Abstract
Fusarium grain mould disease (FGMD) is a part of the grain mould disease complex and is caused by Fusarium species which are capable of infecting spikelet tissues at anthesis or immature grain up to physiological maturity. The disease causes severe losses in quality, viability and germination of sorghum seed. To determine the effect of FGMD associated characters (panicle grain mould score (PGS), seed rot, amount of Fusarium, and non-Fusarium infected seed and seed weight) on seed germination and to find the inter-relationship among these characters, replicated field trials were conducted with 36 sorghum recombinant inbred lines (RILs) during 2009 and 2010 at Hyderabad, India. PGS showed a strong positive relationship with seed rot and seedborne Fusarium (P < 0.01). Seed rot showed a significant positive relationship with seedborne Fusarium and a negative relationship with seed weight and germination (P < 0.01). Frequency of seedborne Fusarium on mould-infected sorghum seed had a strong negative correlation with that of non-Fusarium infection (P < 0.01), suggesting interactions between them in causation of grain mould in sorghum. Path coefficient analysis for seed germination revealed that seed rot (−0.43) and PGS (−0.28) had a maximum direct effect, accompanied with less interference by other factors, on seed germination. Seed rot has emerged as the most important parameter for determining seed germination in moulded sorghum grains. Few promising RILs that produced minimum premature seed rot were identified. The RIL numbers 144, 156 and 159 were superior to controls for many FGMD associated characters and could be useful sources for improvement of FGMD resistance.
Résumé
La moisissure des grains de sorgho (MGS) appartient à un ensemble de maladies des grains causées par certaines espèces de Fusarium qui peuvent infecter les tissus des épillets au stade de l'anthèse ou le grain à tous les stades, et ce, jusqu'à la maturité physiologique. La maladie altère grandement la qualité, la viabilité et la germination des grains de sorgho. Afin de déterminer l'effet des particularités associées à la MGS [degré de gravité de la moisissure des grains de la panicule (PGS), pourriture des semences, quantité de Fusarium, semences infectées par des agents autres que Fusarium, poids des semences] sur la germination et de trouver des relations entre ces particularités, des essais au champ sur des parcelles répétées ont été menés, en 2009 et 2010 à Hyderabad en Inde, avec 36 lignées consanguines recombinantes (LCR). La PGS a affiché une forte corrélation positive par rapport à la pourriture des semences et à Fusarium transmis par les semences (P < 0.01). La pourriture des semences a affiché une corrélation positive significative par rapport à Fusarium transmis par les semences et une corrélation négative par rapport au poids et à la germination des semences (P < 0.01). L'occurrence de Fusarium transmis par les semences sur les graines de sorgho infectées par la moisissure affichait une forte corrélation négative par rapport à l'infection causée par des agents autres que Fusarium (P < 0.01), ce qui suggère des interactions entre ceux-ci quant à l'infection du grain par la moisissure chez le sorgho. L'analyse des pistes causales relative à la germination a révélé que la pourriture des semences (−0.43) et la PGS (−0.28) avaient un effet maximal direct sur la germination et que l'influence des autres facteurs était moindre. La pourriture des semences s'est révélée le critère le plus déterminant quant à l'évaluation de la germination chez les grains moisis de sorgho. Quelques LCR qui affichaient un minimum de pourriture chez les semences vertes ont été identifiées. Les lignées 144, 156 et 159 étaient supérieures aux témoins quant à plusieurs particularités associées à la MGS et pourraient servir à améliorer la résistance à cette maladie.
Introduction
Fusarium grain mould disease (FGMD) occurs on sorghum grain before physiological maturity of the grain and is a part of the grain mould disease complex. The grain mould disease complex is caused by several fungi including Fusarium, Curvularia, Alternaria and Phoma (Forbes et al., Citation1992; Singh & Bandyopadhyay, Citation2000), whereas FGMD is caused by Fusarium species which are capable of infecting spikelet tissues at anthesis or immature grain before physiological maturity. In India, common Fusarium species causing grain mould on sorghum are F. thapsinum Klittich, Leslie, Nelson & Marasas, F. proliferatum (Matsushima) Nirenberg and F. andiyazi Marasas, Raheeder, Lamprecht, Zeller & Leslie (Sharma et al., Citation2011). Damage resulting from the early infection of sorghum florets by Fusarium spp. includes arrest of seed development, decrease in seed weight, density, germination and seedling vigour. Infection that occurs during anthesis is most damaging as the fungi colonize the lodicules and ovary base and then progress in an acropetal fashion as the caryopsis matures (Butler et al., Citation2008). Inoculation of sorghum panicles at anthesis with F. thapsinum results in reduced caryopsis formation, especially in grain mould susceptible cultivars (Little & Magill, Citation2009). If humid conditions persist after infection, Fusarium spp. profusely colonize the developing seed, causing destruction of the endosperm or seed rot before it reaches physiological maturity (Directorate of Sorghum Research, Citation2010). Apart from causing grain mould, Fusarium spp. contaminate grain with hazardous mycotoxins. Some Indian isolates of Fusarium spp. from sorghum are reported to be highly toxigenic (Das et al., Citation2010). An outbreak of food poisoning attributed to the ingestion of mouldy sorghum and maize grain was reported from several villages in India (Bhat et al., Citation1997). Considering the dominance of Fusarium spp. in grain mould disease complex worldwide and huge losses in grain and seed quality, studies on FGMD as a component of the grain mould disease complex is getting increased attention (Das et al., Citation2011; Prom et al., Citation2011).
Seed germination is one of the most important characters that determine seed quality. It is influenced by many factors, including physiological, biochemical, environmental and nutritional characters of seed (Bewley & Black, Citation1978). Seed-mycoflora is another important factor that influences seed health and determines germination of seed. Germination of sorghum seed produced during the rainy season is heavily affected by grain mould (Kannababu et al., Citation2009). Reduction in seed germination varies depending on fungal infection and genotypes (Audilakshmi et al., Citation1999; Garud et al., Citation2000). Fungi causing grain mould also cause reduction in seed weight and loss in seed viability (Glueck & Rooney, Citation1976; Castor, Citation1981). Fusarium spp. as well as other fungi causing grain mould in sorghum are reported to adversely affect seed germination (Prom et al., Citation2011).
Path coefficients have been used to develop selection criteria for complex traits like shoot fly resistance (Omori et al., Citation1983) and grain yield (Ezeaku & Mohammed, Citation2006) in sorghum. It helps to determine the amount of direct and indirect effect of the causal components on the effect component (García del Moral et al., Citation1991). Such association studies are lacking in sorghum for characters related to FGMD and seed germination. The objective of the present study was to determine the effect of seed rot and other FGMD-related characters on seed germination and to find the inter-relationships among these characters.
Materials and methods
Plant materials
Forty sorghum lines including recombinant inbred lines (RILs), parents and elite checks were used for the experiment. A subset of 36 RILs were selected from a population of 200 RILs that were developed by crossing between 296B and B58586 at Directorate of Sorghum Research, Hyderabad, India (Audilakshmi et al., Citation2011). 296B is the female parent of a popular commercial hybrid, CSH9, and is susceptible to grain moulds. B58586 is a grain mould-resistant genetic stock which is photo-insensitive and has greater grain yield as compared with other available resistant sources. The sub-set was selected based on mean performance for panicle grain mould score in multiple locations. Selection was done keeping in mind that the population represents sufficient variation for grain mould reactions (). In addition to the parents, two standard checks (SVD9601 and C43) were used in the experiment. SVD9601 is an elite cultivar and C43 is an elite restorer line with moderate resistance to grain mould.
Table 1. Plant materials used in this study
Fungal isolates
Fungal isolates (SF018 and SF181) were obtained from mouldy sorghum grains collected from fields in Guntur (SF018) and Hyderabad (SF181) districts in India during 2008. For maintenance, the cultures were grown on potato dextrose agar slants and stored in a refrigerator (4 °C) and subsequently sub-cultured on autoclaved sorghum grain at every 4 months. Isolate SF018 produces a pinkish colony on potato dextrose agar, microconidia are produced in chains on monophialides, and macroconidia are thin-walled and 3–5 septate. Isolate SF181 produces a whitish to pinkish colony on potato dextrose agar, and polyphialides are present on which microconidia are produced in long chains. Morphological characterization followed by identification of the cultures was done as per Leslie & Summerell (Citation2006). SF018 was identified as F. thapsinum while SF181 was F. proliferatum.
Field experiment
The selected RILs (F9 and F10 progenies) were used for evaluation of FGMD resistance during rainy seasons in the years 2009 and 2010, respectively. The experiment was conducted in replicated field trials at the Directorate of Sorghum Research, Hyderabad. Each RIL was grown in a single row of 4 m length, with 60 cm row spacing and a plant spacing of 10–15 cm within rows. The trial was laid out in a randomized complete block design with two replications. Standard crop management practices were used to raise the crop (Maiti et al., Citation1985). Five uniform flowering plants in each replication were selected and labelled with tags for artificial inoculation with Fusarium spp. and for further observations.
Artificial inoculation for development of FGMD
Cultures of F. thapsinum and F. proliferatum were revived by transferring mycelium from potato dextrose agar slants to freshly prepared potato dextrose agar plates and placed in an incubator. Four to five agar discs containing mycelium were inoculated on autoclaved sorghum grain kept in a 500 mL conical flask. The flask was incubated in an incubator at 27 ± 1 °C under a 12 h light and 12 h darkness cycle. A conidial suspension was prepared in sterile water from 10-day-old cultures. The labelled panicles in each row (RIL) were spray-inoculated at 80% anthesis with the mixture of spore suspension (1 : 1 v/v; 2 × 106 conidia mL−1) of the above Fusarium spp. Immediately after inoculation, each panicle was covered with a paper bag to maintain humidity and to facilitate fungal infection. Three days after inoculation, bags were removed from the panicles. Overhead sprinklers were used for maintaining high humidity during the grain development stage on rain-free days. During flowering to physiological maturity of grain (i.e. August and September) the experimental location received an adequate amount of rainfall () and thus reduced the frequency of usage of overhead sprinklers.
Table 2. Rainfall, temperature and relative humidity of the experiment location during 2009 and 2010 cropping seasons
Data recording
Plant, panicle and grain characters that are used for evaluation of resistance to grain mould in sorghum include panicle structure, glume cover, grain hardness, panicle grain mould score (PGS), seed weight, seed mycoflora and seed germination (Forbes et al., 1992; Audilakshmi et al., 1999; Das et al., 2011). Among these, panicle structure, glume cover and grain hardness are mainly used for evaluation of resistance against weathering of mature grain in the field. As the present study was on FGMD and observations were limited to physiological maturity stage, these three characters were not considered in our study. Instead, characters associated with FGMD were included. These were PGS, seed rot, seed weight and per cent Fusarium and non-Fusarium infected seed. PGS was recorded at around physiological maturity. Physiological maturity in sorghum can be judged by seeing a black layer formation at the hilar end at around 35 ± 3 days after anthesis, depending on season. Because different lines had different maturity periods, harvesting of treated panicles was done when the line reached physiological maturity. PGS on each treated panicle was measured on a 1–9 scale, where 1 = no visible grain mould in a panicle, 2 = 1–5%, 3 = 6–10%, 4 = 11–20%, 5 = 21–30%, 6 = 31–40%, 7 = 41–50%, 8 = 51–75% and 9 = > 75% grains in a panicle are moulded. While scoring for grain mould, the seeds showing slight to heavy infection/discolouration or complete colonization by fungi were considered as moulded. Mean score of five panicles was considered as a replicate. After recording PGS, the treated panicles from each RIL were used for sampling of panicles for seed rot. Panicle branches containing seeds were randomly sampled from all parts of the treated panicles (base, middle and top). Samples obtained from five panicles were pooled and formed one replicate. Seeds that were fully covered with fungal growth, discoloured, and sometimes soft were considered as rotted seed. Rotted seeds were counted and expressed as per cent of total seed. Once sampling of the panicle branches was completed, the treated panicles from each treatment (RIL) were threshed to obtain grains for further studies on fungal infection and seed germination. Seed weight was based on weight in grams of 100 randomly selected seeds from each replication. Before taking weights, seeds were allowed to dry under sunlight for a week to standardize seed moisture.
Assay for seedborne fungal infection and seed germination
One hundred seeds from each replication were weighed, surface sterilized and used to study seedborne fungi. Seeds were washed aseptically with sterile distilled water twice, then surface-sterilized using 4% sodium hypochlorite solution (NaOCl) for 5 minutes, followed by rinsing with sterile distilled water thrice. The seeds were then air-dried in a laminar flow hood. Twenty-five surface-sterilized seeds were placed on sterile, wet filter paper in a Petri dish and incubated at 28 ± 1 °C for 5 days to facilitate growth of seedborne fungi. After incubation, samples were observed for number of seeds infected with Fusarium spp. (SFu) and non-Fusarium (SNFu) fungi using a magnifying glass. Grain showing even slight growth of fungal mycelium was counted as positive for fungal growth. Seed germination (SG) test was carried out in roll towels (International Seed Testing Association, Citation1999). The seeds (100 seeds/replication) were incubated at 25 ± 1 °C and 90 ± 3% relative humidity in a germinator. After incubation for 9 days, germination counts were taken and percentage germination was determined based on the number of sorghum seedlings produced.
Statistical analysis
Combined analysis of variance for the PGS, per cent seed rot, per cent seed germination, per cent seedborne fungi and seed weight were performed using the command PROC GLM in SAS 9.2 after verifying the homogeneity of trial variance errors. Arcsine transformations of original data for per cent seed rot, seed germination and Fusarium and non-Fusarium infection were used for analysis. Pearson's correlation coefficient was estimated to determine relationships among independent characters. Path analysis was done for the characters to determine the amount of direct and indirect effects of the causal components on the effect component. Correlation, path coefficient analysis and path diagram construction was carried out using statistical software WINDOSTAT (version 7.5).
Results
Variability of characters
Analysis of variance () revealed that genotype (RIL) had a large effect on all the characters while environment (year) had a large effect on all the characters except seed weight. Genotype (RIL) × environment interaction was significant for all the characters except panicle grain mould score (PGS) and seed rot. A high degree of variability was observed in all the characters examined. Few RILS showed significant improvement over the resistant parent for panicle grade mould score, seed germination and 100 seed weight (). The RIL84 (3.1) and RIL156 (3.6) were superior in PGS compared with the resistant parent B58586 (5.0) and the moderately resistant checks C43 (5.5) and SVD9601 (5.7). Per cent seed germination was significantly more in RIL159 (67%) than the parent B58586 (47%). RIL30 (2.3 g−1100 seed) and RIL80 (2.4 g−1100 seed) had significant improvement in seed weight over B58586. RIL159 (13%), RIL90 (15%), RIL144 (15%), RIL124 (20%) and RIL163 (18%) had significantly less seed rot compared with the check SVD9601 (45%). None of the RILs showed significant improvement over the resistant parent for per cent seedborne Fusarium and non-Fusarium. RIL163, RIL156 and RIL172 recorded minimum (<25%) infection of seedborne Fusarium, whereas RIL159, RIL77 and RIL4 recorded minimum (<25%) infection of seedborne non-Fusarium. Among the RILs, RIL159, RIL166, RIL144 and RIL77 recorded maximum germination per cent (>30%). RIL144, RIL156 and RIL159 recorded overall improvement over either the resistant parents or the checks for most of the characteristics examined.
Table 3. Probability values for mean squares from the combined analysis of variance for seed germination and FGMD associated characters examined on 40 sorghum lines artificially inoculated with Fusarium spp. during two seasons (2009 and 2010)
Table 4. Mean values of Fusarium grain mould associated characters of 40 sorghum lines artificially inoculated with Fusarium spp. during two seasons (2009 and 2010, Hyderabad, India)
Correlation studies
Pearson correlation coefficients among seed germination, seed rot, panicle grain mould score, seedborne Fusarium and other grain mould-associated characters of sorghum lines that were artificially inoculated with Fusarium spp. are given in . Seed germination showed a strong negative relationship with PGS, seed rot and seedborne Fusarium (P < 0.01) and had a strong positive relationship with seed weight (P = 0.04). Relationship of seed germination with seedborne non-Fusarium was not significant (P = 0.13). PGS recorded a strong positive correlation with seed rot and seedborne Fusarium (P < 0.01), and a negative correlation with seed weight and seedborne non-Fusarium (P < 0.01). Seed rot had a strong positive relationship with seedborne Fusarium (P < 0.01) and negative with seed weight (P < 0.05). Its relationship with seedborne non-Fusarium was non-significant (P = 0.34). Seedborne Fusarium showed a strong negative relationship with seedborne non-Fusarium and no relationship with seed weight (P = 0.23). Partial R2 (coefficient of determination) was maximum for seed rot (0.27), followed by PGS (0.16), seedborne Fusarium (0.07) and non-Fusarium (0.04).
Table 5. Pearson correlation coefficients among seed germination (SG), seed rot (SR), panicle grain mould score (PGS), seed-borne Fusarium (SFu), seed-borne non Fusarium (SNFu) and seed weight (SW) studied on 40 sorghum lines artificially inoculated with Fusarium spp. at anthesis during two seasons (2009 and 2010)
Path coefficient analysis for seed germination
Path coefficient analysis was performed to obtain further information on the inter-relationship among the characters and their associations with seed germination. Path coefficient diagrams for seed germination showing inter-relationships among five characters of 40 sorghum lines are demonstrated in Results showed that four characters had direct negative associations with seed germination (). Seed rot (−0.43) showed maximum direct association on germination, followed by seedborne non-Fusarium (−0.37), PGS (−0.28) and seedborne Fusarium (−0.28). The direct correlation of PGS on germination was negative and high. Total correlation coefficient (−0.54) between PGS and germination was mainly due to its direct effect (−0.28) plus its effect through seed rot (−0.24). The direct association of seed rot on germination was negative and quite high. Total correlation coefficient (−0.63) between seed rot and germination was mainly due to its direct correlation (−0.43) plus its indirect association through PGS (−0.16). Seedborne Fusarium showed a significant negative correlation (−0.26) with germination and this was due to its direct association (−0.28). Results based on simple correlation studies and path coefficient analysis was different for seedborne non-Fusarium and seed weight. Seedborne non-Fusarium had far greater direct effect on seed germination (−0.37) than shown by total correlation (−0.12). Its effect on seed germination was neutralized by indirect effect of PGS (0.17). Seed weight showed a significant positive correlation with germination (0.16). However, its direct association with germination was negligible (−0.002). Its positive correlation resulted from indirect association with seed rot and PGS.
Fig. 1. Phenotypic path coefficient diagrams for seed germination (SG) showing the inter-relationships among five characters (PGS = panicle grain mould score, SR = seed rot, SFu = Seedborne Fusarium, SNFu = Seedborne non-Fusarium, and SW = seed weight) of 40 sorghum lines (the numbers on the lines indicate path coefficients).
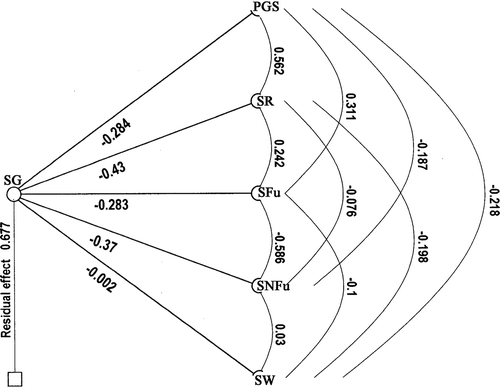
Table 6. Path coefficient analysis of seed germination of 40 sorghum lines artificially inoculated with Fusarium spp. at anthesis during two seasons (2009 and 2010)
Discussion
Combined analysis of the data obtained over 2 years suggested differential responses among the RILs to all the characteristics examined. However, mean squares due to years for all the characters except seed weight were significant, indicating variable responses of these characters with the year of evaluation. The responses of RILs to seed germination, seed weight, seedborne Fusarium and non-Fusarium varied with the 2 experimental years as revealed from significant mean squares due to year × RIL interactions for these characters. Differences in weather variables including rainfall, number of rainy days and relative humidity during grain development stage in 2009 and 2010 () might have played a role in these interactions. Influence of environmental variables, such as relative humidity and temperature, on infection by fungi and grain mould development has been well documented (Indira & Muthusubramanian, Citation2004; Navi et al., Citation2005). RILs revealed a wide range of genotypic variation for all the characters examined. The RIL numbers 144, 156 and 159 recorded significant overall improvement over the parents or checks for most of the characters. Hence, these RILs were considered superior for FGMD resistance and can be utilized in resistance breeding programmes. Reasons for improved resistance of the above three RILs are not yet known. Morphological characters like glume coverage, glume colour (Audilakshmi et al., 1999), panicle compactness (Mansuetus et al., Citation1988) and grain hardness (Aruna & Audilakshmi, Citation2004) have been associated with resistance to grain weathering of white grain sorghum. However, the role of these characters in resistance to infection of the sorghum flower are not clear. The role of antifungal proteins such as chitinases, β-1,3-glucanases, sormatins and PR-10 (Prom et al., Citation2005; Katile et al., Citation2010) has been implicated in resistance to early infection of sorghum by Fusarium spp., indicating their importance in development of mould-resistant sorghum lines. Rodriguez-Herrera et al. (Citation2006) observed that levels of sormatin, chitinases and glucanases were higher in resistant varieties and their F1s in comparison to susceptible ones. Further analysis of the RILs for such characters will shed more light on their resistance to FGMD. In this study, we noted quite a high percentage of non-Fusarium in the harvested grain samples. As the experiment was conducted under field conditions, contamination by airborne fungi (Prom et al., Citation2011) could have occurred. Evaluation under controlled conditions might help reduce this contamination.
PGS was scored taking into consideration the extent of fungal infection on grains in a panicle. In the present experiment, because of pressure of artificial inoculation of panicles with Fusarium spp., visual mould symptoms and seed rot symptoms on the treated panicles were dominated by Fusarium spp. (personal observation). That was perhaps the reason we observed a strong positive relationship between PGS and seed rot. Following incubation, the majority of the visually moulded grains yielded Fusarium spp. That might be the reason for the strong positive relationship of seedborne Fusarium with PGS and seed rot. As fungi other than Fusarium were mostly recovered from seeds that had relatively fewer visual symptoms (i.e. less PGS), seedborne non-Fusarium showed a negative relationship with PGS and its effect on seed rot was negligible. This was evident from the non-significant relationship between these two characters. Moreover, there was a strong negative relationship between seedborne Fusarium and non-Fusarium, which suggested that Fusarium infection might have prevented other fungi from infecting the same floret or seed. Kedera et al. (Citation1994) observed that fungi, particularly F. moniliforme, could provide protection to maize kernels from other invading fungi. Spraying panicles with a high concentration of conidia of weakly virulent species is reported to prevent internal infection by other fungi, perhaps by competition or by defence response (Little & Magill, Citation2003). Competition among fungi indicates an opportunity to manage grain mould through avirulent strains of Fusarium. Similarly PGS and seed rot showed a strong negative influence on seed weight. Fungal infection in florets and its subsequent growth might have affected seed development. Previous researchers have reported severe loss in seed weight due to floral infection by Fusarium (Little & Magill, Citation2009; Prom & Erpelding, Citation2009). Infection by F. moniliforme and C. lunata has been reported to interfere with carbohydrate translocation to developing kernels, thus causing reduction in size and weight of seed (Castor & Frederiksen, Citation1977).
Seed is an important input for agriculture. Germination ability of seed is one of the most important indicators for seed quality and viability. In the present study, an effort was made to understand which of the FGMD-associated characters affects seed germination. The inter-relationships of the characters were studied by correlation and path coefficient analysis. Results suggested significant negative effect of PGS, seed rot, seedborne Fusarium and non-Fusarium on seed germination. These observations were supported by both correlation studies as well as path coefficient analysis. Therefore, all these characters might have played a role in germination of Fusarium mould-infected sorghum seed. Panicle grain mould score (PGS) gives an indication of the extent of fungal infection on sorghum grain and is known to affect seed germination in sorghum (Bandyopadhyay & Chandrashekar, Citation2000; Garud et al., Citation2000; Prom & Erpelding, Citation2009). In this study, the character ‘seed rot’ included grains that were fully covered with fungal growth, discoloured, and sometimes soft. This character gives an indication of inherent susceptibility of a genotype towards invasion of sorghum grain by fungi. Disintegration of sorghum grain due to infection of grain mould causing fungi and resultant loss in germination is well documented (Garud et al., Citation2000). The colonization of sorghum grain by fungi is reported to affect embryo viability and destroy the endosperm (Butler et al., Citation2008). This might be the reason for high negative effect of seedborne Fusarium and non-Fusarium on germination. Fungal infection in many cereal grains causes decrease in germination percentage. The respiration of grain and fungi results in a loss in dry matter as well as the production of heat and moisture which contribute to further spoilage (Sauer, Citation1988). Hare et al. (Citation1999) observed that reduced germination and emergence of seedlings of wheat was associated with infection by F. culmorum, causing Fusarium ear rot in wheat. Apart from seed microflora and other characters that were examined in this study, there are many other factors including physiological, biochemical and nutritional factors that might have governed seed germination (Bewley & Black, Citation1978). This is reflected by high residual effect (0.68) shown by path coefficient analysis. Observations on inter-relationships based on correlation studies were not supported by the results of path coefficient analysis for seed weight. Seed weight had a positive correlation but no pronounced direct association with germination. The direct association was neutralized by a positive indirect association of seed rot and PGS. This finding is in agreement with observations by other researchers who reported that inter-relation of the path analysis may provide a somewhat different picture than does a simple correlation analysis (Gebeyehou et al., Citation1982; García et al., Citation1991). Simple correlation shows relationships among independent characters and their linear relationships. Path analysis, on the other hand, determines the amount of direct and indirect effects of the causal components on the effect component (García et al., Citation1991). Although four characters (seed rot, PGS, seedborne Fusarium and non-Fusarium) showed direct association with seed germination, seed rot and PGS were the two characters that had maximum coefficients of determination and direct association of these two characters was less influenced by indirect association of other characters (seedborne Fusarium, non-Fusarium and seed weight). Hence, these two characters are considered as important determinants of germination of grain mould-infected sorghum seeds.
In summary, this study indicates that per cent seed rot and panicle grain mould score are important characters for determining seed germination in moulded sorghum grains. Seed rot emerged as an important character that showed a strong relationship with PGS and very pronounced effect on seed germination and can be used for evaluation of sorghum genotypes for FGMD resistance. RIL numbers 90, 144, 124 and 163 were promising for resistance to premature seed rot. The RIL numbers 144, 156 and 159 were superior both to the resistant parents or checks for most of the FGMD-associated characters and can provide useful sources for improvement of FGMD resistance in a grain mould breeding programme.
References
- Aruna , C. and Audilakshmi , S. 2004 . Genetic architecture of grain hardness – a durable resistance mechanism for grain moulds in sorghum [Sorghum bicolor (L.) Moench] . Indian J. Genet. Plant Breed , 64 : 35 – 38 .
- Audilakshmi , S. , Stenhouse , J.W. , Reddy , T.P. and Prasad , M.V.R. 1999 . Grain mould resistance and associated characters of sorghum genotypes . Euphytica , 107 : 91 – 103 .
- Audilakshmi , S. , Das , I.K. , Ghorade , R.B. , Mane , P.N. , Kamatar , M.Y. , Narayana , Y.D. and Seetharama , N. 2011 . Genetic improvement of sorghum for grain mould resistance: I. Performance of sorghum recombinant inbred lines for grain mould reactions across environments . Crop Prot , 30 : 753 – 758 .
- Bandyopadhyay , R. and Chandrashekar , A. 18–19 May 2000 . “ Biology and management of sorghum grain mold ” . In Proceedings of Consultative Group Meeting on Technical and Institutional Options for Sorghum Grain Mold Management , Edited by: Chandrashekar , A. , Bandyopadhyay , R. and Hall , A.J. 18–19 May , Patancheru , , India : International Crops Research Institute for the Semi-Arid Tropics .
- Bewley , J.D. and Black , M. 1978 . Physiology and Biochemistry of Seeds in Relation to Germination. Vol. 1. Development, Germination, and Growth , 306 Berlin : Springer-Verlag .
- Bhat , R.V. , Shetty , P.H. , Amruth , R.P. and Sudershan , R.V.A. 1997 . Foodborne disease outbreak due to the consumption of moldy sorghum and maize containing fumonisins mycotoxins . J. Toxicol. Clin. Toxic , 35 : 249 – 255 .
- Butler , D.N. , Noll , L.W. and Little , C.R. 2008 . Tissue-specific colonization of sorghum caryopses by grain mold fungi . Phytopathology , 98 : S28 Abstr
- Castor , L. 1981 . Grain mold, histopathology, damage assessment, and resistance screening with Sorghum bicolor (L.) Moench lines , 177 College Station, TX , , USA : PhD thesis. Texas A & M University . pages
- Castor , L.L. and Frederiksen , R.A. 1977 . Seed molding of grain sorghum caused by Fusarium and Curvularia species . Proc. Am. Phytopath. Soc. Meeting , 4 : 151 Abstr
- Das , I.K. , Vijay Kumar , B.S. , Ratnavathi , C.V. , Komala , V.V. , Annapurna , A. and Seetharama , N. 2010 . Toxigenicity of Fusarium isolates and fumonisin B1 contamination in rainy season sorghum [Sorghum bicolor L. (Moench)] . Indian J. Agric. Sci , 80 : 67 – 72 .
- Das , I.K. , Audilakshmi , S. and Patil , J.V. 2011 . Fusarium grain mold: The major component of grain mold in sorghum (Sorghum bicolor L. Moench) . Eur. J. Plant Sci. Biotechnol , 6 (forthcoming)
- Directorate of Sorghum Research . 2010 . Annual Report 2009–10 , 93 Andhra Pradesh , , India : Directorate of Sorghum Research, Rajendranagar, Hyderabad 500030 . pages
- Ezeaku , I.E. and Mohammed , S.G. 2006 . Character association and path analysis in grain sorghum . African J. Biotechnol , 5 : 1337 – 1340 .
- Forbes , G.A. , Bandyopadhyay , R. and Garcia , G. 1992 . “ A review of sorghum grain mold ” . In Sorghum and millet diseases: A second world review , Edited by: de Milliano , W.A.J. , Frederiksen , R.A. and Bengston , G.D. 265 – 272 . International Crops Research Institute for the Semi-Arid Tropics, Patancheru. 502324 .
- García Del Moral , L.F. , Ramos , J.M. , García Del Moral , M.B. and Jimenez-Tejada , P. 1991 . Ontogenetic approach to grain production in spring barley based on path-coefficient analysis . Crop Sci , 31 : 1179 – 1185 .
- Garud , T.B. , Ismail , S. and Shinde , B.M. 2000 . Effect of two mold causing fungi on germination of sorghum seed . Int. Sorghum Millets Newsl , 41 : 55
- Gebeyehou , G. , Knott , D.R. and Baker , R.J. 1982 . Relationships among durations of vegetative and grain filling phases, yield components, and grain yield in durum wheat cultivars . Crop Sci , 22 : 287 – 290 .
- Glueck , J.A. and Rooney , L.W. 1976 . Physical and chemical characterization of sorghum lines with resistance to grain deterioration . Cereal Foods World , 21 : 436 – 437 .
- Hare , M.C. , Parry , D.W. and Baker , M.D. 1999 . The relationship between wheat seed weight, infection by Fusarium culmorum or Microdochium nivale, germination and seedling disease . Eur. J. Plant Pathol , 105 : 859 – 866 .
- Indira , S. and Muthusubramanian , V. 2004 . Influence of weather parameters on spore production in major mold pathogens of sorghum in relation to mold severity in the field . Indian J. Plant Prot , 32 : 75 – 79 .
- International Seed Testing Association . 1999 . International rules for seed testing . Seed Sci. Technol , 27 ( suppl ) : 27 – 30 .
- Kannababu , N. , Das , I.K. and Seetharama , N. 2009 . Effect of chemicals and bioagents on control of molds and improvement of seed quality of rainy season sorghum (Sorghum bicolor) . Indian J. Agric. Sci , 79 : 461 – 465 .
- Katile , S.O. , Perumal , R. , Rooney , W.L. , Prom , L.K. and Magill , C.W. 2010 . Expression of pathogenesis-related protein PR-10 in sorghum floral tissues in response to inoculation with Fusarium thapsinum . Curvularia lunata. Mol. Plant Pathol , 11 : 93 – 103 .
- Kedera , C.J. , Leslie , J.F. and Claflin , L.E. 1994 . Genetic diversity of Fusarium section Liseola (Gibberella fujikuroi) in individual maize stalks . Phytopathology , 84 : 603 – 607 .
- Leslie , J.F. and Summerell , B.A. 2006 . “ The Fusarium ” . In Laboratory Manual , 1st , 388 Ames , IA : Blackwell Publishing . pages
- Little , C.R. and Magill , C.W. 2003 . Elicitation of defense response genes in sorghum floral tissues infected by Fusarium thapsinum and Curvularia lunata at anthesis . Physiol. Mol. Plant Pathol , 63 : 271 – 279 .
- Little , C.R. and Magill , C. 2009 . The grain mold pathogen, Fusarium thapsinum, reduces caryopsis formation in Sorghum bicolor . J. Phytopathol , 157 : 518 – 519 .
- Maiti , R.K. , Raju , P.S. and Bidinger , F.R. 1985 . Studies on germinability and some aspects of preharvest physiology of sorghum grain . Seed Sci. Technol , 13 : 27 – 35 .
- Mansuetus , A.S.B. , Frederiksen , R.A. , Waniska , R.D. , Odvody , G.N. , Craig , J. and Rosenow , D.T. 1988 . “ The effects of glume and caryopses characteristics of sorghum on infection by Fusarium moniliforme Sheldon ” . In Sorghum Newsl , Vol. 31 , Patancheru , , India : International Crops Research Institute for the Semi-Arid Tropics (CP738) .
- Navi , S.S. , Bandyopadhyay , R. , Reddy , R.K. , Thakur , R.P. and Yang , X.B. 2005 . Effects of wetness duration and grain development stages on sorghum grain mold infection . Plant Dis , 89 : 872 – 878 .
- Omori , T. , Agrawal , B.L. and House , L.R. 1983 . Componental analysis of the factors influencing shoot fly resistance in sorghum (Sorghum bicolor (L.) Moench) . Japan Agric. Res. Quarterly , 17 : 215 – 218 .
- Prom , L.K. and Erpelding , J.E. 2009 . New sources of grain mold resistance among sorghum accessions from Sudan . Tropic. Subtropic. Agroecosys , 10 : 457 – 463 .
- Prom , L.K. , Waniska , R.D. , Kollo , A.I. , Rooney , W.L. and Bejosano , F.P. 2005 . Role of chitinase and sormation accumulation in the resistance of sorghum cultivars to grain mold . J. Agric. Food Chem , 53 : 5565 – 5570 .
- Prom , L.K. , Isakeit , T. , Perumal , R. , Erpelding , J.E. , Rooney , W.L. and Magill , C.W. 2011 . Evaluation of the Ugandan sorghum accessions for grain mold and anthracnose resistance . Crop Prot. J , 30 : 566 – 571 .
- Rodriguez-Herrera , R. , Waniska , R.W. , Rooney , W.L. , Aguilar , C.N. and Contreras-Esquivel , J.C. 2006 . Antifungal proteins during sorghum grain development and grain mould resistance . J. Phytopathol , 154 : 565 – 571 .
- Sauer , D.B. 1988 . Effects of fungal deterioration on grain: nutritional value, toxicity, germination . Int. J. Food Microbiol , 7 : 267 – 275 .
- Sharma , R. , Thakur , R.P. , Senthilvel , S. , Nayak , S. , Reddy , S.V. , Rao , V.P. and Varshney , R.K. 2011 . Identification and characterization of toxigenic fusaria associated with sorghum grain mold complex in India . Mycopathologia , 171 : 223 – 230 .
- Singh , S.D. and Bandyopadhyay , R. 2000 . “ Grain mold ” . In Compendium of Sorghum Diseases , Edited by: Frederiksen , R.A. and Odvody , G.N. 38 – 40 . St. Paul , MN : The American Phytopathological Society .