Abstract
Tobacco rattle virus (TRV), genus Tobravirus, is able to infect a broad range of plant species, possesses worldwide distribution and naturally infects a very large number of cultivated as well as ornamental plants. Members of the plant ectoparasitic nematode genera Trichodorus and Paratrichodorus transmit TRV in a semi-persistent way in a non-replicative process, where virus particles are transferred to the host plant through vector feeding on root epidermal and root hair cells. Our investigations concentrated on ultrastructural and anatomical effects of TRV strain PSG infection in situ following introduction into potato and tobacco tissues by Trichodorus primitivus. Our anatomical observations indicated necrotic changes of rhizodermis and cell wall deformations in primary cortex parenchyma and external phloem layers as a consequence of the interactions between Trichodorus–TRV–host plants. Ultrastructural analyses revealed TRV particles in rhizodermis, cortex and vascular roots of potato and tobacco tissues. These results indicate that TRV strain PSG was transferred into roots and also transported from cell to cell in all root tissues. Complete TRV PSG particles of two lengths were documented in companion cells, phloem fibre and parenchyma, as well as for the first time in immature and mature xylem tracheary elements and xylem parenchyma. Our findings suggested that TRV was systemically transported from the place of direct transfer by the vector to above-ground plant organs (especially leaves). The presence of TRV PSG particles in mesophyll and vascular leaf tissues confirmed our thesis. Moreover, we concluded that TRV systemic movement occurred not only in the phloem, but especially in the xylem, because virus particles were more frequently observed in xylem parenchyma and xylem tracheary elements.
Résumé
Le bruissement du tabac (TRV), genre Tobravirus, peut infecter une vaste gamme d'espèces de plantes, se trouve à peu près partout dans le monde et infecte naturellement un très grand nombre de plantes cultivées et ornementales. Les membres des nématodes ectoparasites des plantes des genres Trichodorus et Paratrichodorus transmettent le TRV sur le mode semi-persistant et par processus non réplicatif au cours desquels des particules virales sont transférées à la plante hôte par des vecteurs qui se nourrissent de cellules épidermiques et pilifères. Nos recherches se sont concentrées sur les effets ultrastructuraux et anatomiques de l'infection in situ par la particule PSG de la souche TRV à la suite de son introduction, dans des tissus de pomme de terre et de tabac, par Trichodorus primitivus. Basées sur les interactions entre Trichodorus, TRV et les plantes hôtes, nos observations anatomiques indiquent des altérations nécrotiques du rhizoderme et des déformations de la paroi cellulaire dans le parenchyme cortical primaire et les couches externes du phloème. Des analyses ultrastructurales ont permis de déceler des particules de TRV dans le rhizoderme, dans le cortex et dans les faisceaux vasculaires des racines de tissus de pomme de terre et de tabac. Ces résultats indiquent que la particule PSG de TRV a été transférée dans les racines et aussi transportée d'une cellule à l'autre de tous les tissus racinaires. Des particules complètes de PSG de TRV de deux longueurs ont été documentées dans des cellules compagnes, des fibres du phloème et du parenchyme ainsi que, pour la première fois, dans des éléments matures et immatures de trachée du xylème et du parenchyme. Nos résultats suggèrent que le TRV a été systématiquement transporté du site de transfert direct par le vecteur aux organes aériens, particulièrement aux feuilles. La présence des particules PSG de TRV dans les tissus mésophylliques et vasculaires des feuilles a confirmé notre thèse. De plus, nous avons conclu que le mouvement systémique du TRV se produit non seulement dans le phloème, mais notamment dans le xylème, et ce, parce que les particules de virus ont été plus fréquemment observées dans le parenchyme du xylème et dans les éléments de trachée du xylème.
Introduction
In a recent classification of viruses, Tobravirus was established as a genus containing three viruses, each named after one of the hosts that the virus infects: Tobacco rattle virus, the type-member of the genus (TRV), Pea early-browning virus (PEBV) and Pepper ringspot virus (PRV) that was originally referred to as the CAM-strain TRV, which occurs only in Brazil (Fauquet et al., Citation2005). Of the three tobraviruses, the most economically important one causing ‘TRV-spraing’ disease of potatoes (Solanum tuberosum L.) is TRV (Taylor & Brown, Citation1997). The disease results in brown necrotic arcs in the potato tuber flesh that render the crop unmarketable.
TRV has a separate genome and is made of two single strands of positive polarity (+)ssRNA. The genetic material undergoes separate encapsidation into simple, rod-shaped, helical capsids, with identical diameters of 22.5 nm. The particles of TRV vary in terms of length: longer particles (L) range from 180 to 197 nm and shorter particles (S) from 55 to 114 nm. Both types of particles have 5% RNA and 95% of protein. It was demonstrated that these particles can have different functions in the processes of viral infection and multiplication in a plant.
TRV has an extensive host range and infects at least 600 species in more than 50 families – dicotyledonous and monocotyledonous (Weidemann, Citation1981; Arias & Bello, Citation1988). Plant species belonging to the Hydrophylaceae, Solanaceae, Primulaceae and Linaceae families can become systemically infected, while those in the Cucurbitaceae and Fabaceae are only infected locally. Members of the Rosaceae and Poaceae families are not hosts for TRV. Crops and ornamental plants most seriously affected by Tobraviruses include potato, tobacco and tulip, narcissi and hyacinth (Maas & Rothuis, Citation1973; Ploeg & Brown, Citation1997). In tobacco, TRV causes necrotic spots and arcs on the leaves of infected plants. Infection results in the leaves crinkling when they become dry and brittle. The English disease name ‘rattle’ derives from the rustling sound of the dry leaves moving in the wind (Heinicke, Citation1983). TRV causes notched leaf in gladiolus, ringspot in aster and pepper, yellow blotch in sugar beet, colour-break in tulip, and unnamed diseases in lettuce, hyacinth and narcissus. TRV induces a wide range of symptoms in different potato cultivars, with about 50% of currently used cultivars showing symptoms of infection (Reepmeyer, Citation1973). Infection and any consequent disease symptomatology are highly dependent on the potato genotype and the strain of TRV. Infection of tubers is also related to soil moisture conditions prevailing during tuber initiation, as a high soil moisture is necessary for vector nematode mobility and hence efficient virus transmission.
Sol et al. (Citation1960) were the first to report that the natural vector of the virus were trichodorid nematodes. Several members of the plant ectoparasitic nematode genera Trichodorus and Paratrichodorus transmit tobraviruses. The nematodes feed on root epidermal and root hair cells. At the commencement of the feeding cycle, the nematode uses its onchiostyle to penetrate several individual cells, but then it abandons the cell, leaving it intact. Eventually, it selects cells upon which to feed, and most of the cell contents are removed during the feeding process, causing death of the cell (Wyss, Citation1975; Fritzsche et al., Citation1985). During the feeding process, virus particles present in a cell are ingested along with the cell cytoplasm and retained by the nematode in its feeding apparatus. Subsequently, when the nematode begins its feeding cycle on an uninfected cell, or plant, these virus particles are transferred into the new cell, resulting in transmission of the virus (Trudgill, Citation1976).
Trichodorids have been reported from all of the main continents, and are particularly widespread and prevalent in Europe and North America. During a comprehensive survey of virus-vector nematode species in the UK, trichodorid nematodes were recovered from 22% of all sites sampled (Alphey & Boag, Citation1976). At least 10–15% and 15–20% of the arable hectarage in Scotland was found to be infested with TRV and with trichodorids, respectively. In other areas of Europe, TRV was present in more than 50% of irrigated land in Switzerland (Gugerli, Citation1977); 12% of the potato area is affected in Great Britain and 15% in Belgium (De Pelsmacker & Coomans, Citation1987). Paratrichodorus pachydermus and T. primitivus are the most prevalent species, and thus most economically important European vectors of TRV. TRV remains viable and can be transmitted by trichodorids after the nematodes have been starved for 20 weeks; trichodorids stored also in soil in plastic bags were able to transmit TRV after 3 years (van Hoof, Citation1970).
As with most plant viruses, the diseases caused by TRV are not easy to control. Nematicides and nematastats can be used to reduce the number of trichodorids or to temporarily immobilize them – consequently, they reduce the impact of TRV at field sites (Cooper & Thomas, Citation1971). However, this type of control is not fully effective because some nematodes always survive. Soil-disinfection products provide efficient control only for a limited number of growing seasons, sometimes only for the one season after application. Other chemicals inhibit nematode movement, thus preventing the nematode from transmitting virus and are effective for only a few weeks after application and have to be applied every growing season. Non-chemical strategies for controlling TRV also have been proposed, but most do not provide a high degree of success or efficiency. New methods must be developed to protect vulnerable crops such as potato growing on land with vector-nematodes and TRV. The most efficient and reliable method to control TRV in commercial crops is to breed virus-resistant cultivars. However, use of resistant potato cultivars is problematic as TRV-strains differentially affect potato cultivars (Xenophontos et al., Citation1998). The most appropriate cultivar for suppressing disease symptoms may not be the preferred marketable cultivar.
The wide knowledge about TRV biology and plant–virus–nematode interactions is very important to develop efficient methods to control pathogens and their vectors. However, in the current literature, ultrastructural research exploring cytopathological changes in plant cells and tissues caused by TRV is lacking. Our investigations focused on ultrastructural and anatomical effects of TRV strain PSG infection of tobacco and potato plants using the nematode vector. Additionally, the objective of these studies was to document TRV transport from roots (transferred site) to above-ground plant organs.
Materials and methods
Plant material
Plants of Solanum tuberosum ‘Glada’ and Nicotiana tabacum ‘Samsun’ were grown in a growth chamber, at a temperature of 20 °C and a 16-h light cycle with light intensity of 400 mmol m–2 s–1 PAR. The study used soil from fields (Dobrzewin, Western Pomeranian Voivodeship) in which the presence of Trichodorus primitivus nematodes was observed (Ciskowska & Cieślewicz, Citation1990). The field soil was mixed in 1:1 ratio with steamed compost soils with peat. Then, the mixture was placed in flower pots, into which two tobacco and potato seedlings were placed in three repetitions, 10 pots each.
Plants with four sets of true leaves were infected with the PSG strain of TRV through the transmitting vector – Trichodorus primitivus. Plants of potato ‘Glada’ and tobacco ‘Samsun’ used as material for further investigations and control plants were tested using the DAS-ELISA procedure at IHAR Młochów (according to Clark & Adams, Citation1977). The plant organs were collected (depending on symptoms) 4 weeks after Tobacco rattle virus infection. Control plant material (healthy potato and tobacco) was grown in soil free from nematodes.
Analysis in transmission electron microscope (TEM)
The plant material was fixed in 2% (w/v) paraformaldehyde and 2% (v/v) glutaraldehyde in 0.05 M cacodylate buffer (pH 7.2–7.4) (Karnovsky, Citation1965) for 2 h at room temperature. Next, the samples were contrasted and fixed in 2% (w/v) OsO4 in cacodylate buffer for 2 h at 4 °C. The material was rinsed with sodium cacodylate and then dehydrated in a series of increasingly strong solutions of ethanol. The material was gradually saturated with epoxy resin Epon 812 (Fluka) and polymerized for 24 h at 60 °C. The ultrathin sections on copper grids were stained with uranyl acetate and in lead citrate. Observations were made using a Morgagni 268D (FEI) transmission electron microscope. Photographic documentation was prepared with a Morada (SIS) digital camera and the iTEM (SIS) computer program.
Results
Morphological and anatomical changes in host-plant tissues
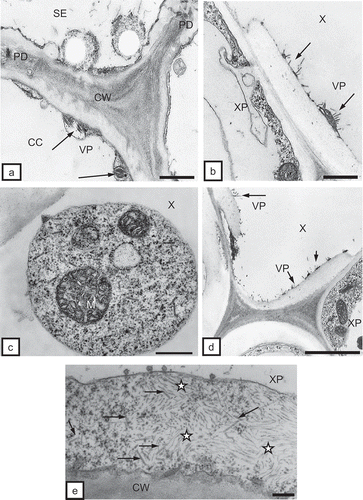
Four weeks after placing the host plants in flower pots with soil containing the ectoparasitic nematode T. primitivus, plants of tobacco and potato responded with deformations and ring-shaped surface necrosis of leaf blades () and external necrosis of stems and petioles (). The root system of the plants was analyzed for presence of the nematodes in soil. Ectoparasitic nematodes feeding on potato () and tobacco plants were detected. The ultrastructural analysis also confirmed the presence of these vectors just at the rhizodermis of the root (). Necrotic anatomic lesions on tobacco and potato roots were detected (, ) and necrosis of rhizodermis () and fractures of cell walls of the primary cortex parenchyma were observed (). Furthermore, necrosis affected primary cortex parenchymal cells of the root (, ), as well as phloem parenchymal cells (, 2c) and pith parenchymal cells () of the root and stem. Anatomic lesions of leaf blades and petioles involved mostly necrosis of the palisade mesophyll () or epidermis.
Fig. 1. a, External surface necrosis (white arrows) and leaflet deformation (black arrows) on tobacco ‘Samsun’ 4 weeks after plants were introduced into soil with nematodes. b, External stem (long arrows) and leaf petioles (short arrows) with necrosis 4 weeks after TRV PSG was transferred by Trichodorus primitivus into tobacco ‘Samsun’. c, Ectoparasitic nematodes Trichodorus primitivus (Nm) feeding on roots of potato (R) ‘Glada’ 3 weeks after the plant was placed in infested soil. Bar = 100 μm. d, Cross-section of the body (asterisk) of the nematode near root hairs (RH) of tobacco. Bar = 1 μm.
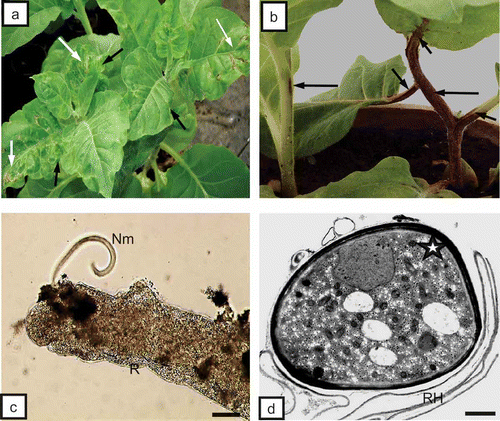
Fig. 2. a, Necrosis of rhizodermis (long arrows) and fracture of cell walls (CW, black asterisk) in primary cortex. Necrosis of primary cortex parenchyma cells (PCP, white asterisks) of tobacco root can be seen. X = xylem tracheary element. Bar = 50 μm. b, Primary cortex cells (PCP) with necrosis of phloem parenchyma (white arrows) and primary cortex (dark arrows) of root of potato ‘Glada’. Ph = phloem, X = xylem tracheary element. Bar = 50 μm. c, Necrosis of phloem parenchymal cells (asterisks), primary cortex parenchyma (PCP, white arrow) and pith parenchymal cells (dark arrow) of tobacco stem. X = xylem tracheary element. Bar = 50 μm. d, Necrosis of palisade mesophyll cells (Pme, asterisks) of tobacco leaf blade. Sme = spongy mesophyll, VB = vascular bundle. Bar = 50 μm.
Ultrastructural changes in potato and tobacco root tissues infected with TRV transmitted by soil nematodes
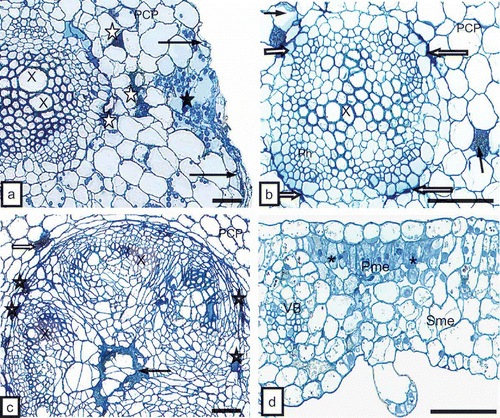
The ultrastructural tissue analysis of roots infected with TRV PSG via the feeding of the T. primitivus vector revealed the presence of complete particles of two lengths of TRV. In non-necrotizing tissues, complete particles of two lengths, L and S, were observed, e.g. in rhizodermis () and in the primary cortex parenchymal cells (, ). TRV particles were located along a cell wall with clearly loosened structure () and in the protoplast area, near dictyosomes of the Golgi apparatus and the numerous vesicles originating from these organelles in the primary cortex of potato and tobacco (). Ultrastructural observations of root tissues revealed also advanced necrotizing processes of cell groups of rhizodermis and primary cortex parenchyma. The tissue system was disrupted due to strong deformations and invagination of cell walls (). In areas isolated by the deformed cell walls, one could observe complete TRV PSG particles (cross-section, ). The studied cultivar of potato (‘Glada’) and tobacco (‘Samsun’) were both susceptible to TRV infection but the changes analysed in vascular tissues of these host plants usually suggested otherwise. In phloem tissues of potato, complete TRV particles were observed mostly in phloem parenchymal cells (, 4d). In the case of tobacco, the presence of complete TRV PSG was visible both in phloem parenchyma and in phloem fibres (). The particles occurred in the cytoplasm, mostly in proximity of dictyosomes of the Golgi apparatus. In companion cells, the particles were observed extremely rarely and they were usually incomplete TRV particles (). TRV particles were also present inside the tracheary elements of potato and tobacco xylem. In young differentiating vessels, complete TRV particles were observed (), and in differentiated (mature) tracheary elements – incomplete particles (, ). In xylem parenchyma, presence of both complete and incomplete forms of TRV PSG particles was detected (). In root tissues, complete forms of TRV particles prevailed. The presence of the virus in root tissues suggests that the particles penetrate the cells of covering layers and external layers of the primary cortex via the vector and are then transported via plasmodesmata to neighbouring cells but they may also be transported systemically through conductive tissues to above-ground plant organs.
Fig. 3. a, Complete TRV particles (VP, arrows) of two lengths (L and S) in tobacco rhizodermis cell (Rh). Bar = 0.2 μm. b, Complete TRV PSG particles (VP) of two lengths (arrows) near cells wall (CW) of primary cortex parenchymal cell (PCP) of tobacco root. Cell wall has clearly loosened structure. M = mitochondria. Bar = 0.2 μm. c, Vast necrosis in rhizodermis (Rh) and primary cortex parenchyma (PCP) of tobacco root. Tissue system disrupted by strong deformations of cell walls (asterisks, CW). Bar = 1 μm. d, Complete TRV particles (VP) at cross-section (arrow) in an area isolated by deformed cell walls (CW) of rhizodermis. Bar = 0.1 μm.
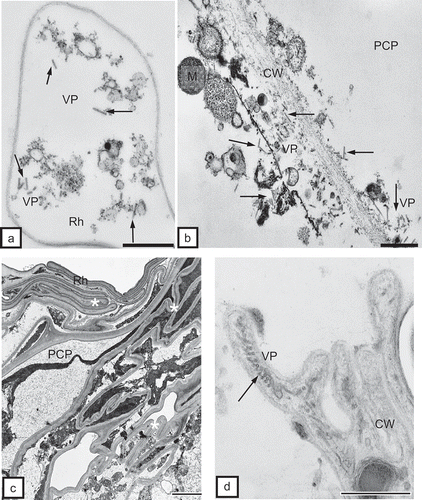
Fig. 4. a, Complete TRV PSG particles (VP) of two lengths near the Golgi apparatuses (GA) and numerous vesicles (vs) in the protoplast of the primary cortex parenchymal cell (PCP) of potato root. CW = cell wall. Bar = 0.2 μm. b, Dispersed incomplete TRV particles (VP) near Golgi apparatus (GA) in phloem fibre (Pf) of tobacco root. CW = cell wall. Bar = 0.5 μm. c, Complete TRV PSG particles (arrow) in phloem parenchymal cell (PP) of potato ‘Glada’ root. The marked fragment is magnified in CC = companion cell, N = nucleus, Pf = phloem fibres, SE = sieve element. Bar = 1 μm. d, Magnified fragment from CW = cell wall, GA = Golgi apparatus, M = mitochondria,N = nucleus, VP = virus particles. Bar = 0.2 μm.
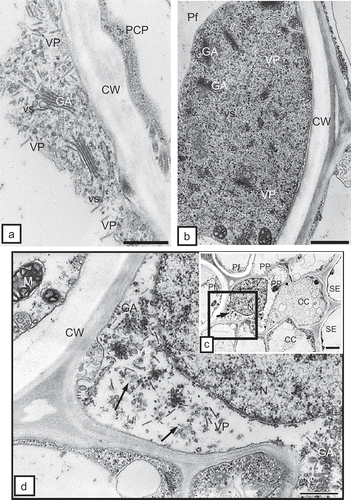
Fig. 5. a, Incomplete TRV PSG particles (VP, arrows) in a companion cell (CC) of tobacco root. CW = cell wall, PD = plasmodesmata, SE = sieve element. Bar = 0.2 μm. b, Incomplete TRV PSG particles (VP, arrows) in a mature tracheary element of xylem (X) of tobacco root. XP = xylem parenchyma. Bar = 0.2 μm. c, Arrows indicate complete virus particles in xylem tracheary element (X). M = mitochondria. Bar = 0.2 μm. d, Incomplete TRV PSG particles (VP, arrows) in a mature tracheary element of xylem (X) of potato root. XP = xylem parenchyma. Bar = 0.5 μm. e, Complete (arrows) and incomplete (asterisks) TRV particles in xylem parenchymal cells (XP) of potato root. CW = cell wall. Bar = 200 nm.
Ultrastructural changes in potato and tobacco above-ground plants organs infected with TRV transmitted by soil nematodes
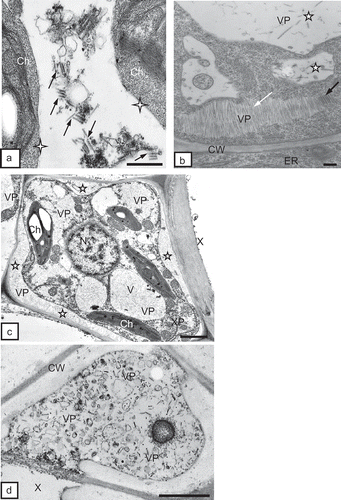
In tobacco leaf tissues, the presence of vast inclusions of incomplete virus particles was observed within mesophyll cells (, 6c). The protoplast of such cells was pulled away from the cell walls. Cell wall fractures were also noted (). In protoplasts of these cells, incomplete TRV particles were prevalent. Incomplete particle forms were visible also in mesophyll intercellular spaces (). In mesophyll cells, dispersed complete TRV particles were observed (). Sometimes, they occurred in more organized groups (), forming at times regular inclusions made of incomplete and complete particles (). In both conductive tissues of tobacco and potato leaves, the presence of TRV PSG was detected. Even though anatomical observations did not reveal necrosis in leaf vascular bundles, a submicroscopic analysis showed single, necrotic cells of phloem parenchyma. In phloem parenchyma cells, complete TRV particles were observed in the proximity of plasmodesmata (data not shown). Inside the tracheary elements and in protoplasts of xylem parenchyma of potato and tobacco, both incomplete () and complete particles were detected (). In xylem, just like in other leaf tissues of tobacco and potato, incomplete TRV PSG forms predominated.
Fig. 6. a, Inclusions of incomplete TRV PSG particles (VP, arrows) in mesophyll cells of tobacco leaves. Protoplasts in a distance to cell wall (asterisks, CW). Ch = chloroplast. Bar = 1 μm. b, Incomplete TRV particles (VP) in the intercellular space (Int) of palisade mesophyll (Pme). Ch = chloroplast, CW = cell wall. Bar = 0.5 μm. c, Fractured cell wall (CW) between cells of palisade mesophyll (Pme). Numerous incomplete TRV particles (VP) of two lengths are present. Changed chloroplast (Ch) structure. ER = endoplasmic reticulum. Bar = 0.5 μm.
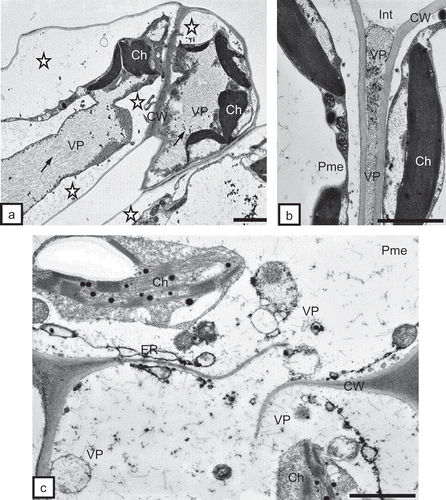
Fig. 7. a, Complete TRV PSG particles (arrows) of two lengths near chloroplasts (Ch) in a potato mesophyll cell. Absence of visible double membrane around chloroplasts (asterisks). Bar = 0.2 μm. b, Regular inclusion of TRV particles (VP) made of complete (dark arrow) and incomplete (white arrow) particles. Complete particles present also inside vacuoles (asterisks) of tobacco mesophyll cells. CW = cell wall, ER = endoplasmic reticulum. Bar = 200 nm. c, Incomplete TRV PSG particles (VP) in xylem parenchyma (XP) of tobacco leaf. Particles are present in the apoplast (asterisks) between the protoplast and the cell wall (CW) and in cytoplasm and vacuoles (V). Ch = chloroplast, N = nucleus, X = xylem tracheary element. Bar = 1μm. d, Complete TRV particles (VP) of two lengths inside the xylem (X) tracheary element of potato leaf. CW = cell wall. Bar = 0.5 μm.
Discussion
Our anatomical observations indicate necrotic changes of rhizodermis as a result of the nematode feeding process. The more appropriate thesis could be that this process is probably a result of chemical or enzymatic reaction to the nematode secretions; however, they may be derived from mechanical injury. The nematodes move to lateral root tips that eventually become stubby and turn brown or black, probably as a result of secondary infection or necrosis. Previously observed symptoms of trichodorid feeding are yellowing on the root surface, root tip swelling, and typically deformed stele of cherry (Coiro & Sasanelli, Citation1994); and cracks in the epidermis and enlargement of tissues underlying feeding points of cells of apple, loss of meristematic activity, browning of epidermal and hypodermal cells and in some cases, shrinkage of these brown cells in apple (Pitcher, Citation1967).
We documented necrotization with cell wall deformation and hypertrophy of the cells in primary cortex parenchyma and external phloem layer caused by TRV infection. Karanastasi et al. (Citation2001) have observed Paratrichodorus (also the vector of TRV), wherein during the later stages of feeding, a significant proportion of feeding episodes are abandoned before completion, leaving the cells alive. Tobravirus particles can be acquired by both juvenile and adult forms of the vector nematode. The nematodes can retain the virus for a long time, e.g. P. pachydermus were able to transmit TRV after a 2-year period of cold storage in soil. Virus is lost from the nematode after moulting, which occurs between each of the four stages of juvenile development and results in the replacement of the external cuticle including the lining of the oesophagus. Brown et al. (Citation1996) observed similar sites of virus retention, and showed transverse sections of TRV particles in anterior oesophagus of Trichodorus similis. The mechanism of the TRV transmission is a semipersistent, non-replicative process where virus particles are retained by binding to specific sites on the surface of the nematode oesophagus (MacFarlane, Citation2003).
Tobravirus TRV is positive-sense, single-stranded RNA virus with two genomic RNAs, the larger reffered to as RNA1 and the smaller as RNA2, that are packaged into separate virus rod-shaped particles (Mayo & Robinson, Citation1996; MacFarlane, Citation1999). RNA1 is highly conserved between isolates, and encodes 4 non-structural proteins. The 134K helicase and 194K RNA-dependent RNA polymerase are both involved in virus replication (Hamilton et al., Citation1987). The 29K protein translated from a subgenomic RNA represents the viral movement protein, which is involved in virus accumulation in N. tabacum (Ziegler-Graff et al., Citation1991). RNA1-encoded 16K protein functions as a pathogenicity factor (Liu et al., Citation2002). RNA2, possessing higher variability between isolates than RNA1, encodes the coat protein (CP) and in some isolates, other non-structural proteins responsible for vector transmission by plant-parasitic trichodorids (Ploeg et al., Citation1993; Hernandez et al., Citation1997). Analysis of different Tobravirus isolates revealed that RNA2 in some isolates encoded only the CP gene and others encoded three additional genes, including partial and complete copies of genes derived from RNA1 (MacFarlane, Citation1999). TRV can cause two types of infection. The first is a multiplying infection (M-type), in which both RNAs are present and the virus produces nucleoprotein particles. The second type is a non-multiplying infection (NM-type), where only uncapsidated RNA1 is present and the virus spreads in the complete absence of RNA2 (MacFarlane, Citation1999). Tobacco rattle virus strain PSG encodes genomic RNA1 (6.3 kb) and RNA2 (1.9 kb) and so represents the M-type of infection, forming two types of nucleoprotein particles. RNA1-PSG sequence contains three open reading frames, encoding the C-terminal region of the 170K protein, a 29K protein and a 16K protein, respectively. RNA2 was found to contain only one significant open reading frame encoding the capsid protein. Sequencing of a subgenomic RNA showed it to be derived from RNA2-PSG. Two other subgenomic RNAs were identified that are probably involved in the expression of the 29K and 16K proteins (Cornelissen et al., Citation1986).
Nixon and Harrison (Citation1959) reported total diameter of TRV particles in cross-section ranged from 17–25 nm in size. Offord (Citation1966) quoted a value of 25.6 nm for these nucleoprotein complexes. The x-diffraction of the cylindrically arranged diameters is reported to be 22.5 nm (Finch, Citation1965). We used the terms ‘complete’ and ‘incomplete’ particles in our analyses, because we observed TRV particles with different diameters. The complete particles were quoted a value of 25 nm and the smaller ones 12.5 nm. Observed TRV PSG particles had bigger and smaller diameters, but were often located in the same arranged cytoplasmic inclusion (, ). We deduced that these are two stages of the same particles in the assembling process, so the nucleoprotein particles with the smaller diameter were named ‘incomplete’ stage of TRV particles.
Previously, TRV particles were found in the cytoplasm and arranged around mitochondria in infected host-plant cells (Brunt et al., Citation1990). In a few plant species, TRV apparently remains localized at the initial site of infection, inducing chlorotic and/or necrotic lesions (hypersensitive reaction resulting in death of the infected cell), regardless of whether infection originated from mechanical inoculation of leaves or root inoculation by virus-vector trichodorids. In other plant species, TRV spreads systemically, eventually infecting the entire plant. TRV-spraing symptoms in potato tuber flesh are regarded as extreme examples of a hypersensitive response in the plant host (Ghazala & Varrelmann, Citation2007).
Our ultrastructural observations revealed TRV particles in rhizodermis, cortex and vascular bundle tissues of tobacco and potato roots. These findings indicate that TRV PSG was transferred into plant cells by the nematode vector and then also efficiently transported from cell to cell in all root tissues. TRV particles were very often documented in root vascular tissues: in companion cells, phloem fibres and parenchyma. In xylem tissues, TRV particles were observed in differentiating and mature tracheary elements, but much more often in xylem parenchyma.
Analysis of constructed infectious cDNA clones of TRV and other Tobravirus PEBV (Pea early browning virus) has allowed identification of which virus genes are involved in transmission and how nematode vector specificity may be determined. Transmission of PEBV SP5 isolate did not occur when the PEBV CP was replaced with that of the highly transmissible TRV PpK20 isolate (MacFarlane & Brown, Citation1995), indicating that the CP is not the sole determination of transmission. Deletion of the 15-amino acid residues at the C-terminus of the capsid protein of PEBV TpA56 and TRV PpK20 prevented the nematode transmission of these pathogens (Hernandez et al., Citation1996). The TRV CP possesses a C-terminal domain between 20–30 amino acid residues that is exposed on the surface of the particle where it is the major antigenic determinant (Legorburu et al., Citation1996). Analysis of particles by using nuclear magnetic resonance and Raman optical analysis confirmed that the C-terminus domain is unstructured and may interact with other viral or nematode vectors to promote transmission (Brierley et al., Citation1993; Blanch et al., Citation2001). The RNA2 of all Tobraviruses encodes in addition to CP two other genes referred to as 2b and 2c. Deletion or mutation of PEBV 2c gene had no obvious effect on virus multiplication and movement but greatly reduced the frequency of nematode transmission of the virus. Deletion of the 2c genes of TRV PpK20 and TRV PaY4 had no effect on virus infection or nematode transmission (Vassilakos et al., Citation2001).
The 2b protein of both Tobravirus TRV and PEBV has a main function in nematode transmission. Deletion of the 2b gene from PEBV TpA56, TRV PpK20 and TRV PaY4 prevented the transmission of all these viruses (MacFarlane, Citation1996; Hernandez et al., Citation1997; Vassilakos et al., Citation2001). The timing of expression of the 2b protein closely follows that of the CP, and both proteins are detected in pelleted and soluble cell fractions (Visser & Bol, Citation1999). The TRV PaY4 mutant lacking the 2b gene can be transmitted when co-inoculated to plants with wild-type TRV PaY4. The TRV PaY4 lacking the 2b was not complemented by TRV PpK20, even though this virus isolate has the same vector – P. pachydermus (Vassilakos et al., Citation2001). This result probably suggests that successful transmission requires cooperation between the 2b and another protein. The TRV PpK20 2b protein can interact with CP but the removal of the C-terminal domain of CP abolished this interaction (Visser & Bol, Citation1999). Immunogold localization studies revealed in vivo binding of the TRV and PEBV 2b proteins to virus particles (Vellios et al., Citation2002). Interaction between 2b and the nematode oesophagus may be responsible for determining which species transmits a particular virus isolate. The CP might directly interact with the nematode but may require interaction between the virus CP and 2b proteins, probably to bring about the necessary conformational change (MacFarlane, Citation2003).
Mutation of the PEBV 2c gene had no obvious effect on virus multiplication and movement but significantly reduced the frequency of virus transmission by the nematode (Schmitt et al., Citation1998). The role of the 2c protein is not clear, although it does participate in the transmission of PEBV TpA56 by T. primitivus. The hybrid study detected an interaction between CP and 2c protein of TRV PpK20. It is possible that this protein is involved in transmission by Trichodorus sp., but not Paratrichodorus sp. nematodes (Vassilakos et al., Citation2001).
Our ultrastructural observations indicated that TRV is systemically transported from the roots (the place of direct transfer) to above-ground organs (especially leaves), resulting in morphological and anatomical changes. Analysis of all leaf tissues of potato and tobacco revealed the presence of virus particles in mesophyll and vascular system (phloem parenchyma, xylem vessels and xylem parenchyma), but here the uncompleted form of TRV PSG particles dominated. In potato and tobacco tissues, systemic movement was not only in phloem elements. Virus particles were often documented in xylem tracheary elements and xylem parenchyma more than in phloem. Our observations confirm the conclusions regarding the transport of infection by GFP-TRV and GFP-PEBV clones in Nicotiana benthamiana and Nicotiana clevelandii plants with the same speed as a non-mutated virus. Moreover, mutants could leave the conductive tissue in systemically infected leaves. Xylem transport by plant viruses was originally proposed in studies of Sobemoviruses such as Rice yellow mottle virus (RYMV) and Blueberry shoestring virus (Urban et al., Citation1989; Opalka et al., Citation1998). In immunogold labelling studies using light and electron microscopy, BNYVV (Beet necrotic yellow vein virus) and SBWMV (Soilborne wheat mosaic virus) were each detected in xylem vessels or xylem parenchyma in infected plant roots (Dubois et al., Citation1994; Verchot et al., Citation2001). SBWMV inclusion bodies were also identified in xylem parenchyma and xylem vessels in infected wheat roots. Otulak and Garbaczewska (Citation2010a, Citation2010b) detected PVY particles and capsid protein in xylem tracheary elements and in xylem parenchyma of potato tissues in the hypersensitive response to PVY infection. RYMV and SBWMV have been detected in immature xylem elements prior to cell death. The virus is likely to move from cell to cell into immature xylem vessels and then undergo replication. After programmed cell death, virus particles are released into the xylem and can move upward in the plant (Verchot et al., Citation2001; Verchot-Lubicz, Citation2003).
Soil-borne viruses transmitted by nematodes like TRV were considered important only because they are causative agents for agricultural diseases. Soil-borne plant viruses have also played a significant role in advancing research into transport and especially resistance to pathogens. Investigations of Tobraviruses may enable the development of new strategies for control of soil-borne virus diseases.
Acknowledgement
We thank inż. Ewa Znojek for expert technical assistance.
References
- Alphey , T.J.W. and Boag , B. 1976 . Distribution of trichodorid nematodes in Great Britain . Ann. Appl. Biol. , 84 : 371 – 381 .
- Arias , M. and Bello , A. 1988 . Status of Trichodoridae in Spain . Nematologica , 34 : 254
- Blanch , E.W. , Robinson , D.J. , Hecht , L. and Barron , L.D. 2001 . A comparison of the solution structures of Tobacco rattle and Tobacco mosaic viruses from Raman optical activity . J. Gen. Virol. , 82 : 1499 – 1502 .
- Brierley , K.M. , Goodman , B.A. and Mayo , M.A. 1993 . A mobile element on a virus particle surface identified by nuclear magnetic resonance spectroscopy . Biochem. J. , 293 : 657 – 659 .
- Brown , D.J.F. , Robertson , W.M. , Neilson , R. , Bem , F. and Robinson , D.J. 1996 . Characterization and vector relation of a serologically distinct isolate of Tobacco rattle tobravirus (TRV) transmitted by Trichodorus similis in northern Greece . Eur. J. Plant Pathol. , 102 : 61 – 68 .
- Brunt , A. , Crabtree , K. and Gibbs , A. 1990 . Viruses of tropical plants: description and lists from the VIDE database , Wallingford , , UK : CAB International .
- Ciskowska , E. and Cieślewicz , I. 1990 . Reakcja roślin ziemniaka porażonych wirusem nekrotycznej kędzierzawki tytoniu TRV . Biul. Inst. Ziemn. , 40 : 5 – 15 .
- Clark , M.F. and Adams , A.N. 1977 . Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses . J. Gen. Virol. , 34 : 475 – 483 .
- Coiro , M.I. and Sasanelli , N. 1994 . The life cycle and reproductive potential of individual Trichodorus sparsus (Nematoda) on S. Lucie cherry . Nematol. Medit. , 22 : 233 – 236 .
- Cooper , J.I. and Thomas , P.R. 1971 . Chemical treatment of soil to prevent transmission of Tobacco rattle virus to potatoes by Trichodorus spp . Ann. Appl. Biol. , 69 : 23 – 34 .
- Cornelissen , B.J.C. , Linthorst , H.J.M. , Brederode , F.T. and Bol , J. F . 1986 . Analysis of the genome structure of Tobacco rattle virus strain PSG . Nucl. Acids Res. , 14 : 2157 – 2169 .
- Dubois , F. , Sangwan , R.S. and Sangwan-Norreel , B.S. 1994 . Spread of Beet necrotic yellow vein virus in infected seedlings and plants of sugar beet (Beta vulgaris) . Protoplasma , 179 : 72 – 82 .
- Fauquet , C.M. , Mayo , M.A. , Maniloff , J. , Desselberger , U. and Ball , L. A . 2005 . Virus taxonomy. Classification and nomenclature of viruses. 8th Report of the International Committee on the Taxonomy of Viruses , Amsterdam: Elsevier .
- Finch , J.T. 1965 . Preliminary X-ray diffraction studies on Tobacco rattle and Barley stripe mosaic viruses . J. Mol. Biol. , 12 : 612
- Fritzsche , R. , Kegler , H. , Decker , H. , Barchend , G. and Thiele , S. 1985 . Einfluß von Nematoden aus der Gruppe der Dorylaimiden mit kurzem Mundstachel auf die Infektion von Pflanzen mit dem bodenbürtigen Tabakrattle-Virus (Tobacco rattle virus) . Arch. Phytopathol. Pflanzenschutz , 21 : 259 – 264 .
- Ghazala , W. and Varrelmann , M. 2007 . Tobacco rattle virus 29 K movement protein is the elicitor of extreme and hypersensitive-like resistance in two cultivars of . Solanum tuberosum. Mol. Plant Microbe Interact. , 20 : 1396 – 1405 .
- Gugerli , P. 1977 . Untersuchungen über die großräumige und lokale Verbreitung des Tabakrattlevirus (TRV) und seiner Vektoren in der Schweiz . Phytopath. Z. , 89 : 1 – 24 .
- Hamilton , W.D.O. , Boccara , M. , Robinson , D.J. and Baulcombe , D.C. 1987 . The complete nucleotide sequence of Tobacco rattle virus RNA-1 . J. Gen. Virol. , 68 : 2563 – 2575 .
- Heinicke , D. 1983 . Eisenfleckigkeit nicht neu aber immer wieder gefürchtet . Der Kartoffelbau , 34 : 335 – 338 .
- Hernandez , C. , Carette , J.E. , Brown , D.J.F. and Bol , J.F. 1996 . Serial passage of Tobacco rattle virus under different selection conditions results in deletion of structural and non-structural genes in RNA2 . J. Virol. , 70 : 4933 – 4940 .
- Hernandez , C. , Visser , P.B. , Brown , D.J.F. and Bol , J.F. 1997 . Transmission of Tobacco rattle virus isolate PpK20 by its nematode vector requires one of the two non-structural genes in the viral RNA2 . J. Gen. Virol. , 78 : 465 – 467 .
- Hoof , H.A. VAN . 1970 . Some observations on retention of Tobacco rattle virus in nematodes . Neth. J. Plant. Path. , 76 : 320 – 330 .
- Karanastasi , E. , Wyss , U. and Brown , D. 2001 . Observations on the feeding behaviour of Paratrichodorus anemones in relation to tobravirus transmission . Meded Rijksuniv Gent Fak. Landbouwkd. Toegep Biol. Wet. , 66 : 599 – 608 .
- Karnovsky , M.J. 1965 . A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy . J. Cell Biol. , 25 : 137A
- Legorburu , F. J. , Robinson , D. J. and Torrance , L. 1996 . Features on the surface of the Tobacco rattle tobravirus particle that are antigenic and sensitive to proteolytic digestion . J. Gen. Virol , 77 : 855 – 859 .
- Liu , H. , Reavy , B. , Swanson , M. and Macfarlane , S.A. 2002 . Functional replacement of the Tobacco rattle virus cysteine-rich protein by pathogenicity proteins from unrelated plant viruses . Virology , 298 : 232 – 239 .
- Maas , P.W.T. and Rothuis , J.H. 1973 . Trichodorus en ratelvirus . Jaarboek Plantenziektenkundige Dienst , : 30 – 33 .
- Macfarlane , S.A. 1996 . Rapid cloning of uncharacterised Tobacco rattle virus isolates using long template (LT) PCR . J. Virol. Meth. , 56 : 91 – 98 .
- Macfarlane , S.A. 1999 . Molecular biology of the tobraviruses . J. Gen. Virol. , 80 : 2799 – 2807 .
- Macfarlane , S.A. 2003 . Molecular determinants of the transmission of plant viruses by nematodes . Mol. Plant Pathol. , 4 : 211 – 215 .
- Macfarlane , S.A. and Brown , D.J.F. 1995 . Sequence comparison of RNA2 of nematode-transmissible and nematode-non-transmissible isolates of Pea early-browning virus suggests that the gene encoding the 29 kDa protein may be involved in nematode transmission . J. Gen. Virol. , 76 : 1299 – 1304 .
- Mayo , M.A. and Robinson , D.J. 1996 . “ Nepoviruses: molecular biology and replication ” . In The plant viruses, Vol. 5: Polyhedral virions and bipartite RNA genomes , Edited by: Harrison , B.D. and Murant , A.F. New York : Planum Press .
- Nixon , H.L. and Harrison , A. D . 1959 . Electron microscopic evidence on the structure of the particles of Tobacco rattle virus . J. Gen. Microbiol , 21 : 582
- Offord , R.E. 1966 . Electron microscopic observations on the substructure of Tobacco rattle virus . J. Mol. Biol. , 17 : 370 – 375 .
- Opalka , N. , Brugidou , C. , Bonneau , C. , Nicole , M. , Beachy , R.N. , Yeager , M. and Fauquet , C. 1998 . Movement of Rice yellow mottle virus between xylem cells through pit membrane . Proc. Natl. Acad. Sci. USA , 95 : 3323 – 3328 .
- Otulak , K. and Garbaczewska , G. 2010a . Ultrastructural events during hypersensitive response of potato cv . Rywal infected with necrotic strains of Potato virus Y. Acta Physiol. Plant. , 32 : 635 – 644 .
- Otulak , K. and Garbaczewska , G. 2010b . Localisation of hydrogen peroxide accumulation during Solanum tuberosum cv. Rywal hypersensitive response to Potato virus Y . Micron , 41 : 327 – 335 .
- Pelsmacker , M. de and Coomans , A. 1987 . Nematodes in potato fields and the relation to some biotic and abiotic factors . Med. Fac. Lanbouwkd. Univ. Gent , 52 : 561 – 569 .
- Pitcher , R.S. 1967 . The host–parasite relations and ecology of Trichodorus viruliferus on apple roots, as observed from an underground laboratory . Nematologica , 13 : 547 – 557 .
- Ploeg , A.T. and Brown , D.J.F. 1997 . “ Trichodorid nematodes and their associated viruses ” . In An Introduction to virus vector nematodes and their associated viruses , Edited by: de , M.S.N , Santos , A. , de , I.M. , Abrantes , O. , Brown , D.J.F. and Lemos , R.M. 41 – 68 . Coimbra , , Portugal : Inst. do Ambiente e Vida .
- Ploeg , A.T. , Robinson , D.J. and Brown , D.J.F. 1993 . RNA-2 of Tobacco rattle virus encodes the determinants of transmissibility by trichodorid vector nematodes . J. Gen. Virol. , 74 : 1463 – 1466 .
- Reepmeyer , H. 1973 . Untersuchungen zur Wirkung eines Phosphorsäureesters auf das Auftreten der Stippigkeit der Kartoffeln und die Überträger des Tobacco-Rattle-Virus . Med. Fac. Landbouww., Univ. Gent , 38 : 1655 – 1662 .
- Schmitt , C. , Mueller , A.M. , Mooney , A. , Brown , D. and Macfarlane , S. 1998 . Immunological detection and mutational analysis of the RNA2- encoded nematode transmission proteins of Pea early browning virus . J. Gen. Virol. , 79 : 1281 – 1288 .
- Sol , Heuven , H.H. , Van , J.C. and Seinhorst , J.W. 1960 . Transmission of rattle virus and Atropa belladonna by nematodes . Tijdschr. Plantenziekten , 66 : 228 – 231 .
- Taylor , C.E. and Brown , D.J.F . 1997 . Nematode vectors of plant viruses , Wallingford , , UK : CABI .
- Trudgill , D.L. 1976 . Observations on the feeding of Xiphinema diversicaudatum . Nematologica , 22 : 417 – 423 .
- Urban , L.A. , Ramsdell , D.C. , Klomparens , K.L. , Lynch , T. and Hancock , J.F. 1989 . Detection of Blueberry shoestring virus in xylem and phloem tissues of high bush blueberry . Phytopathology , 79 : 488 – 493 .
- Vassilakos , N. , Vellios , E.K. , Brown , D.J.F. and Macfarlane , S.A. 2001 . Tobravirus 2b protein acts in trans to facilitate transmission by nematodes . Virology , 279 : 478 – 487 .
- Vellios , E. , Brown , D.J.F. and Macfarlane , S.A. 2002 . Substitution of a single amino acid in the 2b protein of Pea early-browning virus affects nematode transmission . J. Gen. Virol. , 83 : 1771 – 1775 .
- Verchot , J. , Driskel , B.A. , Zhu , Y. , Hunger , R.M. and Littlefield , L.J. 2001 . Evidence that soilborne Wheat mosaic virus moves long distance through the xylem in wheat . Protoplasma , 218 : 57 – 66 .
- Verchot-Lubicz , J. 2003 . Soilborne viruses: advances in virus movement, virus induced gene silencing, and engineered resistance . Physiol. Mol. Plant Pathol. , 62 : 55 – 63 .
- Visser , P.B. and Bol , J.F. 1999 . Nonstructural proteins of Tobacco rattle virus which have a role in nematode-transmission: expression pattern and interaction with viral coat protein . J. Gen. Virol. , 80 : 3272 – 3280 .
- Weidemann , H.L. 1981 . Pfropfenkrankheit und Eisenfleckigkeit: Ursachen und Bekämpfungsmöglichkeiten . Der Kartoffelbau , 32 : 111 – 113 .
- Wyss , U. and Seinhorst , J.W. 1975 . “ Feeding of Trichodorus, Longidorus and Xiphinema ” . In Nematode vectors of plant viruses , Edited by: Lamberti , F. and Taylor , C.E. 203 – 221 . Plenum Press : London .
- Xenophontos , S. , Robinson , D.J. , Dale , M.F.B. and Brown , D.J.F. 1998 . Evidence for persistent, symptomless infection of some potato cultivars with Tobacco rattle virus . Pot. Res. , 41 : 255 – 265 .
- Ziegler-Graff , V. , Guilford , P.J. and Baulcombe , D.C. 1991 . Tobacco rattle virus RNA-1 29K gene product potentiates viral movement and also affects symptom induction in tobacco . Virology , 182 : 145 – 155 .