Abstract
The soilborne necrotrophic fungal pathogen Macrophomina phaseolina (Tassi) Goid causes charcoal rot disease in many economically important crops. There are no effective control methods for this disease and no completely disease-resistant cultivar has been identified in crop species against M. phaseolina, including alfalfa, soybean and sorghum. In our previous study, we have established a pathosystem using the model legume Medicago truncatula to study the molecular interactions between the pathogen and its host. In this study, we investigated the possible variability in host response to M. phaseolina infection among different Medicago ecotypes. We found that disease progression in different ecotypes had little variation, and no ecotype was identified that showed resistance. We have previously found that methyl jasmonate (MJ) and ethylene (ET) could induce partial resistance in Medicago ecotype A17 against M. phaseolina. Here, we compared the disease progression after MJ or ET treatment between A17 and R108, another commonly used ecotype. Unlike A17, our results showed that R108 plants treated with MJ and ET did not show any induced resistance to M. phaseolina. The possible molecular mechanisms that led to these variations are further discussed.
Résumé
L'agent pathogène fongique nécrotrophique terricole Macrophomina phaseolina (Tassi) Goidanich cause la pourriture noire chez de nombreuses plantes agricoles de grande importance économique. Il n'existe pas de méthode de lutte efficace contre cette maladie, pas plus qu'il y a de cultivars de variétés agricoles entièrement résistants à M. phaseolina, y compris chez la luzerne, le soja et le sorgho. Lors de notre étude précédente, afin d'examiner les interactions moléculaires entre l'agent pathogène et son hôte, nous avons établi un pathosystème basé sur la légumineuse Medicago trunculata. Dans celle-ci, nous avons examiné la variabilité possible de la réponse de l'hôte à l'infection par M. phaseolina chez différents écotypes de Medicago. Nous avons trouvé que, chez les différents écotypes, la progression de la maladie affichait peu de variations et aucun écotype n'affichait de résistance. Nous avions déjà constaté que le jasmonate de méthyle (JM) et l'éthylène (ET) pouvaient induire une résistance partielle chez l'écotype A17 de Medicago infecté par M. phaseolina. Ici, nous avons comparé la progression de la maladie après traitement au MJ ou à l'ET chez A17 et R108, un autre écotype utilisé fréquemment. Contrairement à A17, nos résultats montrent que les plants de R108 traités avec MJ et ET n'ont pas affiché de résistance induite à M. phaseolina. Les mécanismes moléculaires probables qui ont mené à ces variations sont discutés plus en détail.
Introduction
Charcoal rot is a plant disease caused by the soil fungus Macrophomina phaseolina (Tassi) Goid. This fungus has a wide range of plant hosts, readily invading over 500 different monocotyledonous and dicotyledonous species, including various important crops such as soybean, sorghum, maize and alfalfa (Wyllie, Citation1989). Plants infected with M. phaseolina develop symptoms ranging from leaf yellowing, wilting to plant death, and the severity of the disease usually increases in hot and dry conditions (Smith & Wyllie, Citation1999). Charcoal rot can result in severe losses due to reduced yield and low seed quality. For instance, the US soybean yield reduction due to charcoal rot was estimated to be around 1.9 million tons in 2003 (Wrather & Koenning, Citation2006). The loss could be more severe in years with hot and dry summers which could become more frequent as a consequence of global warming. Charcoal rot has a wide geographical distribution, and the disease has been reported in southern and north-central regions of the United States (Mihail et al., Citation1990; Bradley & del Rio, Citation2003; Yang & Navi, Citation2005), and in tropical and subtropical regions of the world (Karadimos et al., Citation2002; Gaetan et al., Citation2006; Khangura & Aberra, Citation2009; Mahmoud & Budak, Citation2011).
M. phaseolina exists in soil as compact masses of hardened mycelium called sclerotia, which can remain dormant for many years. Under appropriate conditions, hyphae germinated from the sclerotia infect the roots of plant hosts (Ammon et al., Citation1974). After decay of infected plants, sclerotia are released into the soil and the infection cycle can start again in the following years. The pathogen can also be transmitted through infected seeds (Abawi & Pastorcorrales, Citation1990). The common management strategies that are currently applied to control the disease include crop rotation (Mueller et al., Citation1985; Francl et al., Citation1988), adjusting planting dates (Todd, Citation1993), irrigation (Wyllie, Citation1989), and cover crops (Rothrock et al., Citation1995). Other management approaches including the application of fungicides, plant-derived natural products as well as biological control using competing soil microbes have also been investigated (Ghaffar et al., Citation1969; Abawi & Pastorcorrales, Citation1990; Siddiqui & Mahmood, Citation1993; Brooker et al., Citation2000; Haas & Défago, Citation2005). However, none of these approaches are very effective. Host resistance may be the only feasible approach to manage the disease; however, the search for naturally existing resistant cultivars has not yielded success (Smith & Carvil, Citation1997). Moreover, genetic engineering of resistant plants is not feasible currently due to the lack of knowledge on the molecular processes that occur during M. phaseolina–host interactions.
In order to better understand the host responses to M. phaseolina infection at the molecular level, we have established a model pathosystem for charcoal rot using Medicago truncatula (M. truncatula) in our previous study (Gaige et al., Citation2010). M. truncatula is the primary model legume species for genomic and functional genomic research because of its relatively small genome size and available genomic resources (Thoquet et al., Citation2002; Choi et al., Citation2004). Our inoculation protocol was established using the commonly used M. truncatula ecotype Jemalong A17. This ecotype was used in the Medicago genome sequence project to generate the reference genome (Town, Citation2006). Plants of this ecotype infected with M. phaseolina developed disease symptoms such as wilting and leaf yellowing at 1 day-post-inoculation (dpi), and most of the plants died by 4 dpi. Roots from infected plants showed browning and necrotic lesions (Gaige et al., Citation2010). The disease phenotypes were consistent with what has been observed in the field (Wyllie, Citation1989). Using this ecotype, we have found that A17 plants treated with methyl jasmonate (MJ) and/or ethylene (ET) gained partial resistance to M. phaseolina (Gaige et al., Citation2010). Jasmonic acid (JA) and ET are two plant hormones that are involved in both plant development and defence responses, especially against several necrotrophic pathogens in different plant species (McDowell & Dangl, Citation2000; Glazebrook, Citation2001). For example, Arabidopsis mutants that have defects in JA biosynthesis or signalling were more susceptible to Pythium mastophorum Drechsler, a soil-borne pathogen causing root rot disease (Vijayan et al., Citation1998) and to the necrotrophic fungus Botryotinia cinerea (De Bary) Whetzel (Thomma et al., Citation1998). Arabidopsis plants carrying mutations in EIN2, the key ethylene signalling gene, were more susceptible to B. cinerea (Thomma et al., Citation1999). Similarly, Medicago sickle mutants that are defective in EIN2 ortholog were less tolerant to the necrotrophic pathogens Rhizoctonia solani J.G. Kühn and Phytophthora medicaginis E.M. Hansen & D.P. Maxwell (Penmetsa et al., Citation2008).
To explore naturally existing genetic variations in M. truncatula against M. phaseolina, we first examined the severity of disease symptoms in 323 different M. truncatula ecotypes to evaluate the possible host resistance in this species. These ecotypes include 322 accessions obtained from the USDA National Plant Germplasm System (NPGS) collection and one commonly used ecotype R108. The NPGS collection includes 322 ecotypes collected from different areas around the globe (http://www.ars-grin.gov/cgi-bin/npgs/html/tax_acc.pl) (Bauchan & Greene, Citation2002). Since R108 has been frequently used in genetic studies and it is distinct from A17, we decided to test if the genetic variations existing in these ecotypes led to differences in JA and ET-induced resistance against M. phaseolina. Ecotypes A17 and R108 are phenotypically and genotypically distinct. A17 was originally isolated from Australia, whereas R108 was a derivative of ecotype 108-1 through in vitro regeneration (Hoffmann et al., Citation1997).
Materials and methods
Plant materials
Seeds for 322 M. truncatula accessions were obtained from the USDA National Plant Germplasm System (NPGS) collection (provided by Dr Stephanie Greene). Seeds for Jemalong A17 and R108 were obtained from the Samuel Roberts Nobel Foundation, Ardmore, OK (provided by Dr Srinivasa Rao Uppalapati). M. truncatula seeds were treated with concentrated sulphuric acid for 8 min, rinsed five times with distilled water, followed by surface sterilization with freshly prepared 20% bleach (Chlorox). After sterilization, the seeds were rinsed three times with sterilized distilled water and germinated on half-strength Murashige and Skoog (MS) salt (Murashige & Skoog, Citation1962) (Sigma-Aldrich, St. Louis, MO) with 1% agar at room temperature in the dark for 3 days. The seedlings were then transplanted to plastic pots filled with wet sterilized soil (Sun Gro Horticulture, Bellevue, WA) and grown in the growth chamber (27 °C, 12-h photoperiod, 44% relative humidity [RH], photon flux density 150–200 μmol m−2 s−1).
Fungal inoculum
M. phaseolina isolate and inoculum preparation were described previously (Gaige et al., Citation2010). Briefly, the isolate was cultured on potato dextrose agar (PDA) at 27 °C for 3 days. Then, agar pieces were cut out and transferred to potato dextrose broth (PDB). After incubating at 27 °C for 14 days, the fungal mat from PDB was collected using a sterile spatula and air-dried overnight at room temperature. The dried material containing mostly sclerotia was ground to fine powder and used to prepare the inoculum.
Inoculations
Four-week-old M. truncatula plants were removed from the pots, and rinsed with running tap water to remove potting mix from the roots. The roots then were submerged in M. phaseolina inoculum suspension (1 g of dried sclerotia in 10 mL 0.015% agarose) for 30 s, then re-potted into sterilized soil. Control plants were dipped in 0.015% agarose only (Gaige et al., Citation2010). The low percentage of agarose helped to evenly suspend the sclerotia and also kept the sclerotia from sticking onto plant roots. Treated plants were maintained as described above. Disease symptoms were monitored daily from 1 dpi to 7 dpi using digital imaging with a Nikon CoolPix digital camera under ambient light condition without flash. The disease symptoms were also assessed based on a scoring matrix on a scale of 0–6 (0 = no detectable symptom; 1 = 1–10% chlorotic or 1–5% necrotic; 2 = 10–20% chlorotic or 5–10% necrotic; 3 = 20–40% chlorotic or 10–20% necrotic; 4 = 40–60% chlorotic or 20–40% necrotic; 5 = 60–80% chlorotic or 40–60% necrotic; 6 = > 80% necrotic or dead plant). For the ecotype screen, A17 was used as a reference for every inoculation. For each accession, at least 3 plants were mock-treated, and at least 6 plants were treated with the fungal inoculum. Experiments were repeated for ecotypes that showed altered susceptibility.
Chemical preparation and application
Four-week-old plants were sprayed with either 0.1% MJ (Sigma-Aldrich, St. Louis, MO), or 3.0 mM ethephon (2-chloroethyl phosphonic acid) (Sigma-Aldrich), or the combination of both 3 days before inoculation. Ethephon is a direct ethylene source when applied to plants, and is much easier to handle than ethylene gas (Cooke & Randall, Citation1968; Khan, Citation2004). MJ solution was prepared fresh by diluting the stock solution in water containing 0.01% of Tween-20. Ethephon was freshly prepared by dissolving in water containing 0.01% Tween-20 to a final concentration of 3.0 mM. Mock treatment was water with 0.01% Tween-20. Plants sprayed with the chemicals were covered with a plastic dome and kept in a separate area from untreated plants. For large-scale experiments, 120 4-week-old plants were divided into four groups; each group was treated with either mock control, MJ, ET or MJ plus ET 3 days prior to inoculation. The experiments were performed twice.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA). The statistical significance of ecotype variations in hormone responses was based on two-way ANOVA with P < 0.05 or P< 0.01.
Results and discussion
Using our established model pathosystem for charcoal rot, we plan to identify genetic factors that result in changes in host susceptibility to M. phaseolina using a forward genetics approach. With this in mind, we first conducted the screen with the available 323 accessions to see if there were any naturally existing variations in M. truncatula against M. phaseolina. Any accession that showed different disease symptoms when compared with A17 (either died more quickly or showed partial resistance) was screened again to confirm the results. After completing the screen for 320 accessions (PI-577600, PI-577602 and PI-577608 could not be screened due to low germination rates), we did not observe any major differences in disease progression among different ecotypes (data not shown). Compared with A17 plants, all other ecotypes developed disease symptoms at a similar pace. The inoculated plants started to show leaf yellowing and wilting at 1 day-post-inoculation (dpi). The symptoms became more severe at 2 dpi and 3 dpi, and the plants died at 4 dpi. The roots of the dead plants were completely colonized by M. phaseolina sclerotia (data not shown).
This result is not surprising, considering that previous efforts to identify naturally existing host resistance to M. phaseolina have not been successful (Smith & Wyllie, Citation1999).
In our previous study, we found that the expression of genes in several disease response pathways was affected by M. phaseolina infection, and treating A17 plants with methyl jasmonate (MJ) and/or ethylene (ET) prior to inoculation induced partial resistance against M. phaseolina. Here, we tested the effects of these two hormones on R108 plants. Ecotype A17 plants treated with MJ or ET developed disease symptoms more slowly, and the infected plants survived up to 7 dpi. In addition, A17 plants treated with both hormones showed an increase in resistance compared with the ones treated with either one of the hormones alone. To our surprise, R108 plants did not show a similar response to the hormone treatments. Ecotype R108 plants treated with MJ or ET developed disease symptoms similar to the untreated R108 plants. To test whether the different responses we observed between A17 and R108 were statistically significant, we increased the number of plants in each treatment group and performed the large-scale experiment twice. After hormone treatment and inoculation, the disease symptoms were scored daily for each plant based on a scale of 0 to 6. The numbers of plants with severe symptoms (scored 5 and 6) at each time point were compared between treatment groups (). The impact of hormone treatments on disease progression was significantly different between A17 and R108 from 3 dpi and later (). Mock-treated, infected A17 and R108 plants all died around 4 to 5 dpi, and the same was observed for R108 plants treated with either MJ or ET alone or with both hormones. However, disease progression in A17 plants treated with MJ or ET was slower. A17 plants treated with both hormones showed more resistance to the pathogen, and the difference in disease progression was significant compared with plants treated either MJ or ET alone ( and ).
Fig. 1. Effect of MJ and ET on disease caused by M. phaseolina on Medicago ecotypes A17 and R108. Each treatment was done on 30 plants, and the experiment was performed twice. The percentage of plants that developed severe symptoms (scored 5–6) after M. phaseolina infection in each treatment group was compared at (A) 3 dpi, (B) 4 dpi and (C) 5 dpi. The data were analyzed using two-way ANOVA. * and ** refer to significant differences at P < 0.05 and 0.01, respectively.
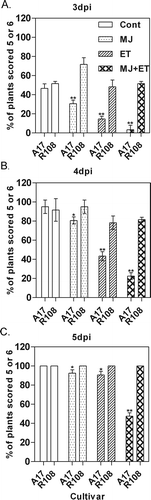
The finding that R108 did not show an induced level of resistance by MJ and ET was intriguing. Ecotypes A17 and R108 have genetic variations that can result in phenotypic differences ranging from salt stress responses to symbiotic interactions with rhizobia (Salzer et al., Citation2004; de Lorenzo et al., Citation2007). It was reported that class IV chitinase gene was inducible by symbiotic rhizobia in A17, but not in R108 (Salzer et al., Citation2004). Several chitinases are JA or ET-inducible in Arabidopsis (van Loon et al., Citation2006). Although the inducible response of chitinases to JA and ET is not well characterized in M. truncatula, it is possible that these genes could be involved in the differences in MJ- and ET-induced partial resistance between A17 and R108. We are also exploring other possible genetic variations between these two ecotypes that could lead to the different hormonal responses.
Acknowledgements
This study was supported in part by a grant to BS from the Kansas Soybean Commission. We thank Dr Srinivasa Rao Uppalapati at the Samuel Roberts Noble Foundation (Ardmore, OK, USA) and Dr Stephanie Greene at the USDA (USDA, Washington State University, Pullman, WA) for providing plant materials for our study.
References
- Abawi , G.S. and Pastorcorrales , M.A. 1990 . Seed transmission and effect of fungicide seed treatments against Macrophomina phaseolina in dry edible beans . Turrialba , 40 : 334 – 339 .
- Ammon , V. , Wyllie , T.D. and Brown , M.F. 1974 . An ultrastructural investigation of pathological alterations induced by Macrophomina phaseolina (Tassi) Goid. in seedlings of soybean, Glycine max (L.) Merril . Physiol. Plant Pathol. , 4 : 1 – 4 .
- Bauchan , G.R. and Greene , S.L. 2002 . Status of the Medicago germplasm collection in the United States . Plant Genet. Resourc. Newsl. , 129 : 1 – 8 .
- Bradley , C.A. and Del Rio , L.E. 2003 . First report of charcoal rot on soybean caused by Macrophomina phaseolina in North Dakota . Plant Dis. , 87 : 601
- Brooker , N.L. , Long , J.H. and Stephan , S.M. 2000 . Field assessment of plant derivative compounds for managing fungal soybean diseases . Biochem. Soc. Trans. , 28 : 917 – 920 .
- Choi , H.K. , Kim , D. , Uhm , T. , Limpens , E. , Lim , H. Mun , J.H. 2004 . A sequence-based genetic map of Medicago truncatula and comparison of marker colinearity with . M. sativa. Genetics , 166 : 1463 – 1502 .
- Cooke , A.R. and Randall , D.I. 1968 . 2-Haloethanephosphonic acids as ethylene releasing agents for the induction of flowering in pineapples . Nature , 218 : 974 – 975 .
- De Lorenzo , L. , Merchan , F. , Blanchet , S. , Megías , M. , Frugier , F. Crespi , M. 2007 . Differential expression of the TFIIIA regulatory pathway in response to salt stress between Medicago truncatula genotypes . Plant Physiol. , 145 : 1521 – 1532 .
- Francl , L.J. , Wyllie , T.D. and Rosenbrock , S.M. 1988 . Influence of crop rotation on population density of Macrophomina phaseolina in soil infested with Heterodera glycines . Plant Dis. , 72 : 760 – 764 .
- Gaetan , S.A. , Fernandez , L. , Madia , M. and De Fitopatologia , C. 2006 . Occurrence of charcoal rot caused by Macrophomina phaseolina on canola in Argentina . Plant Dis. , 90 : 524
- Gaige , A.R. , Ayella , A. and Shuai , B. 2010 . Methyl jasmonate and ethylene induce partial resistance in Medicago truncatula against the charcoal rot pathogen Macrophomina phaseolina . Physiol. Mol. Plant Pathol. , 74 : 412 – 418 .
- Ghaffar , A. , Zentmyer , G.A. and Erwin , D.C. 1969 . Effect of organic amendments on severity of Macrophomina phaseolina root rot of cotton . Phytopathology , 59 : 1267 – 1269 .
- Glazebrook , J. 2001 . Genes controlling expression of defense responses in Arabidopsis – 2001 status . Curr. Opin. Plant Biol. , 4 : 301 – 308 .
- Haas , D. and Défago , G. 2005 . Biological control of soil-borne pathogens by fluorescent pseudomonads . Nat. Rev. Microbiol. , 3 : 307 – 319 .
- Hoffmann , B. , Trinh , T.H. , Leung , J. , Kondorosi , A. and Kondorosi , E. 1997 . A new Medicago truncatula line with superior in vitro regeneration, transformation, and symbiotic properties isolated through cell culture selection . Mol. Plant-Microbe Interact. , 10 : 307 – 315 .
- Karadimos , D.A. , Karaoglanidis , G.S. and Klonari , K. 2002 . First report of charcoal rot of sugar beet caused by Macrophomina phaseolina in Greece . Plant Dis. , 86 : 1051
- Khan , N.A. 2004 . An evaluation of the effects of exogenous ethephon, an ethylene releasing compound, on photosynthesis of mustard (Brassica juncea) cultivars that differ in photosynthetic capacity . BMC Plant Biol. , 4 : 21
- Khangura , R. and Aberra , M. 2009 . First report of charcoal rot on canola caused by Macrophomina phaseolina in western Australia . Plant Dis. , 93 : 666 – 667 .
- Mahmoud , A. and Budak , H. 2011 . First report of charcoal rot caused by Macrophomina phaseolina in sunflower in Turkey . Plant Dis. , 95 : 223
- Mcdowell , J.M. and Dangl , J.L. 2000 . Signal transduction in the plant immune response . Trends Biochem. Sci. , 25 : 79 – 82 .
- Mihail , J.D. , Alcorn , S.M. , Orum , T.V. and Ray , D.T. 1990 . Charcoal rot of guayule in Arizona . Plant Dis. , 74 : 219 – 224 .
- Mueller , J.D. , Short , B.J. and Sinclair , J.B. 1985 . Effects of cropping history, cultivar, and sampling date on the internal fungi of soybean roots . Plant Dis. , 69 : 520 – 523 .
- Murashige , T. and Skoog , F. 1962 . A revised medium for rapid growth and bioassays with tobacco tissue cultures . Physiol. Plant , 15 : 473 – 497 .
- Penmetsa , R.V. , Uribe , P. , Anderson , J. , Lichtenzveig , J. , Gish , J.C. Nam , Y. W. 2008 . The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations . Plant J. , 55 : 580 – 595 .
- Rothrock , C.S. , Kirkpatrick , T.L. , Frans , R.E. and Scott , H.D. 1995 . The influence of winter legume cover crops on soilborne plant-pathogens and cotton seedling diseases . Plant Dis. , 79 : 167 – 171 .
- Salzer , P. , Feddermann , N. , Wiemken , A. , Boller , T. and Staehelin , C. 2004 . Sinorhizobium meliloti-induced chitinase gene expression in Medicago truncatula ecotype R108-1: a comparison between symbiosis-specific class V and defence-related class IV chitinases . Planta , 219 : 626 – 638 .
- Siddiqui , Z.A. and Mahmood , I. 1993 . Biological control of Meloidogyne incognita race-3 and Macrophomina phaseolina by Paecilomyces lilacinus and Bacillus subtilis alone and in combination on chickpea . Fund. Appl. Nematol. , 16 : 215 – 218 .
- Smith , G.S. and Carvil , O.N. 1997 . Field screening of commercial and experimental soybean cultivars for their reaction to Macrophomina phaseolina . Plant Dis. , 81 : 363 – 368 .
- Smith , G.S. and Wyllie , T.D. 1999 . “ Charcoal rot ” . In Compendium of soybean diseases , 4th , Edited by: Hartman , G.C. , Sinclair , J.B. and Rupe , J.C. 29 – 30 . St. Paul , MN : American Phytopathological Society .
- Thomma , B.P. , Eggermont , K. , Penninckx , I.A. , Mauch-Mani , B. , Vogelsang , R. Cammue , B.P. 1998 . Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens . Proc. Natl. Acad. Sci. U.S.A. , 95 : 15107 – 15111 .
- Thomma , B.P. , Eggermont , K. , Tierens , K.F. and Broekaert , W.F. 1999 . Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea . Plant Physiol. , 121 : 1093 – 1102 .
- Thoquet , P. , Gherardi , M. , Journet , E.P. , Kereszt , A. , Ane , J.M. Prosperi , J.M. 2002 . The molecular genetic linkage map of the model legume Medicago truncatula: an essential tool for comparative legume genomics and the isolation of agronomically important genes . BMC Plant Biol. , 2 : 1
- Todd , T.C. 1993 . Soybean planting date and maturity effects on Heterodera glycines and Macrophomina phaseolina in southeastern Kansas . J. Nematol. , 25 : 731 – 737 .
- Town , C.D. 2006 . Annotating the genome of Medicago truncatula. Curr. Opin . Plant Biol. , 9 : 122 – 127 .
- Van Loon , L.C. , Rep , M. and Pieterse , C.M.J. 2006 . Significance of inducible defense-related proteins in infected plants . Annu. Rev. Phytopathol. , 44 : 135 – 162 .
- Vijayan , P. , Shockey , J. , Levesque , C.A. , Cook , R.J. and Browse , J. 1998 . A role for jasmonate in pathogen defense of Arabidopsis . Proc. Natl. Acad. Sci. U.S.A. , 95 : 7209 – 7214 .
- Wrather , J.A. and Koenning , S.R. 2006 . Estimates of disease effects on soybean yields in the United States 2003 to 2005 . J. Nematol. , 38 : 173 – 180 .
- Wyllie , T.D. 1989 . “ Charcoal rot ” . In Compendium of soybean diseases , 3rd , Edited by: Sinclair , J.B. and Backman , P.A. 30 – 33 . St. Paul , MN : American Phytopathological Society .
- Yang , X.B. and Navi , S.S. 2005 . First report of charcoal rot epidemics caused by Macrophomina phaseolina in soybean in Iowa . Plant Dis. , 89 : 526