Abstract
Rhizomania is the most destructive disease in sugar beet throughout the world. The disease is caused by Beet necrotic yellow vein virus (BNYVV). Polymyxa betae (Keskin) is the only known vector of BNYVV, for transmission of the virus between the plants. Developing molecular diagnostic methods has a major impact on forecasting and epidemiological studies as well as screening sugar beet plants used in resistance breeding programmes. In the present study, quantum dots (QDs) were biofunctionalized with a specific antibody against P. betae. The glutathione-S-transferase protein's (GST) corresponding antibody (anti-GST) was effectively conjugated to Tioglicolicacid-modified Cadmium-Telluride QDs (CdTe-QDs) synthesized in an aqueous solution via electrostatic interaction. The dye (Rhodamine) molecules were attached to the GST. Donor–acceptor complexes (QDs-Ab-GST-Rhodamine) were then formed based on the antigen–antibody interaction. The mutual affinity of the antigen and the antibody brought the CdTeQDs and rhodamine together close enough to allow the resonance dipole–dipole coupling required for fluorescence resonance energy transfer (FRET) to occur. The immunosensor constructed showed a high sensitivity and specificity of 100%, acceptable stability and could be used for real sample detection with consistent results. To the best of our knowledge, this is the first report on designing a nano-based biosensing tool for the detection of plant pathogenic fungi.
Résumé
Rhizomania est la maladie de la betterave sucrière la plus destructrice au monde. La maladie est causée par le virus de la rhizomanie de la betterave sucrière (BNYVV). Polymyxa betae (Keskin) est le seul vecteur connu du BNYVV en ce qui a trait à la transmission entre plants. Le développement de méthodes de diagnostic moléculaire a eu une influence majeure tant sur les études prédictives et épidémiologiques que sur le dépistage des plants de betterave sucrière utilisés dans les programmes de sélection pour la résistance. Dans cette étude, des points quantiques (PQ) ont été biofonctionnalisés contre P. betae avec un anticorps particulier. L'anticorps (anti-GST) correspondant de la protéine de la glutathione-S-transférase (GST) a été efficacement conjugué aux PQ en tellure de cadmium modifiés avec l'acide thioglycolique (CdTe-PQ), synthétisés dans une solution aqueuse par interaction électrostatique. Les molécules de la teinture (rhodamine) ont été attachées à la GST. Les complexes donneur-accepteur (PQ-Ab-GST-Rhodamine) ont par la suite été formés en fonction de l'interaction antigène-anticorps. L'affinité réciproque qui existe entre l'antigène et l'anticorps a suffisamment rapproché les CdTe-PQ et la rhodamine pour permettre le couplage dipolaire requis pour que le transfert d'énergie de fluorescence par résonance (FRET) se produise. L'immunosenseur produit a affiché une grande sensibilité et une spécificité de 100 % ainsi qu'une stabilité acceptable. Il pourrait par ailleurs être utilisé pour détecter de vrais échantillons et donner des résultats homogènes. À notre connaissance, il s'agit du premier rapport traitant de la conception d'un outil de biodétection basé sur la GST, destiné au dépistage de ce champignon pathogène.
Introduction
Beet necrotic yellow vein virus (BNYVV), the causal agent of rhizomania, is an economically important viral disease. The plasmodiophoromycete Polymyxa betae Keskin (P. betae), is an obligate parasite of sugarbeet roots and the vector of BNYVV (Putz et al., Citation1990; Tamade et al., Citation1999). The yield losses due to this disease have partially come under control by introducing resistant cultivars now used widely throughout the world. However, specific and accurate diagnostic methods are essential in order to screen different cultivars to ensure resistance to the rhizomania virus and its vector. Conventional methods often rely on identification of disease symptoms, isolation and culturing, and laboratory identification by morphology and biochemical tests, and immunoassay techniques (Mutasa-Gottgens et al., Citation1993; Kingsnorth et al., Citation2003; Ward et al., Citation2004). These techniques are generally costly, lengthen the detection time, and suffer from high detection limits. Among them, however, the immunoassay technique takes advantage of the affinity binding between antibodies and the corresponding antigens to specifically detect their presence in a mixture to be analysed. Moreover, antibodies recognize the overall shape of an epitope rather than particular residues, and can distinguish minor differences in the primary amino acid sequence of antigens as well as differences in charge, optical, and steric conformations (Van Regenmortel, Citation1997; Safarnejad et al., Citation2011). Combining the molecular specificity of a biological recognition entity such as an antigen/antibody with an operationally simple transducer could lead to innovating more advanced diagnostics.
Glutathione-S-transferase (GST), a specific immunogenic protein, is a critical enzyme expressed in P. betae zoospores, sporangia and resting spores and could be a good candidate to develop the serological base of a diagnostic technique (i.e. the biological recognition entity), for the pathogen (Kingsnorth et al., Citation2003). In fact, the pathogen expresses GST at high levels to overcome host defence mechanisms (Mutasa-Gottgens et al., Citation2000). On the other hand, with the achievements of nanotechnology and nano-sciences, a wide variety of nanoscale materials with unique features including size-dependent, bright and extremely stable emissions have attracted a great deal of attention as transducers. One of the most important nanomaterials are fluorescent semiconductor nanocrystals, also known as quantum dots (QDs) which have been widely used for disease diagnosis (Frasco & Chaniotakis, Citation2009). QDs have a number of unique optical properties that are advantageous in the development of bio-analyses based on fluorescence resonance energy transfer (FRET) (Algar & Krull, Citation2007). These biosensors have been widely used in immunoassay (Goldman et al., Citation2005), biomedical sensors (Shi et al., Citation2006), detection of protein (Algar & Krull, Citation2007) and inter-molecular binding assays (Willard et al., Citation2001). QDs have been reportedly used as biosensors by coating them with specific antibodies against various pathogenic agents such as E. coli O157:H7 (Hahn et al., Citation2008).
The aim of this study was to conjugate QDs and a specific antibody (Ab) against GST to develop a specific and sensitive FRET-based QD-antibody biosensor for rapid, accurate, and cost-effective detection of P. betae, the vector of BNYVV.
Materials and methods
Polymyxa culture
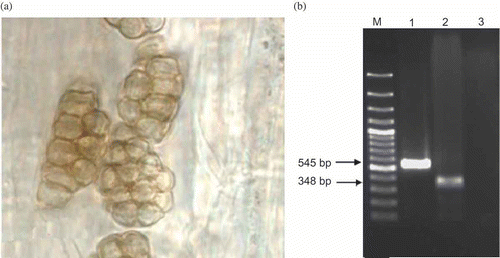
A rather simple test to confirm the presence of Polymyxa spp. is planting the host in naturally infested soils (Abe, Citation1987; Gerik, Citation1992). Ten-day-old sugarbeet (Beta vulgaris L.) and barley (Hordeum vulgare L.) seedlings were inoculated with P. betae and P. graminis using dried root powder inoculum as previously described by Mutasa-Gottgens et al. (Citation1993) and Thompson et al. (Citation2011), respectively. Fifteen days after inoculation, microscopic examination was conducted to confirm the infection.
Detection and identification of Polymyxa spp
The DNA extraction from sugarbeet lateral roots grown in the infested soils as well as barley roots was done according to Mutasa-Gottgens et al. (Citation2000). A primer pair Psp1 (5´ TAGACGCAGGTCATCAACCT 3´) and Psp2rev (5´ AGGGCTCTCGAAAGCGCAA 3´), was used to amplify a 545 and 348 bp region of the nuclear ribosomal DNA of P. betae and P. graminis, respectively (Legrève et al. Citation2002). This region contained the internal transcribed spacer 1 (ITS1), the 5.8S DNA and the internal transcribed spacer 2 (ITS2). The following PCR program was conducted: 95 °C for 2 min, 36 cycles of 96 °C for 45 s, 50 °C for 45 s and 72 °C for 1 min and final synthesis for 10 min 72 °C. Barley roots free of P. graminis were used as negative control.
Antibody specificity test
The specific polyclonal antibody against GST protein of P. betae (kindly provided by M. Basirat, ABRII, Karaj, Iran) was used for detection of fungi in infected plants and bio-conjugation to quantum dots nano-particles. To evaluate the specificity of the polyclonal antibody against the Polymyxa spp., double antibody sandwich (DAS)-ELISA was performed for P. betae and P. graminis as described by Clark & Adams (Citation1977) with modifications. The plant root sap samples were prepared by crushing 0.2 g fresh roots in liquid nitrogen followed by 10-fold dilution in an extraction buffer (2% PVP 25, 0.2% skimmed dried milk in PBS). Microtiter plates (Maxisorp, Nunc) were first incubated (3–4 h, 37 °C) with polyclonal rabbit antibodies (100-fold dilution with coating buffer). Subsequently, they were incubated with fungus-infected or healthy plant root sap at 4 °C for 12–16 h. Then, the plates were washed three times with phosphate buffer saline twin (PBST). After that, 100 μL of alkaline phosphatase conjugated to goat anti-rabbit IgG was added into each well, and the plates were incubated for 2 h at 37 °C. Subsequently, another three rounds of washing were performed. Finally, the plates were incubated for 30 min with alkaline phosphatase substrate solution (1 mg mL−1 p-NPP, pH 9.8, 100 μL per well) and ELISA readings (OD405nm) were carried out by a Sunrise ELISA reader (Tecan, Austria).
CdTe quantum dots preparation
Tellurium powder (99.9%), cadmium chloride (CdCl2, 99.9%) and thioglycolic acid (TGA, 98%) were purchased from Tianjin Chemical Reagents Company (China). Water-soluble CdTe QDs were synthesized by using 5 mM of CdCl2–2.5 H2O dissolved in 110 mL distilled water, followed by the addition of 12 mM TGA under stirring and pH was adjusted to 11 by drop-wise addition of 1 M NaOH solution. The solution was then placed in a three-necked flask and desecrated by N2 bubbling for 30 min. Then, 2.5 mM of oxygen-free NaHTe solution, which was freshly prepared from tellurium powder and sodium borohydride (NaBH4, molar ratio of 1 : 2) in water at 0 °C, was injected into the three-necked flask under stirring. Finally, the resultant mixture was refluxed at 100 °C for 4 h.
Bioconjugation of CdTe QDs with antibodies
One hundred μL of QDs (2 mg mL−1) and 50 μL of the purified GST antibodies (3.5 mg mL−1) were mixed, pH 7.4. Then 150 μL of the freshly prepared 4.2 mg mL−1 1-ethyl-3-(3 dimethylaminopropyl)-carbodiimide EDC (44 mM in borate buffer) was added to the mixture. The samples were then incubated for 2 h at room temperature under shaking in the dark and stored at 4 °C overnight. The overnight QDs-labelled antibodies were then centrifuged at 15 700 g for 20 min (TOMY, Japan). After the lower phase was removed, the upper phase containing QDs-antibody conjugates was decanted, diluted by 1.5 mL Tris-HCl, and then 0.5 mL of 50 mM Tris-HCl containing 1% BSA was added. Finally, the QDs-labelled antibodies were dispersed in Tris-HCl and stored at 4 °C until use.
Conjugation of GST with rhodamine as a quencher
In order to conjugate the GST with rhodamine, initially 360 μL glutaraldehyde (GLA) 10% (100 g L−1) was added to 100 μL GST (5 μg mL−1). Subsequently, 2 μL of dinitropyridine was added whilst stirring and mixed for 30 min. In the next step, 1 mg of NaBH4 was added and the mixture was stored for 1 h at room temperature. GST-GLA conjugates were then separated from the excess GLA via dialysis using Tris-HCl 50 mM for 16 h. After that, 3 mg of rhodamine dissolved in 5 mL ddH2O was added to the dialysed GST-GLA solution. Finally, the rhodamine-GLA-GST conjugates were separated from free rhodamine by dialysis as explained earlier.
Fluorometry instrumentation
Fluorescence spectra were obtained using a Shimadzu 1501 ultraviolet-visible (UV-VIS) spectrophotometer (Shimadzu, Japan). The fluorometer was operated as follows: the excitation wavelength was set at 350 nm (the excitation wavelength of CdTe QDs) and the emission spectra were recorded between 540 and 640 nm as the quencher's (rhodamine) emission was located at the range of 580 to 600 nm. The excitation and emission bandwidth of 5 nm was used.
Kit construction and evaluation
The kit constructed consisted of (1) rhodamine-antigen (GST) solution, (2) QDs-labelled antibodies and (3) Tris-HCl buffer. The test was conducted by first adding 3 mL of Tris-HCl buffer into the fluorometer cell. Then, 50 μL of the rhodamine-antigen solution was added. This was followed by addition of 50 μL of the QDs-labelled antibodies solution. The baseline curve was then evaluated by the fluorometer. This revealed the baseline emission of rhodamine quenching the emission caused by the QDs. At the detection stage, the suspicious roots (0.1–0.5 mm thick) were mashed in Tris-HCl buffer (1 g plant material/500 μL buffer). Twenty μL of the prepared extract was then added to the same fluorometer cell and the second curve was obtained. If no or negligible baseline shift (negative) was observed, the sample was marked as free of P. betae but significant baseline downward shift (positive) would reveal that the sample contained the pathogenic agents.
Sensitivity and specificity
In order to determine the sensitivity and the specificity of the constructed QD FRET-based biosensor kit, 20 P. betae-infected and 10 healthy samples (positive and negative samples, respectively), were tested by the fluorometer in triplicate. In order to compare the immunoassay test with the nanobiosensor, serial dilutions of recombinant GST 3500 μg mL−1 (10 to 0.5 μg mL−1 Tris-HCl buffer) were tested in triplicate with both techniques. Furthermore, the specificity of the nanobiosensor was also checked using 10 P. graminis-positive samples. The baseline shift measured for Immunodominant Membrane Protein (IMP) as the negative control (X ± 3 SD), was used in differentiating healthy and infected samples.
Stability
Fourier Transform Infrared (FT-IR) spectroscopy analysis was used to investigate the stability of the existing chemical bonds such as those between the QDs and antibody molecules as well as between rhodamine and antigens. To achieve that, 150 μL of each QD, QD-Ab and rhodamine-antigen were first freeze-dried. The dried samples were then used to make a tablet for FT-IR analysis by using Nicolet IR100 FT-IR (Madison, WI, USA).
Results
Identification of Polymyxa spp
After microscopic confirmation of infection (), P. betae presence as well as that of P. graminis in the roots of plants grown in the soil samples tested was verified by PCR assay. The primer set Psp1 and Psp2rev produced PCR fragments of about 545 and 348 bp using DNA extracts obtained from P. betae-infected sugarbeet and P. graminis-infected samples, respectively ().
Antibody specificity
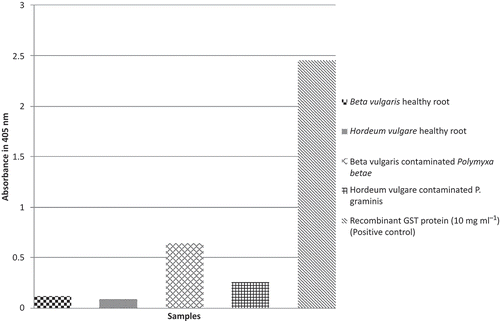
The results of the DAS-ELISA assay conducted to determine the specificity of the produced antibody showed a high specificity for recombinant GST protein and P. betae-infected sugar beet samples. Moreover, it also showed a little cross reactivity to P. graminis ().
QDs synthesis and bioconjugation with antibodies
The synthesized CdTe QDs were surface-modified with TGA and their mean particle diameter was measured at 8.2 nm. The purified antibody was immobilized on the prepared TGA-modified CdTe QDs. This was achieved due to the hydrophilic nature of the QDs leading to the attraction of the Ab molecules on their surfaces (Zhou & Ghosh, Citation2006). The Ab-QDs conjugates showed the maximum fluorescence radiation at approximately 545 nm.
Conjugation of GST with rhodamine and fluorometry analysis
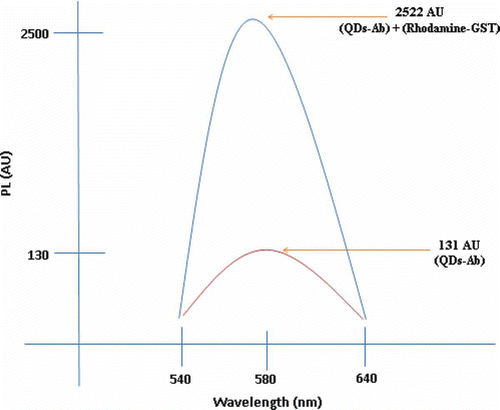
The GST was successfully conjugated to rhodamine using the 19 free amine groups (lysine) of GST linked to GLA. The two conjugates, i.e. Ab-QDs and rhodamine-GST, were further conjugated based on the antibody–antigen interaction phenomenon. Herein, the fluorescence emission wavelength of the Ab-QDs was obtained at 131 AU; however, the emission spectrum of the solution showed an upward shift peaking at 2522 AU when the rhodamine-GST/Ab-QD conjugates were investigated ().
Sensitivity and specificity
The average downward baseline shift measured for IMP as the negative control (−41 ± 30) was used to differentiate the healthy and infected samples (). All 20 P. betae positive samples were successfully detected and 10 out of 10 P. betae negative samples were found healthy by the nanobiosensor method (). Based on these results, the assay has sensitivity and a specificity of 100%. Moreover, the results obtained showed that the developed nanobiosensor failed to differentiate P. betae and P. graminis. This was in agreement with the results of the ELISA method which indicated cross reactivity (). also presents the comparison of the ELISA technique and the nanobiosensor. The detection limit of the ELISA technique in the present study was measured at 2 μg mL−1 recombinant GST protein. The nanobiosensor showed a far lower detection limit of lower than 0.5 μg mL−1 recombinant GST protein.
Table 1. Comparative study to determine sensitivity of the ELISA and the nanobiosensor techniques
Table 2. Analysing real samples (20 P. betae-infected, three healthy sugarbeet and 10 P. graminis infected barley samples) with the constructed bio-nanosensor
Stability
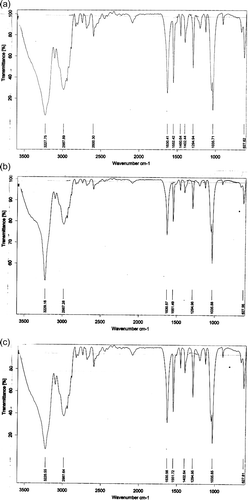
The stability of the TGA-surface modified CdTe QDs, QD-Ab and rhodamine-GST conjugates were determined by FT-IR spectra as shown in The peak obtained at 1630 cm −1 for QDs () should be coincident with carbonyl stretching vibration, indicating that the CdTe QDs were successfully bound to the carboxylic acid functional group of TGA. The same peak (1630 cm −1) was observed for QD-Ab; however, it resulted from the –CO–NH mode or in other words, the contributions of C–N stretching vibration and N–H bending vibration in the CdTe QDs and the antibody molecules, respectively. As for the GST-rhodamine conjugates, the peak obtained at 1550 cm−1 should be coincident with the amine stretching vibration, indicating that amine groups of the rhodamine were successfully bound with the aldehyde functional group (-CHO) of glutaraldehyde. The obtained results were successfully reproduced over the course of time. Therefore, the overall FT-IR analysis of the constructed conjugates revealed their stability.
Discussion
The results of the identification of Polymyxa spp. isolates in the present study using the nuclear rDNA 18S, 5.8S and internal transcribed spacers (ITS1 and ITS2) regions were in agreement with those of the previous studies (Legrève et al., Citation2002; Vaïanopoulos et al., Citation2007).
The reported detection methods for rhizomania so far require multiple steps, and therefore, the present study was set to apply luminescent semiconductor nanocrystals or QDs coupled with FRET analysis to be used as an accurate, cost-effective and rapid immunoassay technique. Traditionally, QDs have been used in imaging techniques acting as fluorescent labels. At the same time, FRET has been used to study the interaction of macromolecules labelled with organic fluorophores. The combination of these two techniques, therefore, could pose several advantages in studying macromolecular interactions (Youn et al., Citation1995).
The synthesis of QDs is a relatively simple process and once synthesized they have a relatively long shelf life. Moreover, minor modifications to the synthetic method lead to a wide range of variations in the dot size and resultant optical properties. On the other hand, QDs are proved to be effective FRET donors due to their broad excitation spectra and tunable, narrow and symmetric photoemission (Youn et al., Citation1995). Therefore, they could enhance FRET as an effective technique for the application in molecular binding event studies, protein conformation change, and biological assays. One of the most important significance of the combination of QDs and FRET system is their application as the QD-FRET based biosensors in designing biomolecule detection systems specially used for pathogen detection in the samples of interest.
In principle, the antibody–antigen attachment is not of robust type. This fact was used in developing the detection basis as when the free GST of P. betae origin was added to the solution, including the rhodamine-GST/Ab-QD conjugates, the rhodamine-GST domain was replaced by the free GST based on the concentration of the free GST in the suspicious sample. This led to a downward shift of the fluorometer curve in comparison with the curve previously obtained for the rhodamine-GST/Ab-QD conjugates (). This could be explained through the optical quenching mechanism of the QD-antibody domain by the rhodamine-antigen domain based on the Forster dipole–dipole interaction model (Zhou & Ghosh, Citation2006). In other words, the inorganic dye i.e. rhodamine (a fluorescence acceptor) conjugated to the antigen (GST) occupies the peptide-binding pocket of the antibody i.e. anti-GST polyclonal antibody resulting in a 3-fold FRET signal. Therefore, when free GST derived from the pathogenic agent was added, it displaced the GST-rhodamine domain in the rhodamine-GST/Ab-QD conjugates, resulting in a fluorescence recovery from the conjugated QD-Ab domain. More specifically, the higher the concentration of the free GST, the GST-rhodamine domain/free GST distribution on the surface of the antibody molecules would shift in favour of the free GST. In another word, higher GST concentrations are translated into the fluorometer curves obtained peaking at lower photoluminescence (PL) intensities.
When using antibodies in a detection system, their specificity against closely related species is of great concern. The cross reactivity observed between the antibody produced against P. betae-GST in this study, and the closely related species P. graminis has also been reported in previous studies where Polymyxa-specific antibodies were developed (Delfosse et al., Citation2000; Mutasa-Gottgens et al., Citation2000). This is due to the high level of homology between the two fungal species. However, fortunately, the two are well-distinguished by their host range, decreasing the risk of false-positive results in the nanobiosensor system developed.
In the nanobiosensor assay, only 20 μL of sample is required, which is critical in cases where sample volumes are small and limited. The detection limit for GST protein using this assay was far lower than 0.5 μg mL−1, which was much better than that of the conventional ELISA (2 μg mL−1). From an economic point of view, the cost of QD synthesis is the sole difference between the ELISA method and the developed nanobiosensor, which is negligible. However, in terms of large-scale operation costs, the nanobiosensor is more economical due to its speed.
Acknowledgement
We greatly appreciate the financial support provided by the Agriculture Biotechnology Research Institute of Iran (ABRII).
References
- Abe , H. 1987 . “ Studies on the ecology and control of Polymyxa betae Keskin, as a fungal vector of the causal virus (Beet necrotic yellow vein virus) of Rhizomania disease of sugar beet ” . In Rept. No , Vol. 60 , 80 – 99 . Hokkaido : Hokkaido Prefect. Kitami Agric. Exp. Stn .
- Algar , W.R. and Krull , U.J. 2007 . Quantum dots as donors in fluorescence resonance energy transfer for the bioanalysis of nucleic acids, proteins, and other biological molecules . Anal. Bioanal. Chem. , 391 : 1609 – 1618 .
- Clark , M.F. and Adams , A. N . 1977 . Characteristics of the microtiter plate method of enzyme linked immunosorbent assay for the detection of plant viruses . J. Gen. Virol. , 34 : 475 – 483 .
- Delfosse , P. , Reddy , A.S. , Legerve , A. , Devi , K.T. , Abdurahman , M.D. , Maraite , H. and Reddy , D.V.R. 2000 . Serological methods for detection of Polymyxa graminis, an obligate root parasite and vector of plant viruses . Phytopathology , 90 : 537 – 545 .
- Frasco , M.F. and Chaniotakis , N. 2009 . Semiconductor quantum dots in chemical sensors and biosensors . Sensors , 9 : 7266 – 7286 .
- Gerik , J.S. 1992 . “ Zoosporic obligate parasites of roots ” . In Methods for Research on Soilborne Phytopathogenic Fungi , Edited by: Singleton , L.L. , Mihailand , J.D. and Rush , C.M. 18 – 24 . St. Paul , MN : American Phytopathological Society Press .
- Goldman , E.R. , Mattoussi , H. , Anderson , P. , Medintz , I. and Mauro , M. 2005 . Fluoroimmunoassays using antibody-conjugate quantum dots . Methods Mol. Biol. , 303 : 19 – 34 .
- Hahn , M.A. , Keng , P.C. and Krauss , T.D. 2008 . Flow cytometric analysis to detect pathogens in bacterial cell mixtures using semiconductor quantum dots . Anal. Chem. , 80 : 864 – 872 .
- Kingsnorth , C.S. , Asher , M.J.C. , Keane , G.J.P. , Chwarszczynska , D.M. , Luterbacher , M.C. and Mutasa-Gottgens , E.S. 2003 . Development of a recombinant antibody ELISA test for the detection of Polymyxa betae and its use in resistance screening . Plant Pathol. , 52 : 673 – 680 .
- Legrève , A. , Delfosse , P. and Maraite , H. 2002 . Phylogenetic analysis of Polymyxa species based on nuclear 5.8S and internal transcribed spacers ribosomal DNA sequences . Mycol. Res. , 106 : 138 – 147 .
- Mutasa-Gottgens , E.S. , Ward , E. , Adams , M.J. , Collier , C.R. , Chwarszczynska , D.M. and Asher , M.J.C. 1993 . A sensitive DNA probe for the detection of Polymyxa betae in sugar beet roots . Physiol. Mol. Plant Pathol. , 43 : 379 – 390 .
- Mutasa-Gottgens , E.S. , Chwarszczynska , D.M. , Halsey , K. and Asher , M.J.C. 2000 . Specific polyclonal antibodies for the obligate plant parasite Polymyxa – a targeted recombinant DNA approach . Plant Pathol. , 49 : 276 – 287 .
- Putz , C. , Merdinoglu , D. , Lemaire , O. , Stocky , G. , Valentin , P. and Wiedemann , S. 1990 . Beet necrotic yellow vein virus, causal agent of sugar beet rhizomania . Rev. Plant Pathol. , 69 : 247 – 254 .
- Safarnejad , M.R. , SalehiJouzani , G.R. , Tabatabaie , M. , Twyman , R.M. and Schillberg , S. 2011 . Antibody-mediated resistance against plant pathogens . Biotechnol. Adv. , 29 : 961 – 971 .
- Shi , X. , Hong , T. , Walter , L.K. , Ewalt , M. , Michishita , E. Hung , T. 2006 . ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression . Nature , 442 ( 7098 ) : 96 – 99 .
- Tamade , T. , Uchino , H. , Kusume , T. and Saito , M. 1999 . RNA 3 deletion mutants in necrotic yellow vein virus do not cause rhizomania disease in sugar beets . Phytopathology , 89 : 1000 – 1006 .
- Thompson , J.P. , Clewett , T.G. , Jennings , R.E. , Sheedy , J.G. , Owen , K.J. and Persley , D.M. 2011 . Detection of Polymyxa graminis in a barley crop in Australia . Austral. Plant Pathol. , 40 : 66 – 75 .
- Vaïanopoulos , C. , Bragard , C. , Moreau , V. , Maraite , H. and Legrève , A. 2007 . Identification and quantification of Polymyxa graminis f. sp. temperate and P. graminis f. sp. tepida on barley and wheat . Plant Dis. , 91 : 857 – 864 .
- Van Regenmortel , M.H.V. 1997 . “ The antigen antibody reaction ” . In Principles and Practice of Immunoassay , Edited by: Price , C.P and Newman , D.J. 14 – 34 . London : Macmillan Press .
- Ward , L.I. , Fenn , M.G.E. and Henry , C.M. 2004 . A rapid method for detection of Polymyxa DNA in soil . Plant Pathol. , 53 : 485 – 490 .
- Willard , D.M. , Carillo , L.L. , Jung , J. and Orden , A.V. 2001 . CdSe-ZnS Quantum Dots as resonance energy transfer donors in a model protein-protein binding assay . Nano Lett. , 1 : 469 – 474 .
- Youn , H.J. , Terpetschnig , E. , Szmacinski , H. and Lakowicz , J.R. 1995 . Fluorescence energy transfer immunoassay based on a long-lifetime luminescent metal-ligand complex . Anal. Biochem. , 232 : 24 – 30 .
- Zhou , M. and Ghosh , I. 2006 . A bright future together . Pept. Sci. , 88 : 325 – 339 .