Abstract
The reaction of plum trees ‘Jojo’ on St. Julien rootstocks, was monitored under field conditions for symptom development following graft inoculation with an isolate of Plum pox virus strain D (PPV-D) that is common and prevalent in the Czech Republic. PPV-D inoculation in 2003 did not cause death, but the virus was detected in some ‘Jojo’ tissues. One, ‘Jojo’ tree died in 2005, and PPV symptoms appeared later in the leaves of the St. Julien rootstock. The presence of PPV-D in leaves of St. Julien rootstock was confirmed both by DAS-ELISA and RT-PCR. The virus was shown to be able to move through the vascular system of ‘Jojo’. Two out of four inoculated ‘Jojo’ trees showed symptoms of PPV-D infection that appeared as necrotic zones on the ‘Jojo’ trunks. The presence of PPV-D was confirmed both by DAS-ELISA and RT-PCR in the green bark close to the necrotic areas in 2007–2009. The growth of PPV-D infected trees was considerably slower than the growth of healthy control trees. Trees of ‘Jojo’ infected with PPV-D began to die out seven years after virus inoculation.
Résumé
La réaction des pruniers ‘Jojo’ sur porte-greffes Saint-Julien à l'inoculation de la greffe avec un isolat de la souche D du virus de la sharka (PPV-D), souche courante en République tchèque, a été suivie dans des conditions naturelles pour étudier l'apparition des symptômes. En 2003, les inoculations avec le PPV-D n'étaient pas létales, mais le virus était détecté dans quelques tissus de ‘Jojo’. En 2005, un prunier ‘Jojo’ est mort et, par la suite, les symptômes sont apparus sur les feuilles du porte-greffe Saint-Julien. L'occurrence du PPV-D dans les feuilles du porte-greffe a été confirmée par DAS-ELISA et RT-PCR. Le virus s'est avéré capable de se mouvoir dans le système vasculaire du cultivar. Deux arbres inoculés sur quatre ont affiché des symptômes d'infection au PPV-D sous forme de zones nécrotiques apparaissant sur les troncs. En 2007 et 2009, les tests DAS-ELISA et RT-PCR ont confirmé l'occurrence du PPV-D dans de l'écorce fraîche avoisinant les zones nécrotiques. La croissance des arbres infectés était beaucoup plus lente que celle des arbres témoins en santé. Les pruniers ‘Jojo’ infectés par le PPV-D ont commencé à mourir sept ans après l'inoculation.
Introduction
Plum pox virus (PPV) is the causal agent of sharka, a viral disease that affects Prunus fruits, including plum, peach, nectarine, cherry and apricot. The disease was first reported in Bulgaria (Eastern Europe) in 1917 and has progressively spread to many other countries, including the USA in 1999, and in Canada in 2000. PPV is the most serious viral disease of Prunus fruits and causes significant damage to Prunus fruit trees.
Breeding programmes to develop resistance to PPV in Prunus domestica L. have been well established, especially in Bulgaria, the former Yugoslavia, and Germany during the last 50 years as summarized by Kegler et al. (Citation1998). After the Second World War, plum cultivars grown in Bulgaria, including ‘Anna Späth’, ‘Bühler Frühzwetsche’ and ‘Montfort’ were reported to be resistant to PPV (Christoff, Citation1947). The most extensive programme of breeding for resistance in plum was developed in Cacak, Yugoslavia in the 1970s and 1980s. This programme resulted in new cultivars e.g. ‘Cacanska Lepotica’, ‘Cacanska Najbolja’, ‘Cacanska Rodna’ and ‘Valjevka’ (Rankoviċ, Citation1986). However, the fruit quality of all plum cultivars resistant to PPV was lower than the PPV susceptible Prunus domestica cvs. such as Bulgarian cv. ‘Požegača’, Romanian cv.‘Bystrická’, Czech cv. ‘Domácí švestka’ or German cv. ‘Deutsche Hauszwetsche’. A plum breeding programme at Hohenheim, Germany during the 1990s was designed to obtain new sharka-resistant cultivars with better fruit quality using the results of qualitative resistance based on hypersensitivity. The result of the study was a hybrid hypersensitive to PPV derived from crossing ‘Ortenauer’ × ‘Stanley 13’ which was registered and patented as plum cv. ‘Jojo’ (Hartmann, Citation1998). This cultivar has been grown from the year 2000 also in the Czech Republic.
PPV is endemic in the Czech Republic (Polak, Citation2002). Plums and myrobalans grown in the wild and showing systemic PPV symptoms are natural sources of infection. Most of the isolates of PPV present in natural sources were classified as PPV-D isolates (Polak & Kominek, Citation2009). Growing the hypersensitive ‘Jojo’ could decrease problems caused by PPV infected trees in the Czech Republic. However, hypersensitivity is extreme susceptibility (Loebenstein & Carr, Citation2005; Polak, Citation2008), and not absolute resistance as proposed by Hartmann (Citation2004). Hypersensitivity can be disrupted by mildly pathogenic strains of a virus (Goodman & Novacky, Citation1996), such as PPV-D. To test this hypothesis, we monitored the reaction of plum trees ‘Jojo’ to three strains of PPV, namely PPV-M, PPV-Rec and PPV-D, present and isolated from prune in the Czech Republic (Polak et al., Citation2005). PPV-M is highly pathogenic, PPV-Rec moderately pathogenic and PPV-D a mildly pathogenic strain. A strong hypersensitive reaction appeared a year after the inoculation with highly pathogenic PPV-M, and the moderately pathogenic PPV-Rec strain. All of the graft inoculated trees died. Inoculation with PPV-D strain did not result in death of the four ‘Jojo’ trees. No trees inoculated by PPV-D died during the first two years (2003–2004) of evaluation although a few side shoots did die (Polak et al., Citation2005). Responses of the ‘Jojo’ PPV-D inoculated trees during the following six years are presented in this contribution.
Materials and methods
Plant material and symptom observations
Trees were grown under natural conditions. Two-year-old trees of virus-free plum ‘Jojo’ grafted onto virus-free ‘St. Julien’ rootstock were planted in the field in October 2002. In April 2003, four trees were inoculated by chip budding with grafts from plum trees infected with PPV-D strain isolated from plum in the Czech Republic. Different PPV strains including the PPV–D strain are maintained in the collections of plant viruses, National Programme of Ministry of Agriculture, in the Department of Virology, Crop Research Institute, Prague. Two infectious buds were used per tree. Four non-grafted trees were used as control. Growth of the infectious buds was checked in the same year. At least one bud was growing on every inoculated tree. Evaluation took place in the following years (2004–2009). Visual evaluation of trees was carried out monthly from May to November.
Serological testing
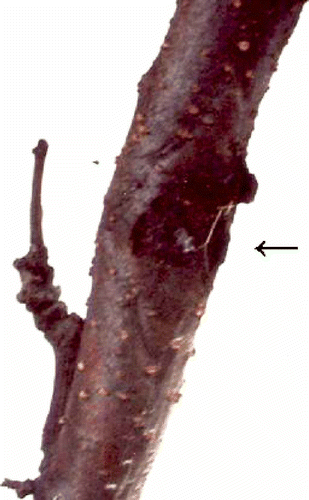
Samples prepared from leaves of plum ‘Jojo’ trees were evaluated serologically in the second half of June every year. Four leaves were sampled from the bottom of four different shoots. Screening for other viruses (Prunus necrotic rinspot virus (PNRSV), Prune dwarf virus (PDV), Apple chlorotic leafspot virus (ACLSV), Apple mosaic virus (ApMV)) was done in 2009 with negative results. Samples prepared from the green bark of the trunk close to the necrotic zones () were evaluated in the second half of June every year, and in the middle of November in 2007–2009. PPV polyclonal antibodies from Bioreba, Switzerland were used in DAS-ELISA (Clark & Adams, Citation1977) to detect PPV in leaves and green bark tissues of plum ‘Jojo’.
Samples for DAS-ELISA were prepared by grinding 0.2 g of leaf or green bark tissues in phosphate buffered saline, pH 7.4 with 2% polyvinylpyrrolidone and 0.2% of egg albumin (1:20) (Polak & Kominek, Citation2009). Microplates were rated by using a MR 5000 Dynatech reader (Dynatech Laboratories, Chantilly, VA, USA) at 405 nm. Samples with an OD405 higher than 0.10 (standard deviations) were considered positive, whereas samples with an OD405 lower than 0.03 were negative. The OD405 of the four healthy controls were 0.03 or less.
RT–PCR evaluation
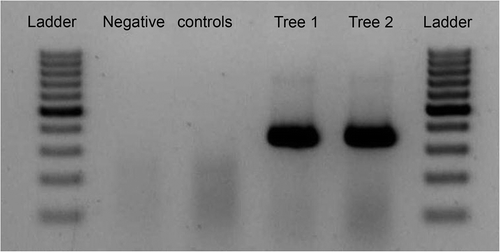
Total RNA was isolated from green bark and leaf tissue from all of the plants using a commercially available extraction kit (Spectrum™ Plant Total RNA Kit) (Sigma-Aldrich) according to the manufacturer's instructions. Concentration and purity of isolated RNA was determined by measuring absorbance at 260, 280 and 230 nm (GeneQuantPro, Biochrom, UK). The concentration varied from 500 to 1000 ng μL−1. One-step RT-PCR was performed with the one-step RT-PCR kit (Qiagen). Upstream and downstream primers () were prepared in microtubes and 1 μL of RNA was added. The reaction was carried out in a thermocycler (BioRad). After the last cycle, a final extension step at 72 °C for 10 min was added. For electrophoretic analysis, PCR products (10 μL) were separated on a 2% agarose gel in TAE buffer, at 80 V for 60 min, with SYBR Green Dye staining. In cases of a positive finding, the reaction resulted in a 345 bp DNA amplicon (see ).
Table 1. Primers used for RT-PCR detection of Plum pox virus
Results
The reaction of plum trees ‘Jojo’ on St. Julien rootstocks, grown under field conditions, was monitored following graft inoculation with the PPV-D strain in 2003. Inoculated trees were evaluated in the following six years. One ‘Jojo’ tree died in 2005. The St. Julien rootstock of this tree continued to grow and manifested strong PPV symptoms. Prior to the death of the ‘Jojo’ scion, no root suckers were evident on any of the ‘Jojo’ trees. The presence of PPV in suckers of rootstock leaves was proven both by ELISA and RT-PCR, showing that PPV-D must have been transmitted from the infectious graft via tissues of ‘Jojo’ into the St. Julien rootstock, which is susceptible to PPV. PPV-infected suckers of rootstock can serve as a source of inoculum for aphid transmission.
Some branches or short parts of branches of other ‘Jojo’ trees wilted and died in the first year after the PPV-D inoculation. Recovery of the other three trees started in the second year and continued into the third year after the virus inoculation. Damaged branches began to grow and no wilting of top of branches appeared. No systemic PPV symptoms appeared in leaves of two (1, 2) ‘Jojo’ trees, and no PPV was detectable by ELISA or RT-PCR in the leaves of two recovered trees. Necrotic spots and zones () appeared on trunks of two recovered trees after the fourth year (2007) following the PPV-D inoculation. Necrotic spots were situated on the main trunk, but not in the location of PPV inoculation. The presence of PPV-D was tested in leaves and in green bark close to the necrotic zones by ELISA and RT-PCR every year in June and in green bark also in November during leaf fall. The green bark of the trunk could be tested only twice a year. The absence of PPV was proven by ELISA and RT-PCR in leaves and in green bark of the trunk close to the necrotic zones in June. Surprisingly, the presence of PPV was proven by ELISA and confirmed by RT-PCR in green bark tissue adjacent to the necrotic zones in November during all three years of testing (). PPV-D was thus confirmed to be present in plant tissue of plum ‘Jojo’. Tree no. 2 died in the vegetative season of 2010.
Table 2. Results of ELISA and RT-PCR detection of PPV in green bark of trunk of plum trees ‘Jojo’ infected with PPV-D
Control trees of plum ‘Jojo’ not inoculated with PPV-D showed no symptoms in all seven years of evaluation and the growth was several times greater compared with the PPV infected trees (). Positive and negative controls were used in ELISA and RT-PCR tests. Leaf samples of suckers from the rootstock St. Julien A infected with PPV-D and showing symptoms (Jojo tree died second year after the virus inoculation) served as a positive control. Results of ELISA and RT-PCR detection of PPV were positive in all the three years of testing of these tissues. Samples of leaves of the healthy control ‘Jojo’ trees and leaves of its St. Julien rootstock served as a negative control and tested negative by ELISA and RT-PCR in all the years of testing.
Discussion
The results of early responses of plum trees ‘Jojo’ to inoculation with the different PPV strains were summarized in Polak et al. (Citation2005). Briefly, all the PPV-M and PPV-Rec inoculated ‘Jojo’ trees died in 2004, whereas no PPV-D inoculated trees died and the all four trees exhibited stunting and some branches partially died during 2004.
Our six-year results (2004–2009) of evaluation of ‘Jojo’ trees infected with PPV-D support the hypothesis that the hypersensitivity of plants to a virus can be disrupted by mildly pathogenic strains of a pathogen. Differences in response of ‘Jojo’ trees inoculated with the mild PPV-D isolate were most probably caused by different quantity of virus in infected buds used for virus inoculation. A high quantity of virus may have caused the death of one ‘Jojo’ tree after two years. This indicates that infection with low pathogenic strain of virus can, in some cases, result in severe systemic infection. Hypersensitivity can be understood as extreme susceptibility, and not the absolute resistance, as was stated by Hartmann (Citation2004). Results of serological and molecular PPV detection in leaves of St. Julien rootstock and in green bark of the trunk close to the necrotic plates on trunks of plum trees ‘Jojo’ with PPV-D provided evidence that: (a) PPV can be transported from the infectious graft via vascular tissues of ‘Jojo’ into the St. Julien rootstock; (b) PPV-D is present in green bark of ‘Jojo’ tree trunks; and (c) necrotic zones on ‘Jojo’ tree trunks are symptomatic of PPV-D infection.
The ability of PPV virus particles to move through the vessel system (phloem and maybe xylem) of hypersensitive genotypes has been reported by Neumüller et al. (Citation2010). However, the virus has not been detectable at any time in the tree tissues until now. The plum cultivar ‘Jojo’ has been grown in regions where the distribution of sharka disease is endemic in the Czech Republic. Most of the PPV isolates identified in the Czech Republic have been classified as mildly pathogenic PPV-D isolates (Polak & Kominek, Citation2009). Plum cultivars highly susceptible to PPV such as ‘Domaci svestka’ respond to Czech Republic PPV-D isolates with severe systemic mosaic symptoms in leaves and malformations of fruits. Plum cultivars with some level of resistance to PPV such as ‘Stanley’ or ‘Cacanska rodna’ respond to PPV-D strain with mild mosaic symptoms. The plum ‘Jojo’ is of a potential risk for all the areas where only the PPV-D strain is present, as in Canada (James & Upton, Citation2001), or the USA (Damsteegt et al., Citation2001), or where PPV-D is highly prevalent as in central Europe, the Czech Republic, Austria and Poland (Polak & Kominek, Citation2009). Hypovirulent PPV-D strains may be able to evoke systemic infection. The use of plum varieties that are hypersensitive to PPV may only be a provisional solution to the problem of growing plums in the presence of PPV. Developing genetically modified plums (e.g. ‘HoneySweet’), and/or breeding of plum varieties truly resistant to PPV is the long-term solution not only in the Czech Republic, but in most of the European countries and other areas where sharka disease remains a serious problem.
Acknowledgements
The authors thank Prof. Vaclav Kudela for valuable advice, Jiři Svoboda for grafting of ‘Jojo’ trees, and Mrs Jitka Pívalová for excellent technical assistance. The authors are also deeply grateful to Rebecca Pace, Entomology and Plant Pathology Department, Oklahoma State University, Stillwater, for proofreading and correcting the article. This study was supported by the Ministry of Agriculture of the Czech Republic, Project No. MZe 0002700604.
References
- Christoff , A. 1947 . Sharka disease of plum. Bulletin de la Chambre de culture Nationale de Sofia . Série de Biologique , 1 : 261 – 296 . (in Bulgarian)
- Clark , M.F. and Adams , A. 1977 . Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses . J. Gen. Virol. , 34 : 851 – 857 .
- Damsteegt , V.D. , Stone , A.L. , Luster , D.G. , Levy , L. , Gildow , F.E. and Welliver , R. 2001 . Preliminary characterization of a North American isolate of Plum pox virus from naturally infected peach and plum orchards in Pennsylvania, USA . Acta Hortic. , 550 : 145 – 152 .
- Goodman , R. N. and Novacky , A. J. 1996 . “ The hypersensitive reaction in plants to pathogens, a resistance phenomenon ” . In St. Paul , 244 MN : APS Press .
- Hartmann , W. 1998 . Breeding of plums and prunes resistant to . Plum pox virus. Acta Virol. , 42 : 230 – 232 .
- Hartmann , W. 2004 . New results from plum breeding in Hohenheim. VIII international symposium on plum and prune genetics, breeding and pomology . Acta Hortic. , 734 : 187 – 192 .
- James , D. and Upton , C. 2001 . Molecular characterization and comparison of the 3′-terminal region of two Canadian isolates of plum pox virus . Phytopathology , 91 : S43 (Abstr.)
- Kegler , H. , Fuchs , E. , Gruntzig , M. and Schwarz , S. 1998 . Some results of 50 years of research on the resistance to Plum pox virus . Acta Virol. , 42 : 200 – 215 .
- Loebenstein , G. and Carr , J.P. 2005 . Natural resistance mechanisms of plants to viruses . Acta Phytopathol. Entomol. Hung. , 40 : 199 – 204 .
- Neumüller , M. , Hartmann , W. , Petruschke , M. and Treutter , D. 2010 . The hypersensitivity resistance of European plum to the Plum pox virus and its potential impact on the epidemiology of the virus . Julius-Kühn-Archiv. , 427 : 147 – 150 .
- Polak , J. 2002 . Distribution of Plum pox virus in the Czech Republic . Plant Protect. Sci. , 38 : 98 – 102 .
- Polak , J. 2008 . Characterisation of different interactions between cultivars of stone fruits and Plum pox virus. Proceedings of the Twentieth International Symposium on Virus and Virus-Like Diseases of Temperate Fruit Crops - Fruit Tree Diseases . Acta Hortic. , 781 : 287 – 293 .
- Polak , J. and Kominek , P. 2009 . Distribution of Plum pox virus strains in natural sources in the Czech Republic . Plant Protect. Sci. , 45 : 144 – 147 .
- Polak , J. , Pivalova , J. and Svoboda , J. 2005 . Prune cv. Jojo resistance to different strains of Plum pox virus . Plant Protect. Sci. , 41 : 47 – 51 .
- Rankovic , M. 1986 . Ispitivanje otpornosti nekih novijih sorti Sljiva prema virusu Sarke . Jugosl.Vocar. , 20 : 601 – 606 .
- Varga , A. and James , D. 2005 . Detection and differentiation of Plum pox virus using real-time multiplex PCR with SYBR Green and melting curve analysis, a rapid method for strain typing . J. Virol. Meth. , 123 : 213 – 220 .
- Varga , A. and James , D. 2006 . Use of reverse transcription loop-mediated isothermal amplification for the detection of Plum pox virus . J. Virol. Meth. , 138 : 184 – 190 .