Abstract
An indigenous strain of Cucumber mosaic virus (CMV) associated with a naturally occurring benign viral satellite RNA (345 bp long), referred to as CMV-KU1, although effective as a protective biocontrol agent against the damaging effects of the virulent CMV-16 strain, produced negative side-effects such as mild stunting, vigour reduction and about 20% yield loss in tomato (Solanum lycopersicon L.) plants. The efficacy of using a mixture of two plant growth-promoting rhizobacteria (PGPR) strains, Pseudomonas aeruginosa and Stenotrophomonas rhizophilia, to compensate for vegetative and yield loss caused by CMV-KU1 in tomato plants, was evaluated in greenhouse experiments. In addition to promoting plant growth, PGPRs are known to enhance systemic defences in plants against foliar pathogens such as viruses that attack tissues distant to PGPR sphere of activity. The use of PGPR and CMV-KU1 together successfully promoted vegetative growth and fruit yield in tomato plants to values equivalent to that of the healthy controls. The combination used also enhanced overall protection of the plants against the severe CMV-16 virus with about 91.3% disease prevention. Serological analysis using enzyme-linked immunosorbent assay (ELISA) also indicated a lower incidence of CMV-16 infection in protected test plants.
Résumé
Une souche indigène du virus de la mosaïque du concombre (CMV), associée à un ARN satellite naturel bénin (long de 345 bp), appelé CMV-KU1, bien qu'efficace à titre d'agent de protection intégrée contre les dommages infligés par la souche virulente CMV-16, a produit des effets secondaires néfastes telles une légère atrophie, une perte de vigueur et une diminution de rendement d'environ 20 % chez la tomate (Solanum lycopersicon L.). Nous avons évalué, en serre, la pertinence d'utiliser un mélange de deux souches de rhizobactéries bénéfiques à la croissance des plantes (RBCP), Pseudomonas aeruginosa et Stenotrophomonas rhizophilia, pour compenser les pertes occasionnées par CMV-KU1 relativement à la croissance et au rendement chez la tomate. En plus de promouvoir la croissance des plantes, les RBCP ont le potentiel de renforcer leurs défenses systémiques contre les agents pathogènes foliaires comme les virus qui attaquent les tissus situés à l'extérieur de la sphère d'influence des RBCP. L'utilisation conjointe de RBCP et de CMV-KU1 a favorisé la croissance végétative et le rendement des plants chez la tomate dans des proportions équivalant à celles des plants témoins sains. La combinaison utilisée a également renforcé, de façon générale, la protection des plants contre les attaques graves de CMV-16 en leur conférant un taux de résistance à la maladie d'environ 91,3 %. L'analyse sérologique fondée sur le test ELISA a également indiqué, chez les plans témoins protégés, un plus faible taux d'infection par CMV-16.
Introduction
Viral diseases pose a serious threat to crop plants, including tomato (Solanum lycopersicon L.). Cucumber mosaic virus (CMV), belonging to the genus Cucumovirus of the Bromoviridae family, is considered to be one of the most economically damaging viruses among field-grown vegetables worldwide (Tomlinson, Citation1987; Roossinck et al., Citation1997; Montasser et al., Citation2006a ). CMV causes systemic mosaic, yellowing, ringspots, deformed fruit and poor fruit set of tomatoes. Some strains of CMV contain viral satellite RNAs while others, such as CMV-16, is a satellite RNA-free strain which causes severe stunting, chlorosis, and malformation of fruits in tomato plants (Sayama et al., Citation1993; Gallitelli et al., Citation1991). Viral disease management strategies employed can include the use of non-pathogenic microorganisms or naturally occurring viral satellites (Montasser et al., Citation1991, Citation2006b ) that can be used as a biological control agent against such viral infections. These control agents act by enhancing the systemic resistance or acquired resistance of the plants against viruses (Murphy et al., Citation2000).
Satellite RNAs are capable of altering the viral phenotype to such an extent that they can modulate − attenuate or exacerbate − the symptoms caused by their cognate helper viruses (Hu et al., Citation2009). Satellite RNAs are small nucleic acids whose nucleotide sequences are unrelated to, but are dependent upon, the viral genome for replication, encapsidation and dispersion; they have a molecular parasitic relationship (Roossinck et al., Citation1992; Xu et al., Citation2000; Montasser et al., Citation1998). According to Kaper & Waterworth (Citation1977), the satellite RNA of CMV which was designated as CMV-associated RNA 5 (CARNA 5) or satRNA (Francki, 1985) modulated the symptom expression of its helper CMV. The viral strain contains a low molecular weight viral satellite RNA associated with its own tripartite genomic RNA and a fourth subgenomic RNA. This viral satellite RNA parasitizes the viral genome at the molecular level by competing with the virulent virus for the host plant enzymatic machinery and replicative enzymes which are essential for virus multiplication and causing infection. This serves as the conceptual basis for experiments on biological control of CMV (Montasser et al., Citation2006b ). Strain KU1 is a mild strain associated with a benign satellite RNA (345 bp long) that induces mosaic symptoms on squash leaves. In tobacco plants, strain KU1 induced mild mosaic on very young leaves but later the plants were symptomless. It is symptomless on tomato (Montasser et al., Citation1991) and its presence in these plants can be detected only by a slight decrease in vegetative growth and a significant yield loss of about 15–20% (Montasser et al., Citation1991, Citation2006b ).
Plant growth-promoting bacteria (PGPR) (Dashti et al., Citation2007), such as nitrogen-fixing rhizobacteria that colonize plant rhizospheres, have been studied for their beneficial role in promoting plant growth (Dashti et al., Citation2000 a; 2000b). The ability to enhance plant growth is limited to specific bacteria and is dependent on: (a) their genetic traits such as motility; (b) chemotaxis to seed and root exudates; (c) production of pili and fimbriae; (d) production of specific cell surface components; (e) ability to use certain cell surface components of root exudates, protein secretion; and (f) quorum sensing (Nelson, Citation2004). The mechanisms of PGPR to promote plant growth are not fully understood, but are thought to influence the plants both directly and indirectly. The direct effect of the PGPRs is the promotion of plant growth and is most often observed in the absence of plant pathogens and other competing soil microbes. Indirectly, PGPRs play a vital role in plant protection against plant pathogens such as viruses and certain fungi (Nelson, Citation2004). The mechanisms by which they enhance protection include: (i) the ability to produce or change the concentration of the plant hormones, such as indoleacetic acid, gibberellic acid, cytokinins and ethylene (Joseph et al., Citation2007); (ii) asymbiotic N2 fixation (Meunchang et al., Citation2006); (iii) antagonism against phytopathogenic microorganisms by production of siderophores that chelate iron (Nelson, Citation2004); production of ß-1,3-glucanase, chitinase, antibiotics and cyanide (Nelson, Citation2004); and (iv) solubilization of mineral phosphates and other nutrients (Chabot et al., Citation1996).
The PGPRs used in this work (Pseudomonas aeruginosa and Stenotrophomonas rhizophilia) are well known for their ability to promote plant growth both individually and in association with one another. Pseudomonas is a diverse group of Gram-negative, aerobic heterotrophic bacteria found in soil; some are aquatic, some can be found in animals. Individual Pseudomonas strains may have biocontrol activity, plant growth-promoting activity, the ability to induce systemic plant defence responses or the ability to act as pathogens (Adesemoye & Ugoji, Citation2009). Many fluorescent Pseudomonas strains (e.g. P. aeruginosa) which colonize the rhizosphere, exert a protective effect on the roots through the production of in situ antibiotic compounds that promote growth and prevent microbial infections (Adesemoye & Ugoji, Citation2009; De Meyer et al., Citation1999). Stenotrophomonas species, belonging phylogenetically to γ-Proteobacteria, have an important ecological role in the elemental cycle of nature, degradation of xenobiotic compounds, promotion of plant growth and as a biocontrol agent against certain pathogenic fungi (Wolf et al., Citation2002). Stenotrophomonas rhizophilia is a xylose utilizing, non-lipolytic, non β-glucosidase-producing Stenotrophomonas species that is capable of growth even at low temperatures (4 °C) (Berg et al., Citation1996). These properties offer a great advantage for symbiotic association with plants. Stenotrophomonas rhizophilia is also known to have remarkable antifungal activity against plant-pathogenic fungi (Wolf et al., Citation2002). This ability to reduce disease and hence promote plant growth is largely due to the ability of Stenotrophomonas species to produce siderophores for iron chelation, antibiosis and production of lytic enzymes (Berg et al., Citation1996). Stenotrophomonas rhizophila is able to colonize various plant tissues in tomato, sweet pepper, cotton and oilseed rape. In general, the population establishment is higher on the roots and stems than on the leaves (Wolf et al., Citation2002). Stenotrophomonas rhizophila has been observed as an endophyte of tomato root hairs. The plant growth-promoting effect of S. rhizophila is mostly via the suppression of pathogens and deleterious microbes, which could lead to a better growth environment for the plant (Schmidt et al., Citation2010).
The objectives of this study were to: (i) investigate the efficacy of applying PGPRs combined with CMV-KU1 associated with viral satellite RNA to enhance plant protection; and (ii) to investigate the effectiveness of PGPRs in compensating for vigour loss caused by CMV-KU1 associated with viral satellite RNA.
Materials and methods
Virus source, maintenance and inoculum preparation
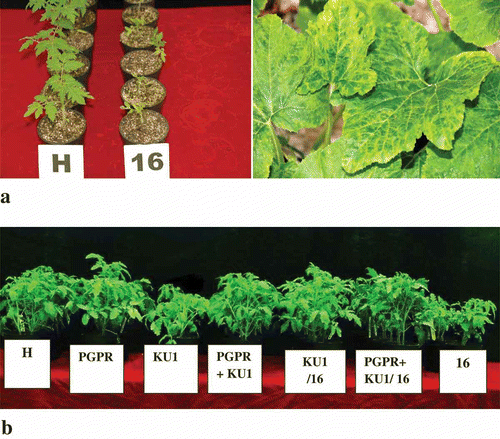
Cucumber mosaic viral strain associated with a benign viral satellite RNA (CMV-KU1) was isolated in Kuwait (Montasser et al., Citation2006b ). This virus is symptomless in tomato. Strain CMV-16, subgroup II, is a Japanese isolate from tomato (kindly provided by H. Sayama, Kikko Foods Corporation, Japan) (Sayama et al., Citation1993) that contains no detectable viral satellite when maintained in tomato, but causes severe stunting and fruit malformation. This virus was used as a challenge strain. Strains were revived from leaves of old desiccated samples that were available in our Molecular Virology Laboratory, University of Kuwait. The viral isolates were invigorated by mechanical passage into fresh squash (Cucurbita pepo L.) and tomato (Solanum lycopersicon L.) plants (, b). The infected leaves were ground in neutral 0.01 M potassium phosphate buffer with a mortar and pestle and the crude sap was used to inoculate the tomato test plants.
Fig. 1. a, Effect of CMV-16 infection on tomato plants and squash plants, respectively. Infected tomato plants (16) appeared severely stunted compared with healthy (H) controls of the same age. Squash leaves infected with cucumber mosaic virus strain-16 showing severe mosaic symptoms, vein clearing and blistering indicating a severe infection. b, Representative tomato plants from different treatments showing the relative differences in height 7 days after challenge with virulent CMV-16 (from left to right) H: healthy control plants; PGPR: plants treated with PGPR mixture alone; KU1: plants preventatively inoculated with CMV-KU1; PGPR+KU1: plants preventatively inoculated with PGPR mixture and CMV-KU1; KU1/16: plants preventatively inoculated with CMV-KU1 virus and challenged with CMV-16; PGPR+KU1/16: plants inoculated with PGPR mixture and CMV-KU1 and challenged with CMV-16 virus; 16: positive control plants infected only with CMV-16. CMV-16 was inoculated at 21 days post-inoculation (dpi) with protective treatments.
Planting methods
Seeds of tomato cultivar ‘Supermamande’ were surface sterilized in sodium hypochlorite (2% solution containing 4 ml L−1 Tween 20) and then rinsed several times with distilled water (Bhuvaneshwari et al., Citation1980; Dashti et al., Citation2000 b). The seeds were planted by hand into pots washed with sodium hypochlorite solution containing sterilized soilless growth medium. The soilless growth medium used was prepared by mixing peat moss (Plantaflor) with Perlite in a ratio of 3 : 1. Following germination, the seedlings were thinned to one plant per pot to ensure better growth. The plants were allowed to acclimatize within the greenhouse for 48 h after reaching the dicotyledonary stage prior to treatment with the PGPR inoculum.
PGPR source and inoculum preparation
Two strains of PGPR were used in this study: Pseudomonas aeruginosa and Stenotrophomonas rhizophilia. The PGPR strains used were obtained locally from the stock cultures available in our laboratory. Both the strains used were isolated in previous work from the Vicia faba rhizosphere. Diluted rhizosphere soil suspensions were plated on solid Pseudomonas medium and Yeast–mannitol agar for P. aeruginosa and S. rhizophilia, respectively (Radwan et al., Citation2007; Dashti et al., Citation2000 a). These were incubated at 30 °C for 7 days and pure colonies were subcultured. The organisms were identified by the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ), Braunschweig, Germany and by various biochemical tests performed at the Kuwait University (Radwan et al., Citation2005). The inoculum of the two PGPR strains was prepared by culturing them separately in nutrient broth and incubating at 20–25 °C with constant shaking at 125 rpm. When the cultures reached log phase, each of the strains was adjusted with distilled water at A420 to give a cell density of 108 CFU mL−1. Equal volumes (1 : 1) of the two strains were mixed and allowed to stand approximately for half an hour at room temperature without shaking before use.
Greenhouse set-up
Three independent experiments were conducted. Plants were divided into three different treatments: (a) plants treated with satellite virus CMV-KU1 alone (referred to as KU1); (b) plants treated only with PGPR mixture (referred to as PGPR); and (c) plants treated with a combination of CMV-KU1 and the PGPR mixture (referred to as PGPR+KU1). One control treatment without any bacterial or viral inoculation was also included. Treatments were arranged in a randomized complete block design with 20 plants in each treatment. Each of the three treatments and the control treatment were divided into two subgroups, one challenged with CMV-16 and the other one without CMV-16 at 21 days post-inoculation (dpi) with treatments. Both the PGPR strains and the satellite associated CMV-KU1 viruses were applied to the plants at the cotyledonary stage.
Mechanical inoculation and maintenance
The PGPRs were applied to the base of the plants close to the roots to ensure better colonization (Dashti et al., Citation2000 a; Citation2000 b). The volume of PGPR inoculated per plant was 10 mL, each containing a cell density of 108 CFU mL−1. The virus was applied onto the plants by mechanical sap transmission (Montasser et al., Citation1991). The leaves of the tomato plants were dusted with abrasive carborundum and then rubbed with infected leaf sap ground in 0.01 M phosphate buffer using a sterile cotton swab. Plants were maintained under greenhouse conditions with alternating 16 h light and 8 h dark periods. Plants were maintained under greenhouse conditions at a temperature of 25° C with 16 h of daylight and 8 h of dark periods. Fertilization was carried out every alternate day using sterile Hoagland's solution. Perforated pots were used to ensure proper drainage of excess amount of solutions. Twenty-eight days after inoculation, the plants were repotted into bigger pots. Plants were scored for symptoms at 28, 35 and 42 days post-inoculation with the biological treatments.
Evaluation of plant growth characteristics and PGPR levels in the soil
Forty-two days post-inoculation with the different treatments, the plants were harvested by following the procedure described by Radwan et al. (Citation2007). Plants were carefully dislodged from the soil, taking special care not to sever the fine root hairs. All soil that was not part of the rhizosphere (i.e. not attached or close to the roots) was carefully removed by gentle tapping. After washing the roots, the heights, fresh weights and fruit yield were measured. The plants were then placed in paper bags and kept in an oven for 2–3 days for dry weight determination. Approximately 10 g of the soil attached to the roots of each treatment was transferred into 90 mL of sterile distilled water. This was shaken for 10–20 min after which 1 mL aliquot from this mixture was taken, serially diluted (10-fold) and finally plated by spreading on nutrient agar. Incubation was done for 48 h at 20–25 °C. Eight dilutions were prepared per treatment with two replicates for each dilution. Control plates were also incubated.
Disease assessments
Enzyme Linked Immunosorbent Assay (ELISA)
The CMV-16 accumulation in the foliar tissue of untreated and treated test plants was investigated using the indirect ELISA method (Clark & Adams, Citation1977). Each plant was sampled at the end of 42 days by collection of three terminal leaflets from three young non-inoculated leaves. For normalizing the samples for ELISA, 0.5 g of plant tissue was mixed with 10 × (w/v) coating buffer (15 mM sodium carbonate, 35 mM sodium bicarbonate, pH 9.6, containing 2% polyvinyl pyrrolidone: CB-PVP), then homogenized using a mortar and pestle for sap extraction. Extracted crude sap was filtered through cheesecloth and centrifuged at 6000 g for 2 min. The clarified extract was pipetted into wells of polystyrene microtitre plates. The antigen solution was stored overnight at 4 °C in glass tubes, before the coating incubation for 2–3 h, or incubated directly in microwell plates either at 4 °C overnight, or at 37 °C for 3 h. After three washes for 3 min each with phosphate-buffered saline (PBS) containing 0.5% Tween-20 (PBS-T), the plates were blocked by incubation in 1% bovine serum albumin (BSA) in PBS, for 30–60 min. The blocking solution was replaced by an appropriate dilution of a specific monoclonal antibody against CMV-16 (Affinity Bioreagents, NJ, USA), that was incubated at 37 °C for 60 min. This was followed by three washes with PBS-T and the addition of goat anti-mouse alkaline phosphatase conjugate diluted in PBS buffer (1 : 1000), after which the solution was incubated at 37 °C for 3–4 h. After three washes with PBS-T, p-nitrophenyl phosphate was added in the substrate buffer (pH 9.8). The absorbance was measured at 405 nm, 15–60 min after the addition of the substrate, using a Biotek Model EL307 (Burlington, VT) or a Dynatech MR700 ELISA Reader (Bio-Rad Laboratories, Inc. UK). Values that exceeded twice that of the untreated/healthy samples and/or the buffer controls were considered positive. Based on the ELISA values, the percentage infection of plants in each treatment was also calculated.
Bioassay
Based on the visibility of symptoms appearing on the plants, the plants were scored from a scale of 0 to 10, where 0 = no symptoms and 10 = severe symptoms (Zehnder et al., Citation2000). A logistic model was fitted to assess disease intensity, area under disease progression curve and disease prevention. This model is given by the following equations: Disease Intensity = 100(Σ sn/SN), where s is the disease score, S is the highest s grade, n is number of plants with the same s value, and N is total number of test plants indexed (Montasser et al., Citation1998; Zehnder et al., Citation2000). Area under disease progress curves (AUDPC) was calculated using the formula: Σ (0.5) (Yi+ Yi+1) (Ti+ Ti+1) where Y = disease severity at time T, and i = the time of the assessment in days (Zehnder et al., Citation2000). Disease prevention was calculated using the formula 100([C – T]/C), where C = disease intensity of control plants inoculated only with CMV-16 and T = disease intensity of three treatments plants challenged with CMV-16 (Montasser et al., Citation1998). Analysis of variance (ANOVA) at P = 0.05 was performed on all the data using the SPSS (Statistical Package for Social sciences) – PASW statistics 18 software and the means were separated with Duncan's Multiple Range Test (DMRT) using PASW statistics 18 and the Michigan University Statistical Package (MSTATC) software. The graphs were constructed using the Slidewrite program. The standard error was calculated using the formula: where SE
is the standard error, ‘s’ is the sample standard deviation and ‘n’ is the number of samples. The error bars were drawn on the graphs based on the standard error calculation. The statistical analysis was done together for all three independent experiments.
Results and discussion
Efficacy of using a combination of CMV-KU1 and PGPR in enhancing protection against CMV-16
The efficacy of the combination of CMV-KU1 and two PGPR strains in enhancing protection was determined based on visual observation for the appearance of virus symptoms and by serological analysis (, ). All PGPR-treated plants showed a lower disease severity rating when compared with the control plants challenged with CMV-16. The protection against CMV-16 infection was highest for plants treated with PGPR and CMV-KU1 in combination and lowest for those treated with CMV-KU1 alone. The disease reducing capacity of the combination was 91.3%. In comparison, the plants treated with either PGPR or CMV-KU1 individually reduced the disease by 83.3% and 76.2 %, respectively ().
Table 1. Effects of PGPRs and viral satellite RNA combinations in lowering disease severity based on Enzyme Linked Immunosorbent Assay and symptom scoring
Fig. 2. Mean disease severity values for different treatments at 28, 35 and 42 dpi (days post-inoculation), respectively. 16: control plants challenged with virulent CMV-16; PGPR/16: PGPR treated plants challenged with CMV-16; PGPR+KU1/16: plants preventatively inoculated with a combination of PGPRs and CMV-KU1 associated satellite virus, challenged with CMV-16; KU1/16: plants inoculated with CMV-KU1 associated satellite virus and challenged with CMV-16. Plant growth-promoting rhizobacteria used: a mixture of Pseudomonas aeruginosa and Stenotrophomonas rhizophilia. Three independent experiments were performed. Standard error (n = 3) represented by error bars.
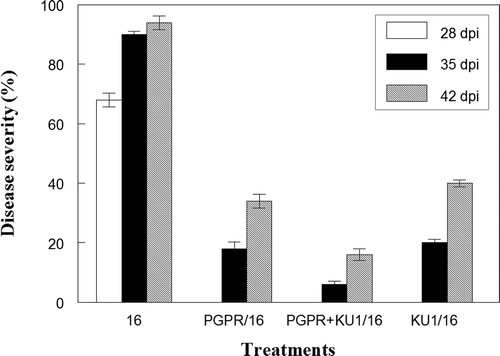
The control plants challenged with CMV-16 (referred to as 16 or positive control in this study) showed severe stunting, outbreak of mosaic symptoms, leaf curling and loss of vigour as a result of infection. The stunting and the mosaic symptoms were observed in all of the positive controls within 7 days of being challenged with CMV-16. The disease severity value of the positive control was calculated to be at 94% by the third week after challenge with CMV-16 (), indicating a high rate of disease incidence and progression. The plant height differences between treatments, one week after challenge with CMV-16, are shown in .
The appearance of symptoms and severity of the disease in the treatment KU1 when challenged with CMV-16 was delayed compared to the positive control. The disease severity ratings at 28, 35 and 42 dpi are shown in . The plants in this treatment were already slightly stunted compared with control plants not challenged with CMV-16 virus (referred to as H or Healthy controls) due to the presence of CMV –KU1. This difference was even more pronounced when challenged with the CMV-16 virus.
Plants treated with a PGPR mixture (PGPR/16 and PGPR+KU1/16) had a comparatively lower disease severity rating compared with those treated with CMV-KU1 alone (). There was also a delay in the onset of symptoms. At 35 dpi, mosaic symptoms began appearing on the plants, although not as severe as the CMV-16 positive controls or CMV-KU1 treated plants. There was, however, a decline in plant height and vigour compared with standard treatment (PGPR) without CMV-16 virus infection. These values, however, were comparable with the healthy control plants without any protective treatments. AUDPC values of the different treatments are shown in .
CMV accumulation determined by ELISA showed that the absorbance values at 405 nm observed at 42 dpi were significantly lower in treated plants compared with the positive controls (). The absorbance value was 0.32 for the healthy controls. Absorbance values that exceed twice that of the healthy values are considered positive (Montasser et al., 2006a). All the treatments not challenged with CMV-16 were less than twice the absorbance value for the healthy control and therefore were considered as negative. For treatments challenged with CMV-16, only PGPR and CMV-KU1 in combination showed absorbance values below the threshold value. The other two treatments showed positive reaction, the degree of infection indicated by ‘+’ sign (). The percentage infection based on the absorbance value of all samples higher than the threshold value per treatment indicated that percentage of plants infected when protected by a combination of PGPRs and viral satellite were lower than those protected by one of them alone (). Analysis for disease severity values, AUDPC values and absorbance values at 405 nm were highly significant at P ≤ 0.05 ( and ).
The use of viral satellite RNA (CMV-KU1) and PGPRs individually for plant protection against viruses and promoting growth has been previously reported by a number of scientists (Murphy et al., Citation2003; Montasser et al., Citation2006b ). The PGPR treatments, when applied to seeds of tomato and cucumber, significantly reduced the AUDPC values of CMV-inoculated plants compared with CMV-inoculated controls without any bacterial inoculations (Murphy et al., Citation2003). Also, PGPR-mediated induced resistance was reported against Tobacco necrosis virus (TNV), Tobacco mosaic virus (TMV) and Tomato mottle virus (ToMoV) (Murphy et al., Citation2003). However, their combined effect in preventing disease has not been researched so far. The results of the present study revealed that PGPR-mediated protection in combination with viral satellite has a positive effect on protection of plants against viral infection and that the combined effect of the protective strain of CMV KU1 and the PGPRs progressively reduced stunting caused by CMV-16 to a great extent. Plants of all three treatments exhibited a delayed response to infection compared with the positive controls. The disease reduction percentage, disease severity values and AUDPC values indicated that the combination of the PGPRs and CMV-KU1 enhanced protection of the tomato test plants by about 10–20% (, ) compared with the individual protection conferred by either the satellite viral or PGPR alone. From the ELISA results, it is clear that plants, when protected with PGPR alone, did not reduce the virus titre. Even though the symptoms of the severe virus are attenuated due to high vegetative growth, the virus accumulation in the tissues, based on the ELISA results, is still comparatively high. Similarly, the loss of vegetative growth in plants treated with the satellite associated helper virus (CMV-KU1) alone may depreciate the protective capability of benign satellite RNA. This is indicated by a high accumulation of CMV-16 in the tissues of these plants (). When PGPRs were combined with CMV-KU1, virus titres were brought down to values equivalent to those of the healthy control. The presence of CMV-KU1 competitively prevents CMV-16 replication in the foliar tissues (Montasser et al., Citation2006b ) while the presence of the PGPRs in the roots enhances the overall natural defences of the plants (Kloepper et al., Citation1999), thus providing double-fold protection against the CMV-16 virus. This is the added advantage compared with using each of them alone. PGPR-mediated biocontrol can be extended to foliar and systemic diseases, even when the PGPRs are applied to seeds and roots (Kloepper et al., Citation1999). The reduction in virus titre due to the presence of PGPRs and satellite virus agrees with previous results reported by Murphy et al. (Citation2003) and McGarvey et al. (Citation1994). We observed that both PGPRs and CMV-KU1 strain associated with the viral satellite RNA require a minimum of 3 weeks to establish themselves and provide protection (data not shown). This allows the PGPRs to successfully colonize the roots and the CMV-KU1 to spread and multiply in the leaves. PGPR and satellite virus-treated plants challenged with severe CMV-16 viral strain without providing sufficient time to establish themselves were infected as severely as the positive controls. The enhanced plant growth due to the presence of PGPRs was also found to be an added advantage for the enhanced protective effect. The protective effect of the PGPRs will therefore vary from strain to strain depending on their ability to promote growth either directly or indirectly.
PGPR ability to compensate for growth loss in CMV-KU1 protected plants
The ability of the PGPRs to compensate for the vigour and yield loss was determined by the mean differences in the shoot length, weight (fresh and dry) and the fruit yield.
There was a significant improvement in the height, weights and the fruit yield when PGPRs were added to CMV-KU1. The growth parameters of the different treatments and healthy control plants are shown in . The growth of plants treated with a combination of PGPRs and CMV-KU1 in the absence of CMV-16 was much higher than that of the healthy controls. In the presence of the virus, there was a small decline in growth. Prior to the application of the treatments, the growth of all the plants was similar to each other. After the application of the protective treatments one week after germination, all the plants receiving PGPRs showed improved growth, root and leaf development, while those treated with the CMV-KU1 without the addition of PGPR showed vegetative stunting. By the third week, this growth difference was very distinct. The leaf area of the PGPR-treated plants was greater compared with the healthy controls without any inoculation and treatments containing CMV-KU1. Similarly, root lengths of the plants with PGPRs were also longer. The application of the challenge virus caused a reduction in the vegetative growth in all the treatments; however, for treatments with PGPR, this decline in growth was not less than healthy plants of the same age without infection.
Fig. 3. Differences in the various growth parameters between treatments. a, Shoot length of tomato plants from different treatments in the presence and absence of the CMV-16 virus, respectively. b, Fresh weight of tomato plants from different treatments in the presence and absence of CMV-16 virus, respectively. c, Dry weight of tomato plants from different treatments in the presence and absence of CMV-16 virus, respectively. d, Fruit yield of tomato plants from different treatments in the presence and absence of CMV-16 virus, respectively. Plant growth-promoting rhizobacteria used: a mixture of Pseudomonas aeruginosa and Stenotrophomonas rhizophila. Control: control plants without any protective treatments; PGPR: plants preventatively inoculated with the PGPR mixture alone; PGPR+KU1: plants preventatively inoculated with PGPR mixture and CMV-KU1; KU1: plants preventatively inoculated with CMV-KU1 virus. Standard error represented by error bars.
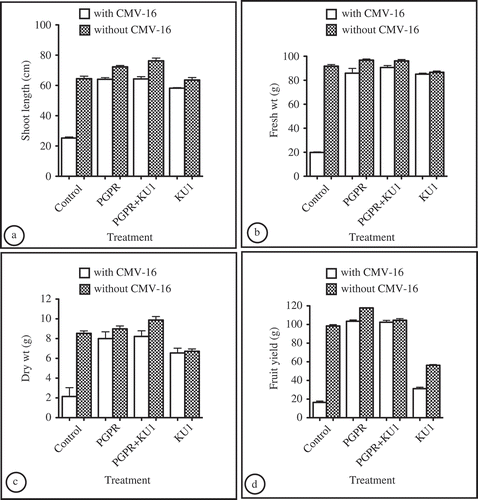
The shoot height, fresh weight and dry weight () showed some differences. The most pronounced differences between treatments were in the yield. Treatments receiving PGPR showed a significant increase in fruit yield (). These values were significantly higher than the healthy controls. For treatments with CMV-KU1 alone, the average fruit yield was lower than the healthy control (). The flower abscissions were lower for all plants treated with a protective biological control agent, whether PGPR, CMV-KU1 or the combination compared with the positive control. However, flower drop was higher in treatments receiving only CMV-KU1. The fruit size and setting was also better for plants receiving PGPR treatments. The average fruit size was very small for positive controls challenged only with CMV-16 compared with the other treatments.
Although it was reported that CMV-KU1 causes vigour and yield loss in tomato plants (Montasser et al., Citation1991), no investigations have been carried out so far to find a way to reduce these losses due to the use of viral satellite-mediated protection. The higher average fruit yield in treatments containing PGPR mixtures indicates that PGPRs not only promoted plant growth but also promoted fruit yield as well. The beneficial ability of the PGPR, however, may vary with the PGPR strains used and its mechanism in promoting plant growth (Dashti et al., Citation2007). The consistency in the effect of PGPRs in compensating for vigour, yield loss, and in enhancing protection has been demonstrated in three consequent experiments. PGPRs used in this work have successfully been tested on different plants, including tomato, cucumber and peppers by other investigators (García et al., Citation2004; Radwan et al., Citation2005). Both of the strains used were indigenous and were capable of degrading hydrocarbons and thereby enhancing nutrient availability in the soil. They also aid in increased nitrogen absorption by plants (Radwan et al., Citation2005). The success of PGPRs is also dependent on the compatibility of the individual PGPR strains used in an inoculum mixture. Adding PGPRs in compatible mixtures has been found to be more successful than using individual strains (Murphy et al., Citation2003; Raupauch & Kloepper, Citation1998). The ability of the PGPRS to increase yield and size of fruits to values higher than healthy controls may have economical benefits.
Assessment of PGPR levels in soil
Plate enumeration at different dilutions indicated that the PGPR levels in the test plant rhizosphere decreased from the original count of 108 cells mL−1 to 105 cells mL−1. Since the soil used was sterile, the cultures were fairly free from any other contamination. The decrease in the PGPR count in the soil rhizosphere may be an indication of declining populations in the rhizosphere.
To our knowledge, this is the first report showing the use of PGPR–viral satellite RNA combination as an effective biological control strategy for disease prevention against virulent viral strain infection as well as yield increase. The effectiveness of the PGPR–viral satellite combination has to be further investigated on a larger scale in field experiments for a potential commercial implementation.
Acknowledgements
This work was funded by the Research Administration (Research Project No. SL 02/07), University of Kuwait, and was carried out at the Molecular Virology Laboratory (MVL), and The Microbiology Central Laboratory, Department of Biological Sciences, Faculty of Science, University of Kuwait, Kuwait. We would also like to thank Dr Patrice Suleman and Prof. Mohammed Afzal for their significant contribution to this paper, especially statistical evaluation of the data, construction of the graphs and proofreading of the manuscript.
References
- Adesemoye , A.O. and Ugoji , E.O. 2009 . Evaluating Pseudomonas aeruginosa as plant growth-promoting rhizobacteria in West Africa . Arch. Phytopathol. Plant Protect , 42 : 188 – 200 .
- Berg , G. , Marten , P. and Ballin , G. 1996 . Stenotrophomonas maltophilia in the rhizosphere of oilseed rape – occurrence, characterization and interaction with phytopathogenic fungi . Microbiol. Res , 151 : 19 – 27 .
- Bhuvaneswari , T.V. , Goodman , R.N. and Bauer , W.D. 1980 . Early events in the infection of soybean (Glycine max L. Merr.) by Rhizobium japonicum. I. Location of infectible root cells . Plant Physiol , 66 : 1027 – 1031 .
- Chabot , R. , Antoun , H. , Kloepper , J.W. and Beauchamp , C.J. 1996 . Root colonization of maize and lettuce by bioluminescent Rhizobium leguminosarum biovar phaseoli . Appl. Environ. Microbiol , 62 : 2767 – 2772 .
- Clark , M. and Adams , A. 1977 . Characteristic of the microplate method of enzyme-linked immunosorbent assay for detection of plant viruses . J. Gen. Virol , 34 : 475 – 483 .
- Dashti , N. , Prithviraj , B. , Zhou , X. , Hynes , R.K. and Smith , D.L. 2000a . Combined effects of plant growth-promoting rhizobacteria and genistein on fixation in soya bean at suboptimal root zone temperatures . J. Plant Nutr , 23 : 593 – 604 .
- Dashti , N. , Prithviraj , B. , Zhou , X. , Hynes , R.K. and Smith , D.L. 2000b . Root and rhizosphere colonization of soybean Glycine max L. Merr. by plant growth promoting rhizobacteria at low root zone temperature and under short season conditions . J. Agron. Crop. Sci , 185 : 15 – 22 .
- Dashti , N. , Montasser , M.S. , Ali , N.Y. , Bhardwaj , R.G. and Smith , D.L. 2007 . Nitrogen biofixing bacteria compensate for yield loss caused by viral satellite RNA associated with Cucumber mosaic virus in tomato . Plant Pathol. J , 23 : 90 – 96 .
- De Meyer , G. , Audenaert , K. and Höfte , M. 1999 . Pseudomonas aeruginosa 7NSK-2 induced systemic resistance in tobacco depends on in planta salicylic acid accumulation but is not associated with PR-1a expression . Eur. J. Plant Pathol , 105 : 513 – 517 .
- Gallitelli , D. , Volvos , C. , Martelli , G.P. , Montasser , M.S. , Tousignant , M.E. and Kaper , J.M. 1991 . Satellite mediated protection of tomato against Cucumber mosaic virus. II. Field test under natural epidemic conditions in Southern Italy . Plant Dis , 75 : 93 – 95 .
- García , J.A.L. , Probanza , A. , Ramos , B. and Manero , F.J.G. 2004 . Effect of inoculation of Bacillus licheniformis on tomato and pepper . Agronomie , 24 : 169 – 176 .
- Hu , C. , Hsu , Y. and Lin , N. 2009 . Satellite RNAs and satellite viruses of plants . Viruses , 1 : 1325 – 1350 .
- Joseph , B. , Ranjan Patra , R. and Lawrence , R. 2007 . Characterization of plant growth promoting rhizobacteria associated with chick pea . Int. J. Plant Prod , 2 : 141 – 152 .
- Kaper , J.M. and Waterworth , H.E. 1977 . Cucumber mosaic virus-associated RNA-5: causal agent for tomato necrosis . Science , 196 : 429 – 431 .
- Kloepper , J.W. , Ubana , R.R. , Zehnder , G.W. , Murfy , J.F. , Sikora , E. and Fernandez , C. 1999 . Plant root-bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases . Aust. Plant. Pathol , 28 : 21 – 26 .
- McGarvey , P.B. , Montasser , M.S. and Kaper , J.M. 1994 . Transgenic tomato plants expressing satellite RNS are tolerant to some strains of Cucumber mosaic virus . J. Am. Soc. Hort. Sci , 119 : 642 – 647 .
- Meunchang , S. , Thongra-Ar , P. , Sanoh , S. , Kaewsuralikhiti , S. and Ando , S. Development of rhizobacteria as a biofertilizer for rice production . International workshop on sustained management of the soil-rhizosphere system for efficient crop production and fertilizer use . October 16th–20th 2006 . pp. 1 – 7 . Bangkok , , Thailand : Land Development Department .
- Montasser , M.S. , Tousignant , M.E. and Kaper , J.M. 1991 . Satellite-mediated protection of vegetable crops against Cucumber mosaic virus. I. Protection of tomato in the greenhouse and under simulated epidemic conditions in the field . Plant Dis , 75 : 86 – 92 .
- Montasser , M.S. , Tousignant , M.E. and Kaper , J.M. 1998 . Viral satellite RNAs for the prevention of Cucumber mosaic virus disease in field-grown pepper and melon plants . Plant Dis , 82 : 1298 – 1303 .
- Montasser , M.S. , Dashti , N.H. , Ali , N.Y. , Bhardwaj , R. and Al-Hamar , B. 2006a . Occurrence of three strains of Cucumber mosaic virus affecting tomato in Kuwait . Plant Pathol. J , 22 : 51 – 62 .
- Montasser , M.S. , Al Hamar , B. and Bhardwaj , R.G. 2006b . Biological control of a severe viral strain using a benign viral satellite RNA associated with Cucumber mosaic virus . Plant Pathol. J , 22 : 131 – 138 .
- Murphy , J.F. , Reddy , M.S. , Ryu , C.M. , Kloepper , J.W. and Li , R. 2003 . Rhizobacteria-mediated growth promotion of tomato leads to protection against Cucumber mosaic virus . Phytopathology , 93 : 1301 – 1307 .
- Nelson, L.M. (2004). Plant growth promoting rhizobacteria: prospects for new inoculants. [Online.] Crop Manage. doi: 10.1094/Cm-2004-0301-05 RV. http://www.plantmanagementnetwork.org/pub/cm/review/2004/rhizobacteria/ (http://www.plantmanagementnetwork.org/pub/cm/review/2004/rhizobacteria/) (Accessed: 4 March 2009 ).
- Radwan , S.S. , Dashti , N. and El-Nemr , I.M. 2005 . Enhancing the growth of Vicia faba plants by microbial inoculation to improve their phytoremediation potential for oily desert areas . Int. J. Phytoremed , 7 : 19 – 32 .
- Radwan , S.S. , Dashti , N. , El-Nemr , I.M. and Khanafer , M. 2007 . Hydrocarbon utilization by nodule bacteria and plant growth promoting rhizobacteria . Int. J. Phytoremed , 9 : 1 – 11 .
- Raupauch , G.S. and Kloepper , J.W. 1998 . Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple Cucumber pathogens . Phytopathology , 88 : 1158 – 1164 .
- Roossinck , M.J. , Sleat , D. and Palukaitis , P. 1992 . Satellite RNAs of plant viruses: structures and biological effects . Microbiol. Rev , 56 : 265 – 279 .
- Roossinck , M.J. , Kaplan , I. and Palukaitis , P. 1997 . Support of a Cucumber mosaic virus satellite RNA maps to a single amino acid proximal to the helicase domain of the helper virus . J. Virol , 71 : 608 – 612 .
- Sayama , H.T. , Kominato , S.M. , Natsuaki , T. and Kaper , J.M. 1993 . Field testing of a satellite containing attenuated strain of Cucumber mosaic virus for tomato protection in Japan . Phytopathology , 83 : 405 – 410 .
- Schmidt , C.S. , Alavi , M. , Cardinale , M. , Muller , H. and Berg , G. 2010 . Root colonization and plant growth promotion by Stenotrophomonas rhizophilia DSM14405 . FEMS Microbiol. Ecol , 74 : 124 – 135 .
- Tomlinson , J.A. 1987 . Epidemiology and control of virus diseases of vegetables . Ann. Appl. Biol , 110 : 661 – 681 .
- Wolf , A. , Fritze , A. , Hagemann , M. and Berg , G. 2002 . Stenotrophomonas rhizophilia sp. nov., a novel plant-associated bacterium with antifungal properties . Int. J. Syst. Evol. Microbiol , 52 : 1937 – 1944 .
- Xu , P. and Roossinck , M.J. 2000 . Cucumber mosaic virus D satellite RNA-induced programmed cell death in tomato . Plant Cell , 12 : 1079 – 1092 .
- Zehnder , G.W. , Yao , C. , Murphy , J.F. , Sikora , E.R. and Kloepper , J.W. 2000 . Induction of resistance in tomato against Cucumber mosaic cucumovirus by plant growth promoting rhizobacteria . Biocontrol , 45 : 125 – 135 .