmi Brasier, Sphaeropsis sapinea (Fr.) Dyko & B. Sutton. Members of the Basidiomycota included Armillaria novae-zelandiae (G. Stev.) Boesew., Phlebiopsis gigantea (Fr.) Jülich and Schizophyllum commune Fr. The one species from kingdom Chromista, phylum Oomycota was Phytophthora cinnamomi Rands. Three isolates of each species were selected for treatment, with the exception of Cladosporium tenuissimum and Cladosporium herbarum, for which there were one and two isolates, respectively. For each species, the isolates were selected to represent as wide a geographic range as possible to capture genetic variation within the species ().
Table 1. Fungi (and one species of Phytophthora) used in this study
Inoculation of wood blocks
Blocks of wood, 30 mm × 10 mm × 5 mm, were cut from Douglas fir (Pseudotsuga menziesii (Mirb.) Franco), Monterey pine (Pinus radiata D. Don), and elm (Ulmus sp.). The blocks were cut from green timber from trees that were felled specifically for this experiment and the time between felling the trees and machining the blocks was minimized by processing the wood within two days of felling. Elm boards from a freshly felled tree were milled offsite, couriered to the laboratory in plastic and machined at the laboratory upon arrival within a week of milling. The blocks were mixed to randomly distribute blocks from the sapwood and heartwood and then autoclaved at 121 °C for 30 min. The blocks were inoculated with the test organisms by aseptically placing 20 wood blocks on 2% malt extract agar in a 95 mm square Petri plate, inoculating the plate with the selected isolate, followed by incubation at 20 °C until the wood blocks were colonized. Ophiostoma novo-ulmi was inoculated onto elm, N. fuckeliana was inoculated onto pine and the rest of the fungi and P. cinnamomi were inoculated onto Douglas fir.
Treatment
For each treatment, a total of six replicate blocks of wood that were colonized by the same isolate were vacuum sealed inside a plastic bag using a Sammic model V421 vacuum sealing machine (Sammic, Spain) and then placed in a water bath set at the temperature required to raise the core temperature of the wood block to the target temperature. Removal of all the air from the plastic bag ensured efficient heat transfer to the blocks by eliminating the insulating effect provided by air trapped inside the bag. Blocks were sealed into the bag immediately prior to treatment to reduce the effect of oxygen starvation. A thermocouple was inserted into the core of a wood block and sealed within a plastic bag so that the temperature of the water bath was set as to achieve the desired core temperature. The temperature of the water bath was set with the same wood block and thermocouple for every target temperature. When the water bath temperature was stabilized and the core target temperature achieved, the blocks were placed in the water bath and then removed after exposure times of < 1 min, 30 min, 60 min or 120 min. To ensure that the < 1 minute exposure was accurate, an experiment was conducted to time how long it took for the wood block to reach the target temperature from room temperature. Therefore, the wood blocks were allowed to reach the target temperature before timing was started. Temperatures that were tested were 25 °C (control), 41 °C, 46 °C, 51 °C, 56 °C, 61 °C, 66 °C, 71 °C and 76 °C. All isolates were treated at each time and temperature combination from 25 °C to 71 °C, but S. commune was subjected to further testing at 76 °C.
All treatments involving F. circinatum, O. novo-ulmi, N. fuckeliana, L. theobromae, S. commune and one isolate of P. gigantea were inoculated, treated and assessed under strict quarantine conditions in the New Zealand Forest Research Institute Ltd. Quarantine and Containment Facility (Ministry of Agriculture and Forestry laboratory reference #2746).
Assessment
Immediately following treatment, the blocks of wood were removed from the vacuum sealed bag and placed onto 2% malt extract agar. All blocks, including those colonized by P. cinnamomi, were incubated at 20 °C and visually assessed for growth at regular intervals for up to 21 days following treatment. The identity of the species emerging from the blocks of wood was confirmed by morphology. If the isolate grew from the wood block following treatment, the treatment was not considered lethal. Of the 972 plates that were assessed, 13 became contaminated and were not included in any of the analyses. These plates were A. novae-zelandiae 2340 at 46 °C < 1 min, and 46 °C for 30 min; A. novae-zelandiae 1026 at 51 °C < 1 min; L. theobromae 2964 at 61 °C < 1 min; C. herbarum 89 at 61 °C for 30 min; C. herbarum 2963 at 41 °C < 1 min; N. fuckeliana 1105 at 41 °C for 60 min and 46 °C for 120 min; P. gigantea 1530 at 46 °C for 120 min and 56 °C < 1 min; P. cinnamomi 979 at 56 °C < 1 min; S. sapinea 15.17 at 46 °C for 120 min; S. sapinea 15.22 at 61 °C < 1 min.
Statistical analysis
A binomial generalized linear model with logit link function was fitted to the proportion (x/6) emerging using R version 2.8.1 (R Development Core Team Citation2009). The model fitted was
where y is the (vector of) observed proportions emerging, n the binomial sample size (here 6 for each observation), and p is corresponding vector of fitted proportions emerging. The coefficients a, b, c were fitted in separate models for each species or in a combined model with separate coefficients for each species. Data for all isolates of each species were combined and the model predictions were calculated at the species level.
At most times and temperatures there was either 100% (6/6) or 0% (0/6) emergence. Often there would be warnings of fitted values being very close to 0 and 1 and very large standard errors. This was a result of data from two species: Armillaria novae-zelandiae and Phlebiopsis gigantea. These two species were omitted to solve this problem (the other nine species fit). These two species had low incidence and very low predicted values at temperature 56 °C/time 30 min, but the coefficients for these species had high standard errors.
A ‘robust deviance’ estimate was calculated based on the nine species fit and used to adjust standard errors. Predicted proportion, , emerging and 95% confidence intervals (q2.5%, q97.5%) for the proportion emerging at temperature 56 °C after time 30 min was calculated. The model was also used to predict the temperature at which 99% and 99.99% mortality occurred after 30 min exposure. The robust deviance estimate was 3.11 and standard errors were adjusted by multiplying by the square root of this quantity, i.e. 1.76. The robust deviance statistic has an asymptotic chi-squared distribution. The reason for using the robust deviance, rather than the standard deviance, is because the latter is distorted by cells with 0% or 100%. As a general rule, it is necessary to have an expected value of five or more alive and five or more dead in each cell (cf. count out of six in the raw data) for the deviance test statistic to be approximately chi-squared. The robust deviance calculation overcomes the problem by pooling cells with similar predicted values until expected values of dead and alive are sufficiently high in each cell.
Results
After treatment at a core temperature of 25 °C, as a control, with exposure for < 1 min, 30 min, 60 min and 120 min, all isolates of every fungus and P. cinnamomi emerged from 100% (six/six replicates) of the treated blocks. Thus, vacuum sealing the wood blocks and depriving the fungi of oxygen for the period of time that was required to complete treatment was not lethal to any of the isolates tested. This result also confirmed that the wood blocks were successfully colonized by the organism of interest.
Mean survival of every species at every temperature/time combination is shown graphically in and b. Armillaria novae-zelandiae was found to be very susceptible to heat treatment, A. novae-zelandiae only survived the < 1 min and 30 min exposure times at 41 °C and < 1 min at 46 °C. The second most susceptible species was P. cinnamomi; isolate 102.16 was killed at all temperature/time combinations greater than 41 °C for 30 min. All temperature/time combinations greater than 46 °C for 120 min were lethal to isolate 979 and greater than 51 °C for 30 min were lethal to isolate 1012. It was observed that S. commune was very heat tolerant. Three of six replicates of isolate 1956 and six of six replicates of isolate 2491 survived 66 °C for < 1 min, but the other exposure times at 66 °C were lethal. When the temperature was raised to 71 °C, three of six and one of six replicates of isolates 1956 and 2491 of S. commune survived 30 min exposure but the rest of the exposure times were lethal. The temperature had to be raised to 76 °C before there was 100% mortality at all exposure times for these two isolates.
Fig. 1. a and b, Survival following heat treatment. Each plot represents mean survival (out of six replicate blocks) ± standard error of the mean for three isolates of each species at each time/temperature combination, with the exception of C. herbarum, for which there were two isolates and C. tenuissimum, for which there was one isolate (and hence no S.E.).
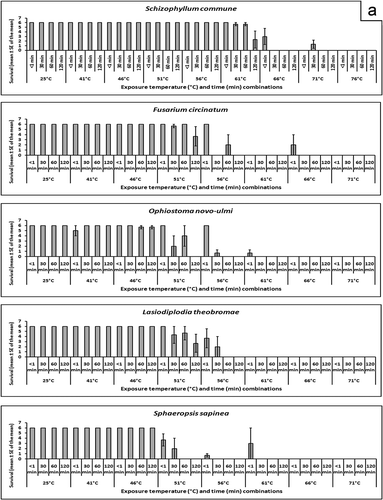
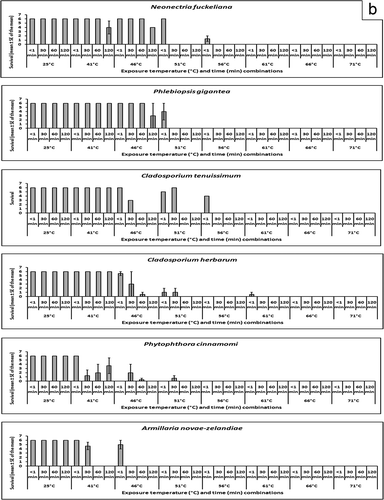
The predicted survival following treatment at 56 °C for 30 min and the temperatures that resulted in predicted 99% and 99.99% mortality following 30 min exposure () demonstrate the variability of susceptibility to heat treatment. Schizophyllum commune was the most heat tolerant species that was tested and the model predicted 99.3% survival following treatment at 56 °C for 30 min (). Of concern from a regulatory standpoint was 27.6% predicted survival of F. circinatum, the causal agent of pitch canker disease, following treatment at 56 °C for 30 min. It is predicted that exposure for 30 min to a minimum temperature of 61.7 °C or 68.9 °C is necessary to cause 99% or 99.99% mortality of F. circinatum, respectively (). Other fungi that were relatively heat tolerant were L. theobromae, O. novo-ulmi and S. sapinea (, ).
Table 2. Regression parameters for the binomial generalized linear model developed in this study, estimated survival ( ) following exposure to 56 °C for 30 min with 95% confidence interval and the predicted temperature (°C) that causes 99% and 99.99% mortality following 30 min exposure
) following exposure to 56 °C for 30 min with 95% confidence interval and the predicted temperature (°C) that causes 99% and 99.99% mortality following 30 min exposure
Discussion
To reduce the probability of movement of invasive organisms, several countries have invoked regulations that state that solid wood packaging material must be treated according to protocols outlined in ISPM No. 15, one of which is heat treatment to a core temperature of 56 °C for 30 min (FAO, Citation2007). Our study has demonstrated that fungi and P. cinnamomi infecting wood can be killed by heat treatment but that different species, as well as different isolates of the same species, vary in their susceptibility to heat ( and b). The ISPM No. 15 protocol results in regulation at the pathway level, rather than individual species, but the results of our in vitrostudy demonstrated that exposure to 56 °C for 30 min is not lethal for all fungi.
The fungi that were selected represented a range of fungal types. Basidiomycetes included A. novae-zelandiae, P. gigantea and S. commune. Armillaria novae-zelandiae is a root disease agent, while P. gigantea and S. commune are white rot decay fungi. The remainder of the fungi selected were ascomycetes and these included L. theobromae, a coelomycete that causes twig dieback and leaf blight, and C. herbarum and C. tenuissimum which are pigmented moulds. Important ascomycete pathogens that were selected included F. circinatum, the causal agent of pitch canker of P. radiata, N. fuckeliana, which is associated with flute canker of P. radiata, S. sapinea, the causal agent of diplodia whorl canker and shoot tip dieback and O. novo-ulmi, the causal agent of Dutch elm disease. Pathogens in the genus Phytophthora have been responsible for some of the worst plant disease problems that have been recorded and P. cinnamomi was selected for this study. These organisms were selected because they represent a broad cross-section of fungal types as well as organisms that colonize different regions of the host plants. Additionally, C. herbarum, P. gigantea and S. commune are common saprophytic fungi that have been recovered from imported and exported wood, while the rest are pathogens that infect wood.
It was observed that the lethal temperature/time combinations for each organism were achieved through either of two treatment regimes: (i) exposure to a critical high temperature for less than one minute; or (ii) a prolonged exposure to a temperature 5 °C lower than the critical temperature that was lethal. For all species that were tested, aside from S. commune, and one isolate each of C. herbarum, O. novo-ulmi, F. circinatum and S. sapinea, exposure to 61 °C for less than one minute was lethal. It was necessary to raise the temperature to 76 °C to kill S. commune with a less than one minute exposure, likely because S. commune is a chlamydospore-forming basidiomycete (Stalpers, Citation1978). Chlamydospores are thick-walled, long-term, survival structures that are produced by many fungi and their presence increases the tolerance of the fungus to heat. The remainder of the fungi in the sample population are non-chlamydospore forming, and they were killed by exposure to lower temperatures. The other chlamydospore-forming organism in the study was P. cinnamomi, yet Gallo et al. (Citation2008) have shown that exposure of P. cinnamomi chlamydospores to 40 °C for one to two hours was lethal, which correlates well with the results of this experiment.
Heat treatment of wood to kill colonizing fungi has been researched in the past, both for wood export purposes (Jones, Citation1973) and to kill decay fungi in wood destined for service, such as poles, railway ties, or structural timbers (Chidester, Citation1937, 1939; Newbill & Morrell, Citation1991). Jones (Citation1973) found that treatment of oak wood infected by the oak wilt pathogen Ceratocystis fagacearum (Bretz) Hunt for 48 h in 43 °C air or 24 h in 54 °C air or 43 °C water for 48 h or 49 °C water for 12 h killed the pathogen. Chidester (Citation1937, 1939) studied the effect of heat on the mortality of Gloeophyllum sepiarium (Wulfen) P. Karst., Meruliporia incrassata (Berk. & M.A. Curtis) Murrill and Neolentinus lepideus (Fr.) Redhead & Ginns, Fomitopsis rosea (Alb. & Schwein.) P. Karst, Gloeophyllum trabeum (Pers.) Murrill and Antrodia serialis (Fr.) Donk infecting wood blocks. It was recommended that core time/temperature combinations of 66 °C for 75 min, 77 °C for 30 min, 82 °C for 20 min, 93 °C for 10 min or 100 °C for 5 min were necessary to kill all of the fungi that were tested. Chidester (Citation1939) concluded that 66 °C is the minimum core temperature necessary to sterilize wood, which was consistent with the results of this study as every fungus, with the exception of S. commune and one isolate of F. circinatum, was killed by exposure to 66 °C or higher ( and b).
From an import/export treatment perspective, a rapid exposure to a core temperature of 76 °C would result in mortality of all the organisms that were tested in this experiment. It is also likely that the regime of 70 °C at the core for 4 h as specified by the Import Standard for Sawn Wood (MAF, Citation2003) would also be lethal to all species that were tested, as 70 °C was the minimum temperature required to achieve 99.99% mortality of all species after 30 minutes' exposure (). Although the 56 °C core temperature for 30-min regime of ISPM No. 15 killed many of the species that were treated in this experiment, not all species were killed. If the objective of a heat treatment is to kill all fungi infecting wood products, the results of this study suggest that the ISPM No. 15 protocol of 56 °C core temperature for 30 min is not sufficient to guarantee mortality of all fungi present in the wood. Indeed, for 99.99% predicted mortality, all but two species that were tested (A. novae-zelandiae and P. gigantea), required exposure to a temperature higher than 56 °C for 30 min.
Acknowledgements
The authors thank Jamie Agnew, Elizabeth Orton, Rita Tetenburg, Rebecca Ganley and Anna Hopkins for technical assistance. Suggestions from two anonymous referees strengthened the manuscript. This research was funded by the New Zealand Ministry of Agriculture and Forestry.
Notes
†Scion is the trading name of the New Zealand Forest Research Institute Ltd.
References
- Allen , E.A. and Humble , L.M. 2002 . Nonindigenous species introductions: a threat to Canada's forests and forest economy . Can. J. Plant Pathol. , 24 : 103 – 110 .
- Brockerhoff , E.G. , Bain , J. , Kimberley , M. and Knížek , M. 2006 . Interception frequency of exotic bark and ambrosia beetles (Coleoptera: Scolytinae) and relationship with establishment in New Zealand and worldwide . Can. J. For. Res. , 36 : 289 – 298 .
- Chidester , M.S. 1937 . Temperatures necessary to kill fungi in wood . Proc. Am. Wood Preservers' Assoc. , 33 : 316 – 327 .
- Chidester , M.S. 1939 . Further studies on temperatures necessary to kill fungi in wood . Proc. Amer. Wood Preservers’ Assoc. , 35 : 319 – 324 .
- FAO . 2007 . International Standards for Phytosanitary Measures, ISPM No. 15, Guidelines for regulating wood packaging material in international trade (2002), updated 2006. International Standards for Phytosanitary Measures 1 to 29 , 197 – 205 . Rome : Produced by the Secretariat of the International Plant Protection Convention, Food and Agriculture Organisation of the United Nations .
- Gallo , L. , Siverio , F. and Rodriquez-Pérez , A.M. 2008 . Thermal sensitivity of Phytophthora cinnamomi and long-term effectiveness of soil solarisation to control avocado root rot . Ann. App. Biol. , 150 : 65 – 73 .
- Haack , R.A. 2006 . Exotic bark- and wood-boring Coleoptera in the United States: recent establishments and interceptions . Can. J. For. Res. , 36 : 269 – 288 .
- Haack , R.A. , Hérard , F. , Sun , J. and Turgeon , J.J. 2010 . Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: a worldwide perspective . Annu. Rev. Entomol. , 55 : 521 – 546 .
- Jones , T.W. 1973 . Killing the oak wilt fungus in logs . Forest Prod. J , 23 ( 11 ) : 52 – 54 .
- MAF (2003). Import Health Standard, Sawn Wood from All Countries, Issued 16 April 2003. http://www.biosecurity.govt.nz/imports/ forests/standards/non-viable-forest-produce/sawn-wood.htm. (http://www.biosecurity.govt.nz/imports/ forests/standards/non-viable-forest-produce/sawn-wood.htm.)
- MAF (2006). Import Health Standard, Wood Packaging Material from All Countries, Issued 1 May 2006. http://www.biosecurity. govt.nz/files/imports/forests/standards/non-viable-forest-produce/wood-packaging-ihs.pdf. (http://www.biosecurity. govt.nz/files/imports/forests/standards/non-viable-forest-produce/wood-packaging-ihs.pdf.)
- Newbill , M.A. and Morrell , J.J. 1991 . Effect of elevated temperatures on survival of Basidiomycetes that colonize untreated Douglas-fir poles . Forest Prod. J. , 41 ( 6 ) : 31 – 33 .
- R Development Core Team (2009). R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. ISBN 3-900051-07-0 http://www.R-project.org. (http://www.R-project.org.)
- Smith , R.S. 1992 . “ Eradication of pinewood nematodes in softwood lumber ” . In Proceedings of the 13th Annual Meeting of the Canadian Wood Preservation Association 185 – 206 . Toronto, ON
- Stalpers , J.A. 1978 . Identification of wood inhabiting Aphyllophorales in pure culture . Stud. Mycol. , 16 : 1 – 248 .
- Uzunovic, A., Byrne, T., Allen, E., Morrell, J., Ormsby, M., & Britton, K. (2006). Time-temperature schedules to kill wood inhabiting fungi: Proposed test method. http://www.forestry-quarantine.org/Sept08/2006_Uzunovic-HT-protocol.pdf. (http://www.forestry-quarantine.org/Sept08/2006_Uzunovic-HT-protocol.pdf.)