Abstract
The rhizosphere is a rich source of actinobacteria and some members of this bacterial group present strong abilities in the biocontrol of plant diseases. In the present study, 72 strains of actinobacteria were isolated from different rhizospheric semi-arid soils collected in Algeria. Their in vitro antagonistic activity was assayed toward the following plant pathogenic fungi: Verticillium dahliae, Fusarium culmorum, Drechslera teres, Microdochium nivale, Bipolaris sorokiniana, Botrytis fabae and Fusarium oxysporum. All isolates showed chitinolytic activity and six isolates (Lac1, Lac3, Vic8, Pin10, Pru14 and Pru16), which inhibited the growth of five or more of the fungi tested, were selected for further study. According to morphological and physiological characteristics, as well as phylogenetic analysis of 16S rRNA gene sequences, isolates Lac1, Lac3, Pru14 and Pru16 were identified as members of the genus Streptomyces, namely S. griseus, S. rochei, S. anulatus and S. champavatii, respectively. Two isolates, Vic8 and Pin10, were associated with the Nocardiopsis genus and identified as N. dassonvillei subsp. dassonvillei and N. alba, respectively. While antagonism of Streptomyces has been demonstrated against a wide variety of plant pathogens, only a few studies have reported production of antifungal products by the genus Nocardiopsis.
Résumé
La rhizosphère est riche en actinobactéries et certains membres de ce groupe bactérien présentent de fortes aptitudes dans lutte biologique contre des maladies végétales. Dans la présente étude, 72 souches d'actinobactéries ont été isolées de différents sols rhizosphériques échantillonnés dans la région semi-aride de l'Algérie. L'antagonisme de ces isolats a été testé envers les sept champignons phytopathogènes suivants : Verticillium dahliae, Fusarium culmorum, Drechslera teres, Microdochium nivale, Bipolaris sorokiniana, Botrytis fabae et Fusarium oxysporum. Tous les isolats ont montré une activité chitinolytique mais six isolats (Lac1, Lac3, Vic8, Pin10, Pru14 et Pru16), inhibant la croissance d'au moins cinq des sept champignons testés, ont été choisis pour des études plus approfondies. L'étude des caractères morphologiques et physiologiques ainsi que l'analyse phylogénétique des séquences du gène ARNr 16S ont permis d'identifier les isolats Lac1, Lac3, Pru14 et Pru16 en tant que membres du genre Streptomyces, soient S. griseus Lac1, S. rochei Lac3, S. anulatus Pru14 and S. champavatii Pru16, respectivement. Les deux isolats Vic8 et Pin10 ont été classés dans le genre Nocardiopsis et ont été identifiés comme N. dassonvillei subsp. dassonvillei Vic8 et N. alba Pin10, respectivement. Alors que l'antagonisme des Streptomyces est bien connu contre une grande variété d'agents phytopathogènes, peu d'études ont montré la production de produits antifongiques chez le genre Nocardiopsis.
Introduction
Actinobacteria are Gram-positive bacteria characterized by a genome with a high G + C content. Most members of this group are soil-dwelling saprophytic microorganisms (Khamna et al., Citation2009) and are abundant in the rhizosphere (Sardi et al., Citation1992). This environment has been found to be a rich source of biocontrol agents and plant growth promoters (Crawford et al., Citation1993; Jiménez-Esquilin & Roane, Citation2005; Yilmaz et al., Citation2008). For instance, rhizospheric actinobacteria from Morocco (Errakhi et al., Citation2009; Loqman et al., Citation2009), Thailand (Khamna et al., Citation2009), the UK (Crawford et al., Citation1993), Egypt (El-Mehalawy et al., Citation2004), Turkey (Orakci et al., Citation2010), Canada (Valois et al., Citation1996) and the USA (Jiménez-Esquilin & Roane, Citation2005) have been identified as potential biocontrol agents against fungal plant pathogens.
Streptomyces is the largest genus of actinobacteria, comprising 588 species with validly published names. It includes filamentous bacteria, which are well known for their ability to produce antibiotics and lytic enzymes (Rugthaworn et al., Citation2007). The Streptomyces genus has been extensively explored for the selection of biocontrol agents of plant diseases (El-Tarabily & Sivasithamparam, Citation2006) as 75% of biologically active compounds are produced by members of this genus. The modes of action employed by these biocontrol agents are varied and include antibiosis, lysis of fungal pathogens, competition and hyperparasitism (Doumbou et al., Citation2001; Rugthaworn et al., Citation2007).
The Algerian territory is characterized by a Mediterranean ecosystem in the North, the Sahara desert in the South and an intermediate semi-arid region. This semi-arid region is characterized by low annual rainfall (about 320 mm per year) and an elevation of 900 to 1200 m. Average minimum and maximum temperatures are of 4 and 33 °C, respectively (Chibane et al., Citation2010). The search for actinobacteria that produce new antibiotic molecules in Algeria has been conducted in various environments such as lakes, salt pans and Saharan soil (Sabaou et al., Citation1983; Boudemagh et al., Citation2005; Boughachiche et al., Citation2005; Kitouni et al., Citation2005; Reghioua et al., Citation2006).
Several actinobacteria have been isolated from Algerian Saharan soils. Some of them belong to rare genera such as Actinomadura, Nonomuraea, Nocardiopsis, Saccharothrix, Spirillospora and Streptosporangium (Hacéne et al., Citation1998, Citation2000; Badji et al., Citation2005, Citation2007; Zitouni et al., Citation2005). The isolation of some of these strains has led to the characterization of new antimicrobial molecules. For instance, the novel species Saccharothrix algeriensis NRLL B-24137 has been characterized (Zitouni et al., Citation2004b ) and found to produce new dithiolopyrrolone antibiotics (Chorin, Citation2009). Furthermore, some strains of Saccharothrix sp. and Streptosporangium sp. have been shown to produce new antimicrobial molecules termed mutactimycin PR and angucyclinone r2 (Zitouni et al., Citation2004a ; Boudjella et al., Citation2010). The diversity of actinobacteria colonizing semi-arid ecosystems has, however, not been extensively explored, and little information has been reported regarding the antimicrobial potential of actinomycetes isolated from bulk soil, water and salt pan samples (Boughachiche et al., Citation2005; Kitouni et al., Citation2005).
To the best of our knowledge, no research has focused on the isolation of actinomycetes from rhizospheric semi-arid Algerian soils or on their possible activity against phytopathogenic fungi. The aim of the present study was to isolate actinobacteria from different rhizospheric semi-arid Algerian soils and to screen them for in vitro antagonism towards seven plant pathogenic fungi of economic importance in Algeria: Verticillium dahliae Kleb., Fusarium culmorum (W.G. Smith) Sacc., Drechslera teres (Sacc.) Shoemaker, Microdochium nivale (Fr.) Samuels & Hallett, Bipolaris sorokiniana (Sacc.) Shoemaker, Botrytis fabae Sard. and Fusarium oxysporum Schlecht. The selected antagonistic actinobacteria were taxonomically identified using morphological, physiological, biochemical and molecular methods.
Materials and methods
Isolation of rhizospheric actinomycetes
Rhizosphere-associated semi-arid soil was sampled from seven sites in North-Eastern Algeria (). To collect rhizospheric soil from trees (Prunus domestica L., Pinus halepensis Mill.), roots were extricated and 100 g of soil firmly attached to the roots was then collected with a sterile spatula and placed into sterile plastic bags (Yilmaz et al., Citation2008). The root systems of cereals (Triticum durum Desf., Hordeum vulgare L.) and vegetables (Lactuca sativa L., Vicia faba L., Allium cepa L.) were collected and gently scraped and rinsed with sterile distilled water to separate rhizospheric soil (Jiménez-Esquilin & Roane, Citation2005). Samples were diluted in sterile water and plated on yeast casamino-acids-extract dextrose agar (YCED) and water yeast extract agar (WYE) (Crawford et al., Citation1993). Plates were incubated at 25 °C for 14 days. Actinobacteria colonies were picked and purified by serial subcultures. Spores were collected using glass beads rolled over sporulating colonies. Beads were then washed with 20% (v/v) glycerol and spore suspensions were kept at −20 °C (Jiménez-Esquilin & Roane, Citation2005).
Table 1. Number of actinobacteria isolated from various Algerian rhizospheric soil
Screening for antagonistic activity against phytopathogenic fungi
Phytopathogenic fungi used in this study are listed in . These fungi were obtained from the Institut National de la Recherche Agronomique d'Algérie (INRAA, Constantine, Algeria). The fungi were grown on potato dextrose agar (PDA) plates at 28 °C for 4 to 6 days. Stock cultures of fungi were maintained on PDA slants and stored at 4 °C.
Table 2. Fungal plant pathogens used in antagonism assays
Antagonistic activity of actinobacteria isolates against the above-mentioned fungal plant pathogens was evaluated according to Soares et al. (Citation2006) with modifications. Each actinomycetes isolate was streaked as a straight line (c. 7 mm wide) across the centre of PDA plates (145 mm diameter) and incubated at 28 °C for 5 days. Two 7-mm diameter discs from an 8-day-old fungal culture were transferred at a distance of 1.5 cm from the edge of both halves of the plates. Plates were then incubated at 28 °C for 5 days and fungal growth was assessed by measuring mycelial development from the disk to the edge of the mycelium, perpendicularly to the bacterial line. Control treatments were prepared similarly without actinomycetes.
Morphological identification of antagonistic isolates
Antagonistic isolates of actinomycetes were grown on yeast-malt extract agar (ISP2), oatmeal agar (ISP3), inorganic salts-starch agar (ISP4) and glycerol-asparagine agar (ISP5) as described by Shirling & Gottlieb (Citation1966). These cultures were examined for pigmentation and spore mass. Colour was visually estimated by comparing the culture with chips from ISSC-NBS colour charts (Kelly, Citation1964). Morphology of spore-bearing hyphae was determined on ISP4 medium (Shirling & Gottlieb, Citation1966) using the cover-slip method (Williams et al., Citation1983b ). Spore surface ornamentation was determined by scanning electron microscopy, from 14- or 22-day-old cultures on yeast-malt extract agar as follows. Spores' mass harvested with aluminium mounts were prefixed and fixed with 1% (w/v) osmium tetroxide (Rueda et al., Citation2001). The samples were then treated with platinum in a Sputter Coater Emitech K550 (Soquelec, Canada) and observed with a scanning electron microscope S-3000N (Hitachi, Canada). Spore-bearing hyphae and spore surface ornamentation were categorized according to the description of Locci (Citation1994) and Williams et al. (Citation1983b ), respectively.
The isomeric form of diaminopimelic acid and the predominant whole-cell sugars were determined following standard procedures described by Becker et al. (Citation1964) and Staneck & Roberts (Citation1974), respectively.
Physiological characterization
Each isolate was examined for the following characteristics: carbon and nitrogen source utilization, melanoid pigment production, degradation activities, enzymatic activities, growth in the presence of inhibitors and growth at different pH and temperatures. A total of 70 characteristics were studied (). Carbon sources were added at a final concentration of 1% (w/v) into ISP9 basal medium (Shirling & Gottlieb, Citation1966 ), except for organic acids which were used at a concentration of 0.1% (w/v). Assimilation of 10 nitrogen sources (added at 0.1%, w/v) was determined by the method of Williams et al. (Citation1983b ). The growth was scored after 14 days by comparing test plates with both negative and positive controls.
Table 3. Morphological and physiological characteristics of the six antagonistic actinobacteria isolates
Peptone-yeast extract-iron agar (ISP6 medium) and tyrosine agar (ISP7 medium) were used to determine melanoid pigment production (Shirling & Gottlieb, Citation1966).
Degradation tests of tyrosine (0.5%), adenine (0.5%), hypoxanthine (0.4%), xanthine (0.4%), xylan (0.4%) and casein (1% skimmed milk) were made according to Williams et al. (Citation1983a ) on modified Bennett's agar (MBA) as the basal medium. Activities were detected after 7, 14 and 21 days (clearing of insoluble compounds around colonies was scored as positive).
Allantoin (0.3%) degradation was studied in tubes; a positive result was scored by a change of the indicator from orange to red or purple. The degradation of arbutin and aesculin (0.1%, w/v) was determined by the method described by Williams et al. (Citation1983a ). Starch (1%) and gelatin (0.4%) degradation were determined on MBA after 7 days by flooding plates with iodine and MgCl2 solution, respectively, and scoring zones of clearing as positive. Degradation of Tween 20 and Tween 80 (1%) was determined using Sierra medium, which was examined for opacity after 3, 7 and 14 days. Urea utilization was tested by using urea broth tubes containing 2% urea.
Chitinolytic activity was detected by the appearance of clear zones around colonies grown on ISP9 medium supplemented with 1% chitin. Nitrate reductase and ß-galactosidase activities were determined as described by Williams et al. (Citation1983b ) and Flores et al. (Citation1990), respectively.
Tolerance to temperature, pH and resistance to chemical inhibitors was tested on MBA as recommended by Williams et al. (Citation1983b ). Growth at pH 4.3 or pH 7 (at a fixed temperature of 25 °C), as well as 4, 10, 25, 37 and 45 °C (at fixed pH 7) was measured after 14 days or 6 weeks. In growth inhibition tests, MBA was supplemented with the following potential inhibitors: phenol (0.1%, w/v), sodium azide (0.01%, w/v), crystal violet (0.0001%, w/v), thallous acetate (0.01%, w/v), potassium tellurite (0.01%, w/v) and tolerance to NaCl (4, 7, 10 and 13%, w/v). Sensitivity to lysozyme (10, 50 and 100 μg mL−1) was studied using the method of Gordon & Barnett (Citation1977). For these tests, the presence or absence of growth was scored after 7 and 14 days.
Sequencing of the 16S rRNA gene
Genomic DNA from selected actinobacteria was isolated according to Pospiech & Neuman (Citation1995). The 16S rRNA gene was amplified using the universal primers F27 [5'AGAGTTTGATCCTGGCTCAG3'] and R1492 [5'TACGGCTACCTTGTTACGACTT3'] (Heuer et al., Citation1997; Monciardini et al., Citation2002). PCR was performed using a Biometra thermal cycler; PCR conditions were 5 min at 95 °C, followed by 35 cycles of 1 min at 95 °C, 1 min at 60 °C and 2 min at 72 °C. PCR products were sequenced by Genome Quebec Innovation Centre (Montreal, Canada). Sequences were deposited in GenBank under the accession numbers HQ184949, HQ184950, HQ184951, HQ184952, HQ184953 and HQ184954 for isolates Lac1, Lac3, Vic8, Pru14, Pru16 and Pin10, respectively. The 16S rDNA sequence of each isolate was analyzed using the BLAST (Basic Local Alignment Search Tool) program from Genbank database (http://www.ncbi.nlm.gov/BLAST). DNA sequences were aligned using CLUTALW2 (Larkin et al., Citation2007). A tree was constructed with the MEGA-4 software package (Tamura et al., Citation2007) using a neighbour-joining method (Saitou & Nei, Citation1987) with Kimura 2 parameter model (Kimura, Citation1980). Bootstrap analysis was performed using 1000 resamplings, the root position of the neighbour-joining tree was deduced using Bacillus subtilis as an outgroup.
Statistical analysis
The data were analyzed with repeated measures analysis of variance (ANOVA) followed by the least significant difference (LSD) test using SAS 9.1 statistical software (SAS Institute Inc., Cary, NC).
Results and discussion
Isolation and antifungal activity
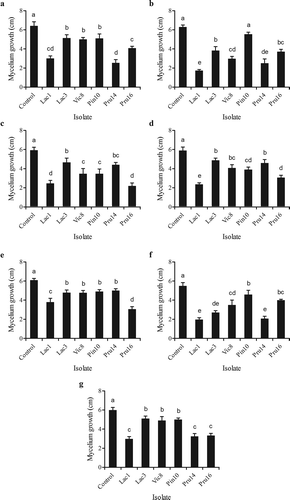
Isolation of actinobacteria was performed from seven rhizosphere samples from vegetables (broad bean, garlic and lettuce), cereals (barley, wheat) and trees (Aleppo pine, plum tree) soils. A total of 72 actinomycetes were isolated, 39 and 33 isolates were respectively purified from YCED and WYE (). Rhizosphere soils from Aleppo pine and plum tree provided the highest number of isolates (). From these 72 actinomycete isolates, two exhibited no antagonistic activity, 22 isolates inhibited the growth of only one of the phytopathogenic fungi and 48 isolates displayed inhibition against at least two fungal species. The highest rates of active isolates were obtained against F. oxysporum (54%), B. sorokiniana (46%) and V. dahliae (33%), while the lowest rates were obtained against F. culmorum (16%) and M. nivale (17%). This high antagonism is not surprising. Sessitsch et al. (Citation2004) found that 43% of potato-associated bacteria exhibited antagonist activities against the pathogenic agent S. scabies. Nevertheless, the proportion of isolates active against V. dahliae was higher in this study than the 8% of actinobacteria isolates from Moroccan rhizospheric soil reported by Barakate et al. (Citation2002). Furthermore, the percentage of isolates active against F. oxysporum was also higher than what was obtained by Kitouni et al. (Citation2005) with non-rhizospheric soil samples from a semi-arid region in North-Eastern Algeria. Further experiments were conducted on the six isolates that inhibited the growth of five or more of fungi tested (Lac1, Lac3, Vic8, Pru16, Pru14 and Pin10). The antagonistic activities of these six isolates against the fungal pathogens are presented in . Isolates Lac1 and Pru16 exhibited the highest antagonistic activities ().
Fig. 1. Effect of antagonistic actinobacterial isolates on mycelium growth of phytopathogenic fungi. Results are the means of triplicates. Values from bars with the same letter do not differ statistically (LSD). (a) Fusarium culmorum, (b) Microdochium nivale, (c) Fusarium oxysporum, (d) Drechslera teres, (e) Verticillium dahliae, (f) Bipolaris sorokiniana and (g) Botrytis fabae.
Genera associated with the antagonistic isolates
All six selected isolates produced mycelium and spores and could thus be associated with actinobacteria. Chemotaxonomy analysis revealed that isolates Lac1, Lac3, Pru14 and Pru16 belonged to the Streptomyces genus. LL-diaminopimelic acid (LL-DAP) and glycine were detected in the whole-cell hydrolysates of these four isolates. These traits are characteristic of the genus Streptomyces (Lechevalier & Lechevalier, Citation1970). Electron microscopy confirmed the classification of these four isolates into the genus Streptomyces. All strains produced spores with a smooth surface that were borne in spiral (S) chains for isolate Lac3, in retinaculum-apertum chains (RA) for isolate Pru14 and rectiflexibiles (RF) chains for isolates Pru16 and Lac1 (). These chain morphologies are typical of the Streptomyces and related genera (Shirling & Gottlieb, Citation1966).
Fig. 2. Spore chain morphology of (a) Streptomyces griseus Lac1, (b) Streptomyces rochei Lac3, (c) Streptomyces anulatus Pru14, (d) Streptomyces champavatii Pru16, (e) Nocardiopsis dassonvillei subsp. dassonvillei Vic8 and (f) Nocardiopsis alba Pin10 observed with scanning electron microscopy. Bars represent 1 μm.
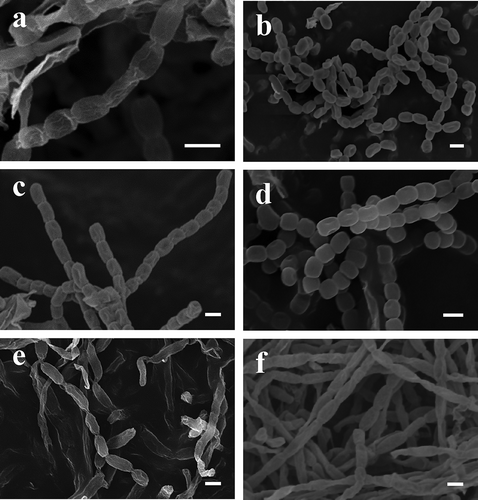
On the other hand, the whole cell hydrolysates of isolates Pin10 and Vic8 contained meso-diaminopimelic acid (meso-DAP). The diagnostic sugars arabinose, madurose and xylose were not detected. According to Yassin et al. (Citation1997), these features suggest a cell wall chemotype III and type C whole cell sugar pattern typical of the genus Nocardiopsis (Meyer, Citation1976). Electron microscopy revealed a smooth spore surface for both strains. Single zigzag hyphae as well as long chains of spores with zigzag forms were observed () and confirm the classification of isolates Pin10 and Vic8 within the Nocardiopsis genus.
Identification of the antagonistic Streptomyces isolates
The sequencing of the 16S rRNA genes and alignment with sequences retrieved from GenBank databases also confirmed that Lac1, Lac3, Pru14 and Pru16 belonged to the Streptomyces genus. A phylogenetic tree was constructed based on the 16S rRNA gene sequences for the six selected isolates and related species (). The phylogenetic dendrogram is divided into distinct clades, one of which includes various Streptomyces species as well as the Streptomyces isolates reported in the present study.
Fig. 3. Phylogenetic tree based on 16S rRNA gene sequences of the six antagonistic strains isolated in this study and their closest relatives. The tree was constructed using the neighbour-joining algorithm. Bootstrap values based on 1000 replicates are shown at the nodes of the tree. The bar indicates a distance of 0.02 substitutions per nucleotide position. Bacillus subtilis was used as an outgroup.
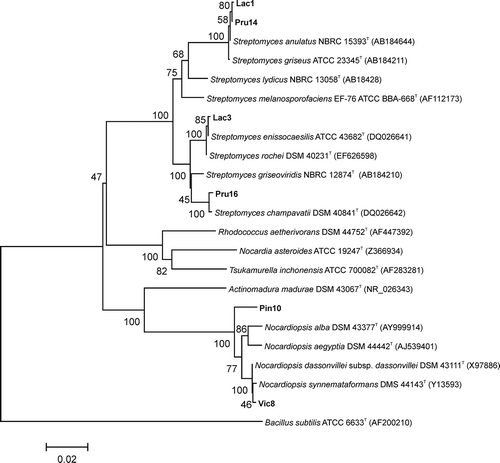
Isolates Pru14 and Lac1 appeared in one cluster with S. anulatus (Beijerinck) Waksman NBRC 15393T and S. griseus (Krainsky) Waksman & Henrici ATCC 23345T as closest neighbours, respectively. Isolate Pru16 formed a phyletic line with S. champavatii Uma & Narasimha Rao DSM 40841T supported by 100% of bootstrap replicates. Isolate Lac3 was phylogenetically related to two species: S. enissocaesilis Sveshnikova ATCC 43682T and S. rochei Berger et al. DSM 40231T.
Several studies have reported the use of physiological and biochemical features to identify Streptomyces species (Faucher et al., Citation1992; Bencheikh & Setti, Citation2007; Khamna et al., Citation2009). Differences in physiological and biochemical features of the selected isolates are represented in . In conjunction with the molecular analysis and the morphological traits, the physiological and biochemical features allowed the placement of each isolate within a species. Physiological characteristics and data reported by Shirling & Gottlieb (Citation1968) and Liu et al. (Citation2005) suggested that Lac1 belongs to S. griseus. Like S. griseus, Lac1 produced a yellow/orange soluble pigment, utilized fructose, galactose, lactose, mannitol, mannose, ribose, xylose and propionate and degraded adenine, Tween 20 and Tween 80.
The characteristics of S. anulatus reported by Shirling & Gottlieb (Citation1972) and Gebhardt et al. (Citation2002) indicate that isolate Pru14 belongs to this species. Like S. anulatus strains, Pru14 did not produce melanoid pigments and utilized glucose, arabinose fructose, mannitol, rhamnose and xylose as carbon sources but not inositol, raffinose or sucrose.
Isolate Pru16 and the type strain of S. champavatii both developed yellow aerial mycelium. They have similar chain-spore morphology (rectiflexibiles) and smooth spore ornamentation. According to data from Locci (Citation1994), both strains could assimilate arabinose, galactose, glucose, mannitol, xylose but not raffinose, rhamnose or sucrose. The two strains differed only in regard to fructose and inositol assimilation. Intraspecific variation within Streptomyces strains has been widely reported (Clayton et al., Citation1995; Anderson & Wellington, Citation2001).
A high degree of 16S rRNA sequence similarity was observed between isolate Lac3, S. enissocaesilis and S. rochei. Nevertheless, Lac3 could be clearly distinguished from S. enissocaesilis as Wink (Citation2001) reported that this species is characterized by a white spore mass, rectiflexibiles spore chains morphology and production of melanoid pigment on ISP7 medium. Comparison with data from Shirling & Gottlieb (Citation1968) confirms that isolate Lac3 should be assigned to S. rochei. They both develop spiral spore-chains, grey spore masses and do not biosynthesize melanoid pigments. In addition, these strains show similar physiological characteristics such as assimilation of glucose, fructose, inositol, mannitol, rhamnose and xylose, and the inability to use sucrose.
The Streptomyces genus has the capacity to produce a wide variety of extracellular enzymes and antibiotics (Trejo-Estrada et al., Citation1998) which may explain their ability to colonize various environments. Antagonism of fungi by Streptomyces has been demonstrated for a wide variety of plant pathogens such as Alternaria (Khamna et al., Citation2009), Rhizoctonia (Chamberlain & Crawford, Citation1999), Verticillium (Berg et al., Citation2000), Fusarium (El-Shanshoury et al., Citation1996) and Macrophomina spp. (Etebarian, Citation2006).
However, few studies have focused on antifungal activity of S. griseus, S. champavatii, S. rochei and S. anulatus against V. dahliae, F. culmorum, D. teres, M. nivale, B. sorokiniana, B. fabae and F. oxysporum. Nevertheless, production of the antifungal molecules endophenazines A-D by S. anulatus has been reported (Gebhardt et al., Citation2002). S. champavatii is known to produce polyenic and non-polyenic antifungal antibiotics called champamycin A, champamycin B and champavatin (Rao & Narasimha Rao, Citation1967). S. rochei has been shown to produce 1-propanone, 1-(4-chlorophenyl) involved in Phytophthora biocontrol (Ezziyyani et al., Citation2007). S. griseus is a producer of several antifungal antibiotics, such as actidione (Whiffen et al., Citation1946), candicidin (Campelo & Gil, Citation2002) and bafilomycins (Werner & Hagenmaier, Citation1984). Tu (Citation1988) has also reported the use of a S. griseus strain to control the phytopathogen Colletotrichum lindemuthianum. In the latter study, the biocontrol efficiency was attributed to antibiosis. Hoster et al. (Citation2005) also reported that a strain of S. griseus controlled the growth of F. culmorum by way of chitinolytic activity. Interestingly strain Lac1, as well as all the actinobacteria selected in this study, were also shown to produce chitinases ().
Identification of antagonistic Nocardiopsis isolates
The dendrogram presented in shows that both isolates Vic8 and Pin10 clustered with Actinomadura madurea DSM 43067T and Nocardiopsis species. This cluster, which is supported by 100% bootstrap replicates, is composed of isolate Pin10, N. alba corrig. Grund & Kroppenstedt DSM 43377T, N. aegyptia Sabry et al. DSM 44442T and isolate Vic8 with its close neighbours N. synnemataformans Yassin et al. DSM 44143T and N. dassonvillei subsp. dassonvillei (Brocq-Rousseau) Meyer DSM 43111T.
By comparing the data of our phenotypical analysis to those of Yassin et al. (Citation1997), isolate Vic8 should be assigned to N. dassonvillei subsp. dassonvillei rather than N. synnemataformans due to differences in colour spore mass and the absence of synnema in N. synnemataformans (). According to Hamedi et al. (Citation2010) and Yassin et al. (Citation1997), N. dassonvillei subsp. dassonvillei like isolate Vic8 utilized galactose, rhamnose, threhalose, xylose, gluconate, propionate, alanine and proline but not fructose, inositol, lactose, maltose, mannose, melibiose, raffinose, mannitol or adonitol. They also showed nitrate reductase and urease activities.
Among the Nocardiopsis clade, the nearest species to isolate Pin10 was N. alba. The comparison of characteristics, based on data from Hamedi et al. (Citation2010) and Yassin et al. (Citation1997), showed that isolate Pin10 developed a white aerial mycelium like N. alba. It degraded adenine and xanthine and was unable to produce melanin pigments or to grow at either 10 or 45 °C. Pin10 could not utilize arabinose, melibiose, fructose, lactose, raffinose, xylose, rhamnose, mannose, mannitol and adonitol but was able to assimilate glycerol, sucrose, threhalose, maltose, glutamate, propionate and proline.
There are a few papers reporting the production of antifungal products by Nocardiopsis dassonvillei. Ali et al. (Citation2009) reported the production of WA52-A, a macrolide antibiotic, by N. dassonvillei while Schumacher et al. (Citation2001) reported the isolation of two indole nucleoside kahakamides from N. dassonvillei. Furthermore, Sabaou et al. (Citation1983) reported a strain of N. dassonvillei showing antibiotic, mycolytic and parasitic activities against the vegetative hyphae of Fusarium oxysporum f. sp. albedinis. Few potentially bioactive N. alba strains have been reported. To our knowledge, no study has reported antibiotic production by N. alba. However, production of a lipopeptide biosurfactant by marine N. alba MSA 10 has been reported (Gandhimathi et al., Citation2009).
The present study reveals the presence of chitinolytic activity in Nocardiopsis strains as reported in N. prasina OPC-131 (Tsujibo et al., Citation2003) and Nocardiopsis sp. strain F96 (Matsui et al., Citation2004). Chitinolytic activity may thus be one of the mechanisms employed by Nocardiopsis isolates to inhibit fungal growth.
Conclusion
In the present investigation, we report the isolation of six rhizospheric actinomycetes with antibiotic properties against seven phytopathogenic fungi. These isolates are of interest for the biocontrol of fungal plant pathogens since they not only produced antifungal molecules but they also all showed chitinolytic activities (Mahadevan & Crawford, Citation1997). Among them, two isolates were affiliated with the Nocardiopsis genus. According to El-Tarabily & Sivasithamparam (Citation2006), Streptomyces spp. have been extensively investigated because of their abundance in soil and because their antibiotic production is of commercial importance. Commercial biocontrol products containing live Streptomyces cells as active ingredients (Mycostop, Actinovate and Actino-Iron) (Tahvonen & Avikainen, Citation1987; Cross & Polonenko, Citation1996; Crawford et al., Citation2005) are available to farmers. Conversely, biological control of plant pathogens by non-Streptomyces actinomycetes is rarely reported in the literature (El-Tarabily & Sivasithamparam, Citation2006). Members of the Nocardiopsis genus could be promising candidates leading to the development of such products. Further studies are, however, necessary to complete this preliminary work and evaluate the efficiency of these potential biocontrol agents.
Acknowledgements
We are grateful to Dr. Abdelhafid Hamidechi (University of Constantine) for his assistance in the construction of the phylogenetic tree. We also thank Sandra Allen and Isabelle Madore (Université de Sherbrooke) for technical assistance.
References
- Ali , M.I. , Ahmed , M.S. and Hozzein , W.N. 2009 . WA52-A macrolide antibiotic produced by alkalophile Nocardiopsis dassonvillei WA52 . Aust. J. Basic Appl. Sci , 3 : 607 – 616 .
- Anderson , A.S. and Wellington , E.M.H. 2001 . The taxonomy of Streptomyces and related genera . Int. J. Syst. Evol. Microbiol , 51 : 797 – 814 .
- Badji , B. , Mostefaoui , A. , Sabaou , N. , Lebrihi , A. , Mathieu , F. , Seguin , E. and Tillequin , F. 2007 . Isolation and partial characterization of antimicrobial compounds from a new strain Nonomuraea sp. NM94 . J. Ind. Microbiol. Biotechnol , 34 : 403 – 412 .
- Badji , B. , Riba , A. , Mathieu , F. , Lebrihi , A. and Sabaou , N. 2005 . Antifungal activity of a saharan Actinomadura strain against various pathogenic and toxinogenic fungi . J. Med. Mycol , 15 : 211 – 219 .
- Barakate , M. , Ouhdouch , Y. , Oufdou , K. and Beaulieu , C. 2002 . Characterization of rhizospheric soil streptomycetes from Moroccan habitats and their antimicrobial activities . World J. Microbiol. Biotechnol , 18 : 49 – 54 .
- Becker , B. , Lechevalier , M.P. , Gordon , R.E. and Lechevalier , H.A. 1964 . Rapid differentiation between Nocardia and Streptomyces by paper chromatography of whole cell hydrolysates . Appl. Microbiol , 12 : 421 – 423 .
- Bencheikh , M. and Setti , B. 2007 . Characterization of Streptomyces scabies isolated from common scab lesions on potato tubers by morphological, biochemical and pathogenicity tests in Chlef region in western Algeria . Sci. & Technol. C , 26 : 61 – 67 .
- Berg , G. , Kurze , S. , Buchner , A. , Wellington , E.M. and Smalla , K. 2000 . Successful strategies for the selection of new strawberry-associated rhizobacteria antagonistic to Verticillium wilt . Can. J. Microbiol , 46 : 1128 – 1137 .
- Boudemagh , A. , Kitouni , M. , Boughachiche , F. , Hamdiken , H. , Oulmi , L. Reghioua , S. 2005 . Isolation and molecular identification of actinomycetes microflora, of some saharian soils of south east Algeria (Biskra, El-Oued and Ourgla) study of antifungal activity of isolated strains . J. Med. Mycol , 15 : 39 – 44 .
- Boudjella , H. , Zitouni , A. , Coppel , Y. , Mathieu , F. , Monje , M.C. , Sabaou , N. and Lebrihi , A. 2010 . Antibiotic r2, a new angucyclinone compound from Streptosporangium sp. Sg3 . J. Antibiot , 63 : 709 – 711 .
- Boughachiche , F. , Reghioua , S. , Zerizer , H. , Oulmi , L. , Boudemagh , A. , Kitouni , M. and Boulahrouf , A. 2005 . Production and preliminary characterization of antibiotics produced by an actinomycetale strain isolated from Ain Mlila Saltpans (Algeria) . Antibiotiques , 7 : 234 – 238 .
- Campelo , A.B. and Gil , J.A. 2002 . The candicidin gene cluster from Streptomyces griseus IMRU 3570 . Microbiology , 148 : 51 – 59 .
- Chamberlain , K. and Crawford , D.L. 1999 . In vitro and in vivo antagonism of pathogenic turfgrass fungi by Streptomyces hygroscopicus strains YCED9 and WYE53 . J. Ind. Microbiol. Biotechnol , 23 : 641 – 646 .
- Chibane , B. , Boutaleb , A. and Lacroix , M. 2010 . Geochemistry study and isotopic approach in semi-arid region: case of the Djelfa syncline (Algeria) . Eur. J. Sci. Res , 45 : 270 – 290 .
- Chorin , A.C. 2009 . Synthèse enzymatique de nouveaux dérivés dithiolopyrrolones par Saccharothrix algeriensis , Toulouse , , France : Ph.D. Thesis, University of Toulouse .
- Clayton , R.A. , Sutton , G. , Hinkle , P.S. Jr , Bult , C. and Fields , C. 1995 . Intraspecific variation in small-subunit rRNA sequences in GenBank: why single sequences may not adequately represent prokaryotic taxa . Int. J. Syst. Bacteriol , 45 : 595 – 599 .
- Crawford , D.L. , Lynch , J.M. , Whipps , J.M. and Ousley , M.A. 1993 . Isolation and characterization of actinomycetes antagonists of a fungal root pathogen . Appl. Environ. Microbiol , 59 : 3899 – 3905 .
- Crawford , D.L. , Kowalski , M. , Roberts , M.A. , Merrell , G. and Deobald , L.A. 2005 . Discovery, development, and commercialization of a microbial antifungal biocontrol agent, Streptomyces lydicus WYEC108: history of a decade long endeavor . Soc. Ind. Microbiol. News , 55 : 88 – 95 .
- Cross , J.V. and Polonenko , D.R. 1996 . An industry perspective on registration and commercialization of biocontrol agents in Canada . Can. J. Plant Pathol , 18 : 446 – 454 .
- Doumbou , C.L. , Salove , M.K. , Crawford , D.L. and Beaulieu , C. 2001 . Actinomycetes, promising tools to control plant diseases and to promote plant growth . Phytoprotection , 82 : 85 – 102 .
- El-Mehalawy , A.A. , Hassanein , N.M. , Khater , H.M. , Karam el Din , E.A. and Youssef , Y.A. 2004 . Influence of maize root colonization by the rhizosphere actinomycetes and yeast fungi on plant growth and on the biological control of late wilt disease . Int. J. Agric. Biol , 6 : 599 – 605 .
- El-Shanshoury , A.R. , Abu El-Sououd , S.M. , Awadalla , O.A. and El-Bandy , N.B. 1996 . Effects of Streptomyces corchorusii, Streptomyces mutabilis, pendimethalin, and metribuzin on the control of bacterial and Fusarium wilt of tomato . Can. J. Bot , 74 : 1016 – 1022 .
- El-Tarabily , K.A. and Sivasithamparam , K. 2006 . Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters . Soil Biol. Biochem , 38 : 1505 – 1520 .
- Errakhi , R. , Lebrihi , A. and Barakate , M. 2009 . In vitro and in vivo antagonism of actinomycetes isolated from Moroccan rhizospherical soils against Sclerotium rolfsii: a causal agent of root rot on sugar beet (Beta vulgaris L.) . J. Appl. Microbiol , 107 : 672 – 681 .
- Etebarian , H.R. 2006 . Evaluation of Streptomyces strains for biological control of charcoal stem rot of melon caused by Macrophomina phaseolina . Plant Pathol. J , 5 : 83 – 87 .
- Ezziyyani , M. , Requena , M.E. , Egea-Gilabert , C. and Candela , M.E. 2007 . Biological control of Phytophthora root rot of pepper using Trichoderma harzianum and Streptomyces rochei in combination . J. Phytopathol , 155 : 342 – 349 .
- Faucher , E. , Savard , T. and Beaulieu , C. 1992 . Characterization of actinomycetes isolated from common scab lesions on potato tubers . Can. J. Plant Pathol , 14 : 197 – 202 .
- Flores , M. , Ford , E.G. and Janda , J.M. 1990 . Value of the O-nitrophenyl-D-galactopyranoside test to differentiate among the aerobic actinomycetes . J. Clin. Microbiol , 28 : 2142 – 2144 .
- Gandhimathi , R. , Kiran , G.S. , Hema , T.A. , Selvin , J. , Raviji , T.R. and Shanmugapriya , S. 2009 . Production and characterization of lipopeptide biosurfactant by a sponge-associated marine actinomycetes Nocardiopsis alba MSA 10 . Bioprocess. Biosyst. Eng , 32 : 825 – 835 .
- Gebhardt , K. , Schimana , J. , Krastel , P. , Dettner , K. , Rheinheimer , J. , Zeeck , A. and Fiedler , H.P. 2002 . Endophenazines A to D, new phenazine antibiotics from the arthropod associated endosymbiont Streptomyces anulatus. I. Taxonomy, fermentation, isolation and biological activities . J. Antibiot , 55 : 794 – 800 .
- Gordon , R.E. and Barnett , D.A. 1977 . Resistance to rifampicin and lysozyme of strains of some species of Mycobacterium and Nocardia as a taxonomic tool . Int. J. Syst. Bacteriol , 27 : 176 – 178 .
- Hacène , H. , Boudjellal , F. and Lefebvre , G. 1998 . AH7, a non-polyenic antifungal antibiotic produced by a new strain of Streptosporangium roseum . Microbios , 96 : 103 – 109 .
- Hacène , H. , Daoudi-Hamdad , F. , Bhatnagar , T. , Baratti , J.C. and Lefebvre , G. 2000 . H107, a new aminoglycoside anti-Pseudomonas antibiotic produced by a new strain of Spirillospora . Microbios , 102 : 69 – 77 .
- Hamedi , J. , Mohammadipanah , F. , von Jan , M. , Pötter , G. , Schumann , P. , Spröer , C. Klenk , H.P. 2010 . Nocardiopsis sinuspersici sp. nov., isolated from sandy rhizospheric soil . Int. J. Syst. Evol. Microbiol , 60 : 2346 – 2352 .
- Heuer , H. , Krsek , M. , Baker , P. , Smalla , K. and Wellington , E.M. 1997 . Analysis of actinomycetes communities by specific amplification of genes encoding 16S rRNA and gel electrophoretic separation in denaturing gradients . Appl. Environ. Microbiol , 63 : 3233 – 3241 .
- Hoster , F. , Schmitz , J.E. and Daniel , R. 2005 . Enrichment of chitinolytic microorganisms: isolation and characterization of a chitinase exhibiting antifungal activity against phytopathogenic fungi from a novel Streptomyces strain . Appl. Microbiol. Biotechnol , 66 : 434 – 442 .
- Jiménez-Esquilin , T.M. and Roane , A.E. 2005 . Antifungal activities of actinomycetes strains associated with high altitude sagebrush rhizosphere . J. Ind. Microbiol. Biotechnol , 32 : 378 – 381 .
- Kelly , K.L. 1964 . Inter-society Color Council – National Bureau of Standards Color Name Charts Illustrated with Centroid Colors , Washington , DC : US Government Printing Office .
- Khamna , S. , Yokota , A. and Lumyong , S. 2009 . Actinomycetes isolated from medicinal plant rhizosphere soils: diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production . World J. Microbiol. Biotechnol , 25 : 649 – 655 .
- Kimura , M. 1980 . A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences . J. Mol. Evol , 16 : 111 – 120 .
- Kitouni , M. , Boudemagh , A. , Oulmi , L. , Reghioua , S. , Boughachiche , F. Zerizer , H. 2005 . Isolation of actinomycetes producing bioactive substances from water, soil and tree bark samples of the north-east of Algeria . J. Med. Mycol , 15 : 45 – 51 .
- Larkin , M.A. , Blackshields , G. , Brown , N.P. , Cenna , R. , Mc Gettigan , P.A. Mc William , H. 2007 . Clustal W and Clustal X version 2.0 . Bioinformatics , 23 : 2947 – 2948 .
- Lechevalier , M.P. and Lechevalier , H. 1970 . Chemical composition as a criterion in the classification of aerobic actinomycetes . Int. J. Syst. Bacteriol , 20 : 435 – 443 .
- Liu , Z. , Shi , Y. , Zhang , Y. , Zhou , Z. , Lu , Z. Li , W. 2005 . Classification of Streptomyces griseus (Krainsky 1914) Waksman and Henrici 1948 and related species and the transfer of Microstreptospora cinerea to the genus Streptomyces as Streptomyces yanii sp. nov . Int. J. Syst. Evol. Microbiol , 55 : 1605 – 1610 .
- Locci , R. 1994 . “ Streptomyces and related genera ” . In Bergey's Manual of Systematic Bacteriology , Edited by: Williams , S.T. , Sharpe , M.E. and Holt , J.G. Vol. 4 , 2451 – 2492 . Baltimore , MD : Williams and Wilkins .
- Loqman , S. , Ait Barka , E. , Clément , C. and Ouhdouch , Y. 2009 . Antagonistic actinomycetes from Moroccan soil to control the grapevine gray mold . World J. Microbiol. Biotechnol , 25 : 81 – 91 .
- Mahadevan , B. and Crawford , D.L. 1997 . Properties of the chitinase of the antifungal biocontrol agent Streptomyces lydicus WYEC108 . Enzyme Microb. Tech , 20 : 489 – 493 .
- Matsui , T. , Kumasaka , T. , Endo , K. , Sato , T. , Nakamura , S. and Tanaka , N. 2004 . Crystallization and preliminary X-ray crystallographic analysis of chitinase F1 (ChiF1) from the alkaliphilic Nocardiopsis sp. strain F96 . Acta Christ. D , 60 : 2016 – 2018 .
- Meyer , J. 1976 . Nocardiopsis, a new genus of the order Actinomycetales . Int. J. Syst. Evol. Microbiol , 26 : 487 – 493 .
- Monciardini , P. , Sosio , M. , Cavaletti , L. , Chiocchini , C. and Donadio , S. 2002 . New PCR primers for the selective amplification of 16S rDNA from different groups of actinomycetes . FEMS Microbiol. Ecol , 42 : 419 – 429 .
- Orakci , G.E. , Yamaç , M. , Amoroso , M.J. and Cuozzo , S.A. 2010 . Selection of antagonistic actinomycete isolates as biocontrol agents against root-rot fungi . Fresenius Environ. Bull , 19 : 417 – 424 .
- Pospiech , A. and Neuman , B. 1995 . A versatile quick-prep of genomic DNA from gram-positive bacteria . Trends Genet , 11 : 217 – 218 .
- Rao , U.K. and Narasimha Rao , P.L. 1967 . Actinomycetes. II. Purification and pharmacological properties of champamycin-B from Streptomyces champavatii . Ind. J. Exp. Biol , 5 : 39 – 42 .
- Reghioua , S. , Boughachiche , F. , Zerizer , H. , Oulmi , L. , Kitouni , M. , Boudemagh , A. and Boulahrouf , A. 2006 . Antibacterial activity of rare actinomycetes isolated from arid soil samples of the south-east of Algeria . Antibiotiques , 8 : 147 – 152 .
- Rueda , B. , Miguélez , E.M. , Hardisson , C. and Manzanal , M.B. 2001 . Mycelial differentiation and spore formation by Streptomyces brasiliensis in submerged culture . Can. J. Microbiol , 47 : 1042 – 1047 .
- Rugthaworn , P. , Dilokkunanant , U. , Sangchote , S. , Piadang , N. and Kitpreechavanich , V. 2007 . Search and improvement of actinomycete strains for biological control of plant pathogens . Kasetsart J. (Nat. Sci.) , 41 : 248 – 254 .
- Sabaou , N. , Bounaga , N. and Bounaga , D. 1983 . Actions antibiotique, mycolytique et parasitaire de deux actinomycètes envers Fusarium oxysporum f. sp. albedinis et autres formae speciales . Can. J. Microbiol , 29 : 194 – 199 .
- Saitou , N. and Nei , M. 1987 . The neighbour-joining method: a new method for reconstructing phylogenetic trees . Mol. Biol. Evol , 63 : 406 – 425 .
- Sardi , P. , Saracchi , M. , Petrolini , B. , Borgonovi , G.E. and Merli , S. 1992 . Isolation of endophytic Streptomyces strains from surface-sterilized roots . Appl. Environ. Microbiol , 58 : 2691 – 2693 .
- Schumacher , R.W. , Harrigan , B.L. and Davidson , B.S. 2001 . Kahakamides A and B, new neosidomycin metabolites from a marine-derived actinomycetes . Tetrahedron Lett , 42 : 5133 – 5135 .
- Sessitsch , A. , Reiter , B. and Berg , G. 2004 . Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities . Can. J. Microbiol , 50 : 239 – 249 .
- Shirling , E.B. and Gottlieb , D. 1966 . Methods for characterization of Streptomyces species . Int. J. Syst. Bacteriol , 16 : 313 – 340 .
- Shirling , E.B. and Gottlieb , D. 1968 . Cooperative description of type cultures of Streptomyces III. Additional species descriptions from first and second studies . Int. J. Syst. Bacteriol , 18 : 279 – 392 .
- Shirling , E.B. and Gottlieb , D. 1972 . Cooperative description of type strains of Streptomyces V. Additional descriptions . Int. J. Syst. Bacteriol , 22 : 265 – 394 .
- Soares , A.C.F. , Sousa , C.D.S , Garrido , M.D.S , Perez , J.O. and Santos de Almeida , N. 2006 . Soil Streptomycetes with in vitro activity against the yam pathogens Curvularia eragrostides and Colletotrichum gloeosporioides . Braz. J. Microbiol , 37 : 456 – 461 .
- Staneck , J.L. and Roberts , G.D. 1974 . Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography . Appl. Microbiol , 28 : 226 – 231 .
- Tahvonen , R.T. and Avikainen , H. 1987 . The biological control of seedborne Alternaria brassicicola of cruciferous plants with a powdery preparation of Streptomyces sp . J. Agr. Sci. Finland , 59 : 199 – 208 .
- Tamura , K. , Dudby , J. , Nei , M. and Kumar , S. 2007 . MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0 . Mol. Biol. Evol , 24 : 1596 – 1599 .
- Trejo-Estrada , S.R. , Paszczynski , A. and Crawford , D.L. 1998 . Antibiotics and enzymes produced by the biocontrol agent Streptomyces violaceusniger YCED-9 . J. Ind. Microbiol. Biotechnol , 21 : 81 – 90 .
- Tsujibo , H. , Kubota , T. , Yamamoto , M. , Miyamoto , K. and Inamori , Y. 2003 . Characterization of chitinase genes from an alkaliphilic actinomycete, Nocardiopsis prasina OPC-131 . Appl. Environ. Microbiol , 69 : 894 – 900 .
- Tu , J. C. 1988 . Antibiosis of Streptomyces griseus against Colletotrichum lindemuthianum . J. Phytopathol , 121 : 97 – 102 .
- Valois , D. , Fayad , K. , Barasubiye , T. , Garon , M. , Déry , C. , Brzezinski , R. and Beaulieu , C. 1996 . Glucanolytic actinomycetes antagonistic to Phytophthora fragariae var. rubi, the causal agent of raspberry root rot . Appl. Environ. Microbiol , 62 : 1630 – 1635 .
- Werner , G. , Hagenmaier , H. , Drautz , H. , Baumgartner , A. and Zähner , H. 1984 . Metabolic products of microorganisms. 224. Bafilomycins, a new group of macrolide antibiotics. Production, isolation, chemical structure and biological activity . J. Antibiot , 2 : 110 – 117 .
- Whiffen , A. , Bohonqs , J.N. and Emerson , R.L. 1946 . The production of an antifungal antibiotic by Streptomyces griseus . J. Bact , 52 : 610 – 611 .
- Williams , S.T. , Goodfellow , M. , Alderson , G. , Wellington , E.M.H. , Sneath , P.H.A. and Sackin , M.J. 1983a . Numerical classification of Streptomyces and related genera . J. Gen. Microbiol , 129 : 1743 – 1813 .
- Williams , S.T. , Goodfellow , M. , Wellington , E.M.H. , Vickers , J.C , Alderson , G. Sneath , P.H.A. 1983b . A probability matrix for identification of some streptomycetes . J. Gen. Microbiol , 129 : 1815 – 1830 .
- Wink, J.M. 2001. Compendium of Actinobacteria [online]. http://www.gbif-prokarya.de/microorganisms/wink_pdf/DSM41454.pdf (http://www.gbif-prokarya.de/microorganisms/wink_pdf/DSM41454.pdf)
- Yassin , A.F. , Rainey , F.A. , Burghardt , J. , Gierth , D. , Un-gerechts , J. Lux , I. 1997 . Description of Nocardiopsis synnematafomans sp. nov., elevation of Nocardiopsis alba subsp. prasina to Nocardiopsis prasina comb. nov., and designation of Nocardiopsis antarctica and Nocardiopsis alborubida as later subjective synonyms of Nocardiopsis dassonvillei . Int. J. Syst. Bacteriol , 47 : 983 – 988 .
- Yilmaz , E.I. , Yavuz , M. and Kizil , M. 2008 . Molecular characterization of rhizospheric soil streptomycetes isolated from indigenous plants and their antimicrobial activity . World J. Microbiol. Biotechnol , 24 : 1461 – 1470 .
- Zitouni , A. , Boudjella , H. , Lamari , L. , Badji , B , Mathieu , F. , Lebrihi , A. and Sabaou , N. 2005 . Nocardiopsis and Saccharothrix genera in Saharan soils in Algeria: isolation, biological activities and partial characterization of antibiotics . Res. Microbiol , 156 : 984 – 993 .
- Zitouni , A. , Boudjella , H. , Mathieu , F. , Sabaou , N. and Lebrihi , A. 2004a . Mutactimycin PR, a new anthracycline antibiotic from Saccharothrix sp. SA 103, I. Taxonomy, fermentation, isolation and biological activities . J. Antibiot , 57 : 367 – 372 .
- Zitouni , A. , Lamari , L. , Boudjella , H. , Badji , B. , Sabaou , N. Gaouar , A. 2004b . Saccharothrix algeriensis sp. nov., isolated from Saharan soil . Int. J. Syst. Evol. Microbiol , 54 : 1377 – 1381 .