Abstract
Sixty-one field collections of Puccinia striiformis, primarily from central Alberta during 2007–2008, were identified to be P. striiformis f. sp. tritici (Pst) or P. striiformis f. sp. hordei (Psh) based on virulence on differential seedlings. Wheat differentials separated 38 Pst isolates into 13 pathotypes, with most pathotypes consisting of single isolates. Two pathotypes consisted of two isolates, and three pathotypes were represented by three, seven and 16 isolates. Wheat lines with resistance genes Yr1, Yr5, Yr15 and YrSP were resistant to all 13 pathotypes, while wheat lines with genes Yr10, Yr24 and Yr28 were resistant to 82–92% of the isolates. Differentials YrA, Yr2, Yr6, Yr7, Yr8, Yr9, Yr17, Yr26, Yr27, Yr31 and YrCV were susceptible to 71–100% of the isolates. Twenty-three Psh isolates were identified to 15 Psh pathotypes, with virulence on two to eight barley differentials, including ‘Topper’, ‘Hiproly’, ‘Varunda’, ‘Abed Binder 12’, ‘Trumpf’, ‘Mazurka’, ‘Bigo’, ‘I 5’ and ‘Bancroft’ and avirulent on ‘Heils Franken’, ‘Emir’ and ‘Astrix’. Ten pathotypes consisted of a single isolate, four pathotypes had two isolates and one pathotype consisted of five isolates. The possible origin and virulence of P. striiformis pathotypes in central Alberta is discussed in relation to using cultivar resistance for disease control.
Résumé
Soixante-et-une collections de Puccinia striiformis, provenant principalement du centre de l'Alberta et prélevées en 2007–2008, ont été identifiées comme appartenant à Puccinia striiformis f. sp. tritici (Pst) ou Puccinia striiformis f. sp. hordei (Psh), selon leur virulence pour des séries différentielles de semis. Les séries différentielles de blé ont permis de répartir 38 isolats de Pst en 13 pathotypes comportant pour la plupart des isolats uniques. Deux pathotypes comportaient 2 isolats et 3 pathotypes, 3, 7 et 16 isolats. Les lignées de blé possédant les gènes de résistance Yr1, Yr5, Yr15 et YrSP étaient résistants aux 13 pathotypes, tandis que celles possédant les gènes Yr10, Yr24 et Yr28 étaient résistantes à 82–92 % des isolats. Les séries différentielles possédant les gènes YrA, Yr2, Yr6, Yr7, Yr8, Yr9, Yr17, Yr26, Yr27, Yr31 et YrCV étaient réceptives à l'égard de 71–100 % des isolats. Vingt-trois isolats de Psh ont été identifiés à 15 isolats de Psh virulents à l'égard de 2 à 8 séries différentielles d'orge, y compris ‘Topper’, ‘Hiproly’, ‘Varunda’, ‘Abed Binder 12’, ‘Trumpf’, ‘Mazurka’, ‘Bigo’, ‘I 5’ et ‘Bancroft’, et avirulent à l'égard de ‘Heils Franken’, ‘Emir’ et ‘Astrix’. Dix pathotypes comportaient un isolat unique, quatre, deux isolats et un en comportait cinq. L'origine possible et la virulence des pathotypes de P. striiformis dans le centre de l'Alberta sont discutées relativement à l'utilisation de la résistance des cultivars en tant que moyen de lutte contre la maladie.
Introduction
Stripe (yellow) rust of cereals and grasses is caused by different formae speciales of Puccinia striiformis Westend. The most economically important formae speciales are P. striiformis f. sp. tritici Eriks. & Henn. (Pst), causing stripe rust of wheat (Triticum aestivum L.) and triticale (× Triticosecale Wittm. ex A. Camus.), and P. striiformis f. sp. hordei Eriks. & Henn. (Psh) causing stripe rust on barley (Hordeum vulgare L.) in many areas around the world. Wheat stripe rust has become a more frequent problem in the central and southeastern states of the USA since 2000 (Chen et al., Citation2010). In 1975, barley stripe rust was found for the first time in the western hemisphere, near Bogota, Colombia and since then has spread throughout western South America, causing yield losses of 30–70% in barley (Dubin & Stubbs, Citation1986). Since 1991, barley stripe rust has become established and caused considerable damage in the northwestern USA (Marshall & Sutton, Citation1995).
Stripe rust in Canada was first identified in Alberta in 1918 (Fraser & Conners, Citation1925). In subsequent years, it was found in British Columbia, southern Alberta, and western Saskatchewan, but was not found in Manitoba or eastern Saskatchewan during that time (Newton & Johnson, Citation1936). The main area of concern for stripe rust in Canada has been central and southern Alberta, where it is an annual production problem. Inoculum appears to overwinter in the Pacific Northwest (PNW) of the USA, then spreads by wind to Alberta and initiates epidemics (Chen, Citation2005). Epidemics of wheat stripe rust have occurred in central Alberta since the late 1990s, but were particularly devastating in Alberta in 2005 when severities of up to 100% were observed in some wheat nurseries, resulting in premature ripening of portions of the heads and prolific production of urediniospores between kernels and glumes (McCallum et al., Citation2006). The disease has also been observed in barley breeding plots and fields in central Alberta where both barley and wheat are major crops (Anonymous, Citation2010).
Pathogen specialization and changes in virulence of P. striiformis have been studied extensively in Europe (Zadoks, Citation1961), the UK (Manners, Citation1950), Canada (Newton & Johnson, Citation1936) and the western USA (Purdy & Allan, Citation1963). The stripe rust pathogen is highly variable in virulence, which has rendered cultivar resistance ineffective in some instances (Wan et al., Citation2004; Chen, Citation2007). Analysis of stripe rust severity in Denmark from 1985 to 1999, found several outbreaks when the Yr9 and Yr17 resistance (R) genes became ineffective (Hovmøller, Citation2001). Since 2000, the geographic range of stripe rust in the eastern USA has expanded, and the old population of races has been replaced by an invasive population (Milus et al., Citation2009). Puccinia striiformis f. sp. tritici has become endemic and progressively adapted to the commercial wheat production in Australia through step-wise mutations (Wellings, Citation2007). Several of these mutant pathotypes became more frequent in the Pst population, causing widespread infection and significant cost to production in certain cultivars and seasons.
While studies on Pst population dynamics and virulence have been intensively conducted in relation to host genetics, information on the virulence dynamics of Psh is limited. A study was conducted in Texas, USA, during 1991–1994 to determine the occurrence and spread of stripe rust, the identity and relative frequency of races present, and the amount of yield loss attributed to the disease on barley cultivars having different levels of resistance (Marshall & Sutton, Citation1995). These researchers reported that from a total of 273 isolates, only three races were present. Subsequent changes in P. striiformis virulence on barley have been reported in Peru and the PNW (Rossi et al., Citation2006; Wan & Chen, Citation2012).
More information is needed regarding stripe rust virulence dynamics in Alberta in order to select effective R genes for use in breeding. While fungicides have been used as a disease management strategy, use of genetic resistance is more desirable because it reduces producer inputs, is not affected by timing of application as fungicides are, and minimizes potential environmental harm. Virulence of Pst from 1984 to 2002 in western Canada has been reported (Su et al., Citation2003), but no study on virulence dynamics of Pst and Psh in central Alberta has been conducted. Thus, the objectives of the present study were to identify the pathotypes of Pst and Psh that are present in central Alberta. This will provide an understanding of the epidemiology of Pst and Psh and information on potentially effective host genes that are useful for breeding resistance in wheat and barley.
Materials and methods
Wheat and barley leaves with stripe rust pustules were sampled from experimental plots and commercial fields at various locations in Alberta. Diseased leaves were placed in a paper envelope and stored in a portable cooler. Samples were then stored at 4 °C if P. striiformis urediniospores were to be processed within one week, or at −20 °C if samples were to be processed later. Urediniospores were collected by rubbing and tapping infected leaves over a piece of aluminium foil, and from infected plants that were inoculated by directly rubbing urediniospores of an infected leaf onto susceptible wheat or barley seedlings. Spores were transferred to 1.5 mL cryo vials, dried in vacuum desiccator for 12 hr, and were stored in liquid nitrogen or a −80 °C freezer until needed.
To purify and propagate a single pustule isolate, urediniospores collected from wheat and barley were used to inoculate the host species from which they were collected. Vials carrying urediniospores were heat shocked at 40 °C in a water bath for 5 min after they were removed from a −80 °C freezer. Susceptible wheat (‘Avocet S’ or ‘Morocco’) and barley (HB522 or ‘Mahigan’) were planted in Pro-Mix BX®, a soilless potting mix nutrient medium [Premier Horticulture Inc. Quakertown, PA], in 18-cell plastic trays in a greenhouse. A slow-release fertilizer (N–P–K, 14–14–14) was added at approximately 350 g per 30 kg medium. Trays were seeded with a single cultivar/line, with four seeds per cell, and grown until the 2-leaf stage. Plants were misted using an atomizer containing one drop of Tween 20 per litre of distilled water to facilitate infection. Urediniospores of an isolate were mixed with a fine grade talcum powder [Fisher Scientific, Fair Lawn, NJ] at an approx. 1 : 20 ratio by volume (Chen et al., Citation2010) for inoculation. The mixture was then filtered through four layers of cheesecloth lining a mesh strainer onto the tray of seedlings. Maleic acid hydrazine (MAH) was added at 1 L per tray in a concentration of 0.033 g L−1 to slow down plant growth at the time of inoculation. The inoculated tray was covered immediately with a plastic dome with a cheesecloth top and then a second plastic dome. The tray with the domes was covered by a black plastic bag and incubated in the dark at 10 °C for 24 hr in a growth chamber. The trays were then uncovered, except for the plastic dome with cheesecloth on it to prevent cross contamination while maintaining aeration. Inoculated plants were incubated at 12/16 °C with a 16-hr photoperiod. When pustules began to develop, leaf sections bearing a single pustule were cut from the plants and placed in individual Petri dishes containing 0.5% water agar with 10 mg kinetin L−1 with the cut end of the leaf embedded in the agar to allow the leaf to hang freely in an inverted Petri dish. The infected leaves were incubated under constant fluorescent light for 2–4 days at 20 ± 1 °C. Urediniospores were collected by tapping the plate gently so the spores fell onto a piece of aluminium foil, and stored immediately in a refrigerator at 4 °C. Spores were harvested several times from the incubated leaf, vacuum-dried overnight, and stored in vials in a −80 °C freezer. Once a single pustule isolate was obtained, it was then re-inoculated onto the seedlings of the susceptible host and harvested again until a quantity of spores sufficient for testing had been acquired.
In order to identify pathotypes, virulence or avirulence of isolates on a set of differentials with known resistance genes was examined. To differentiate Pst pathotypes, we used 19 wheat differentials, of which 18 were near-isogenic lines, each carrying a single R gene, and ‘Avocet S’ (Null) was used as a susceptible check (Wellings et al., Citation2004), supplemented with barley differentials (). To differentiate Psh races, we used 12 barley differential cultivars (Chen & Line Citation2002; Chen, Citation2007), supplemented with the wheat differentials. Isolates were also evaluated using wheat and barley cultivars/genotypes grown in Alberta or used in a local breeding programme (). Using the same procedure described above, three seeds of each differential and cultivar/line were planted in root trainers. Seedlings at the 2-leaf stage were inoculated with one isolate. The inoculated plants were incubated in a growth chamber as described earlier. Three weeks after inoculation, disease reactions were scored using infection type (IT) and intensity of sporulation on a 0–9 disease scale (Line & Qayoum, Citation1992). The experiments were carried out twice for each isolate. The mode of the ratings from all 12 plants was used for deciding the IT for each differential and cultivar/line. The cut-off point between virulence and avirulence described by Chen et al. (Citation2010) was used, where ITs of 0–4 are considered to be avirulent and 5–9 are virulent. Isolates with distinct virulence phenotypes on the differentials of their primary host were scored as different pathotypes and assigned a number. Pathotypes were numbered in order of decreasing virulence complexity. Virulence profiles of P. striiformis isolates were transformed to binary codes based on a rating of 1 (virulence) or 0 (avirulence). Cluster analysis was performed using unweighted pair grouping by mathematical average algorithms (UPGMA) from simple matching similarity values (Sneath & Sokal, Citation1973). Phenograms were then constructed using the SAHN program of the NTSYS-pc package (Rohlf, Citation2000).
Table 1. Virulence frequency of Puccinia striiformis f. sp. tritici (Pst) and f. sp. hordei (Psh) on wheat and barley differentials
Table 2. Virulence frequency of Puccinia striiformis f. sp. tritici (Pst) and f. sp. hordei (Psh) on barley and wheat cultivars/genotypes
Results
Samples collected from central AB (2007 and 2008), Creston, BC (2009) and Winnipeg, MB (2005) were purified and propagated on susceptible ‘Avocet S’ or ‘Morocco’ wheat and susceptible HB522 or ‘Mahigan’ barley. This resulted in 38 single pustule isolates from wheat and 23 from barley. Isolates collected from wheat were virulent on 9 to 14 wheat differentials and avirulent on all barley differentials except for ‘Topper’, ‘Abed Binder 12’, ‘Trumpf’ and ‘Hiproly’ (). Cluster analysis showed no grouping of isolates by location or year (), but clearly separated isolates based on forma specialis. All isolates collected from wheat were Pst, but two isolates (T-B-08-EDM-51 and T-B-08-LAC-45) originally collected from barley were virulent on wheat (12 and 9 differentials, respectively) and not on any barley differentials. Thus, based on cluster analysis and virulence assessment, 38 isolates were identified to be Pst. Isolates identified as Pst were then identified to pathotypes based on their reaction on 19 wheat differentials (). Of the 38 Pst isolates, 16 (42%) were avirulent on genes Yr1, Yr5, Yr10, Yr15, Yr24, Yr28 and YrSP and virulent on the other Yr genes used. The second most common pathotype was from seven isolates (18%) and was similar to the dominant pathotype but lacked virulence on YrCV. The next three most common pathotypes were similar to the dominant pathotype but with virulence on one or both of genes Yr24 and Yr28. The remainder of the Pst pathotypes, each consisting of a single isolate, differed in their virulences from the most common pathotype by up to three differentials ().
Fig. 1. Hierarchical classification of 61 Puccinia striiformis isolates collected from central AB, Creston, BC and Winnipeg, MB during 2007–2009 based on virulence on wheat and barley differentials.
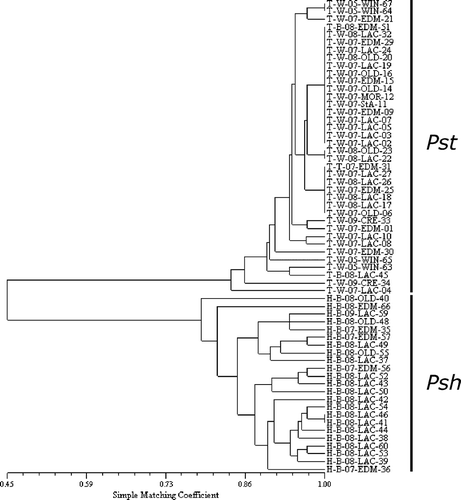
Table 3. Virulence spectra and pathotype frequency of Puccinia striiformis f. sp. tritici isolates sampled in 2007, 2008 and 2009 from central AB, Winnipeg, MB and Creston, BC, determined using wheat and barley differentials
The virulence frequencies of the P. striiformis isolates to wheat differential isogenic lines are reported in . Isogenic lines with genes Yr1, Yr5, Yr15 and YrSP were resistant to all 13 pathoypes, while differentials YrA, Yr2, Yr6, Yr7, Yr9 and Yr26 were susceptible to all. Isogenic lines Yr10, Yr24, Yr28 and YrCV were susceptible to 7.9%, 21%, 18% and 71% of Pst isolates, respectively, while virulence to differentials Yr8, Yr17, Yr27 and Yr31 occurred in over 90% of isolates.
The 38 Pst isolates were also inoculated on wheat, triticale and barley cultivars/genotypes. The spring wheat cultivars ‘AC Barrie’, ‘AC Crystal’, ‘AC Bellatrix’ and ‘Glenlea’ were susceptible to all isolates and the spring triticale ‘AC Ultima’ was susceptible to all but five isolates (). Winter wheat ‘Radiant’ was resistant to all but three Pst isolates. Barley ‘Seebe’, ‘Condor’ and M79530001 were resistant to all Pst isolates, ‘Falcon’ was susceptible to two isolates, while ‘Mahigan’ and HB522 barley were susceptible to 84% and 95% of the Pst isolates, respectively (). As was found when using differential lines, all Pst isolates formed a cluster distinct from all Psh isolates when grouped by virulence using cultivars/lines ().
Fig. 2. Hierarchical classification of 61 Puccinia striiformis isolates collected from central AB, Creston, BC and Winnipeg, MB during 2007–2009 based on virulence on wheat and barley cultivars/genotypes.
Isolates collected from barley were evaluated on both barley and wheat differentials. All isolates were avirulent on most wheat differential lines except ‘Avocet S’, YrA, Yr1, Yr6, Yr26 and Yr31 (). All isolates were virulent on the barley differential ‘Topper’, avirulent on ‘Heils Franken’, ‘Emir’ and ‘Astrix’ and variable across the remaining barley differentials (). Additionally, previous cluster analysis of isolates using differential lines clearly separated isolates into two groups (). Thus, the 23 isolates from barley were identified to be P. striiformis f. sp. hordei (Psh).
Table 4. Virulence spectra and pathotype frequency of Puccinia striiformis f. sp. hordei isolates sampled in 2007 and 2008 from central AB, determined using wheat and barley differentials
The Psh isolates were capable of attacking two to eight barley differentials in addition to a number of wheat differentials (). There were 15 Psh pathotypes identified based on their reaction to the barley differentials. There were 10 pathotypes consisting of a single isolate, four consisting of two isolates and one pathotype consisting of five isolates. There were 22 of 23 Psh isolates virulent on both barley and wheat, while one isolate (H-B-08-LAC-43) was only virulent on barley. Inoculation of Psh isolates on barley cultivars showed that ‘Mahigan’ and HB522 were susceptible to all Psh isolates (). The barley cultivars/genotypes ‘Seebe’, ‘Falcon’, ‘Condor’ and M79530001 were susceptible to 8 to 13 of the 23 isolates tested (). All wheat and triticale cultivars were resistant except for ‘AC Bellatrix’, which was susceptible to nearly 75% of the Psh isolates.
Discussion
The present study showed that the majority of Pst and Psh pathotypes were virulent on both wheat and barley cultivars/genotypes, such as ‘AC Bellatrix’, ‘Mahigan’ and HB522. Virulence of Pst towards susceptible barley and Psh towards susceptible wheat has previously been reported in North America (Chen et al., Citation1995). Whether these shared virulences are relevant under natural conditions is unknown. Whether the occurrence of members of a forma specialis on a species considered a non-host occurs frequently enough to produce substantial yield losses or results in sufficient primary inoculum for infection of the main host remains unexamined and unknown.
Cluster analysis clearly defined the two formae speciales based on virulence phenotypes using differentials and cultivars/genotypes, and there was no apparent separation of the sampled isolates by year and location (, ). The vast majority of Pst isolates examined shared virulence on Yr2, Yr6, Yr8 and Yr9. That particular combination of virulences is considered to be indicative of an invasive population that has been rapidly spreading around the world since 2000 (Hovmøller et al., Citation2008). Hovmøller et al. (Citation2008) characterized two strains within the population, the first of which was present in Europe and western and central Asia since 2000–2001, and the second occurring in the USA and Australia since 2000 and 2002, respectively. Isolates with this unusual virulence phenotype quickly became the dominant form of stripe rust in the USA, western Australia, the Mediterranean and southern Europe (Chen et al., Citation2002; Wellings, Citation2007; Markell & Milus, Citation2008; Bahri et al., Citation2009).
All Pst isolates examined in the present study also had virulence towards YrA. Although not included in the differential set commonly used in the USA (Wan & Chen, Citation2012) or that of Hovmøller et al. (Citation2008), the work of Wellings (Citation2007) and Bahri et al. (Citation2009) showed that virulence towards YrA is typical of this invasive population. Virulence on Yr8 and Yr9 together, which is characteristic of this invasive population, was unknown in North America prior to 2000. During 2000, isolates with virulence on Yr8 and/or Yr9 composed the vast majority of stripe rust isolates recovered in the USA and were found in all regions of the USA where stripe rust was present (Chen et al., Citation2002). The same situation occurred in Canada during the same year (Su et al., Citation2003). These invasive isolates have remained the dominant members of the Pst population in the USA through 2007 (Chen et al., Citation2010). Since the USA acts as the source of stripe rust in Canada, the continued presence of these isolates in Canada is not surprising.
The majority of Pst isolates collected differed from their closest relative by only a single virulence factor. This suggests that the stripe rust pathotypes found in Alberta have evolved in a stepwise manner, where single avirulence genes are gained or lost to produce new races. Stepwise evolution has been shown to be the main process that generates new stripe rust races in other regions (Wellings & McIntosh, Citation1990; Hovmøller et al., Citation2002) as well as leaf rust races in North America (Ordoñez & Kolmer, Citation2009). Chen et al. (Citation2010) postulated that the majority of new races that have occurred in the USA since 2000 can be explained by the stepwise gain or loss of virulence.
The majority of pathotypes in this study were also virulent on R genes not commonly associated with the invasive population. Virulence frequency towards Yr17 was approximately 95%. Although virulence on Yr17 occurred previously in Denmark (Hovmøller, Citation2001), it was not present in the invasive population in Europe and north Africa (Hovmøller et al., Citation2008; Bahri et al., Citation2009). The invasive population that arrived in western Australia, presumed to be the same as the invasive population in North America, also lacked virulence on Yr17, but mutated within 3 years to gain virulence on Yr17 (134 E16 A + Yr17 +) (Wellings, Citation2007). It is logical to conclude that the invasive population that arrived in North America was also avirulent and that the presence of virulence towards Yr17 is a mutation that occurred later. Yr17 has been widely used in breeding programmes, as it confers resistance to both rust and eyespot (McIntosh et al., Citation1995). Therefore, isolates with that virulence would have an advantage due to a wider range of cultivars as susceptible hosts. The timing and location of the development of virulence towards Yr17 in the invasive population within North America is unknown. Limited testing of American isolates up to 2005 revealed no virulence towards Yr17 (Hovmøller et al., Citation2008). Virulence towards Hyak (Yr17, YrTye) in combination with differential lines Yr8 and Yr9 was not detected until 2007 and seems to be confined to areas of the USA west of the Rocky Mountains (Chen et al., Citation2010). Previous studies in Canada did not include a Yr17 differential, thus there is no prior knowledge of the reaction of Canadian isolates to Yr17 (Su et al., Citation2003).
As with Yr17, virulence towards Yr27 is not associated with the invasive population, but occurred in approximately 92% of isolates. It has not been detected in Australia (Wellings, Citation2007), and it was extremely rare in European and Asian collections (Bahri et al., Citation2009). Virulence towards Yr27 may have evolved after the invasive population entered North America, but as there is no Yr27 differential line within the differential set commonly used in the USA, this is unclear. Yr27 has been widely used in spring wheat breeding programmes across North America due to its linkage to leaf rust resistance genes Lr13 and Lr23 and association with the stem rust resistance gene Sr10 (McDonald et al., Citation2004). Virulence on Yr27 would be favoured on cultivars containing this gene, thus increasing the amount of inoculum arriving in Canada. Yr27 has been overcome in recent years in parts of Asia (Wellings et al., Citation2009).
Virulence towards resistance genes Yr26, Yr31 and YrCV were also present in the majority of isolates. Differentials known to contain resistance genes Yr26, Yr31 and YrCV are not commonly used, so it is unknown if the corresponding virulences are typical or not. Yr26 has been reported to be the same gene as Yr24 (Li et al., Citation2006; Wen et al., Citation2008). Virulence to Yr26 was common while virulence to Yr24 was rare. Similar findings have occurred in Ethiopia (Dawit et al., Citation2009). It has been reported that the ‘Avocet’ Yr26 isogenic line does not contain Yr26, but a different unknown Yr gene (X.M. Chen, personal communication). This would explain the discrepancy between the reaction on the Yr24 and Yr26 differential. Virulence to YrCV has been reported in Ethiopia in isolates with virulence spectra typical of the invasive population, so it is possible that the virulence to YrCV arrived in Canada with the invasive population (Dawit et al., Citation2009).
During 2007 and 2008, the R genes that remained effective were Yr1, Yr5, Yr10, Yr15 and YrSP. Virulence on Yr10 in combination with Yr8 and Yr9 was first detected in 2000 in the western USA where it was thought to be contained (Chen et al., Citation2002). However, it was found in pathotypes from Creston, BC and Edmonton, AB. Although no isolates were able to cause disease on Yr1, it is unlikely to be of any use for long-term stripe rust resistance as races virulent on Yr1, Yr8 and Yr9 have emerged within the western USA (Chen et al., Citation2010). Isogenic line Yr5 has remained resistant to all stripe rust races known in the Americas (Wan & Chen, Citation2012). As a result, R genes such as Yr5 and Yr15 are tempting targets for use in breeding programmes, and markers to assist in molecular breeding have been identified for both genes (Chagué et al., Citation1999; Peng et al., Citation2000; Chen et al., Citation2003; Yan et al., Citation2003). Despite Yr5 and Yr15 being deployed in few commercial cultivars, pathotypes have been identified that are capable of overcoming those genes (Zeybek & Yigit, Citation2004; Hovmøller & Justesen, Citation2007). Virulence to YrSP has been detected previously in a minority of races in Canada prior to 2000 (Su et al., Citation2003). Like Yr5 and Yr15, YrSP has not been deployed in common cultivars (Wellings, Citation2007). The deployment of Yr5, Yr15 and YrSP in combination within a cultivar might help provide reasonably durable stripe rust resistance.
In early studies, two races were considered to be the major components in the P. striiformis f. sp. hordei populations of North and South America (Marshall & Sutton, Citation1995; Brown et al., Citation2001). Many more Psh races in the USA have recently been detected as a result of changes in virulence (Chen, Citation2007). All Psh pathotypes detected in the present study () were different from the original races identified by Dubin & Stubbs (Citation1986). In comparison with the identified Psh races in the USA, all pathotypes except numbers 2, 4 and 15 are different from those reported previously in the USA (Chen, Citation2007, Citation2008). Two major differences between USA races and the similar pathotypes from Alberta are the occurrence of virulence on ‘Varunda’ and lack of virulence on ‘Bancroft’ in the majority of central Albertan isolates.
The widespread and common occurrence of Pst isolates with Yr8 and Yr9 virulence in Alberta indicated that the wheat stripe rust population in Alberta is dominated by members or close relatives of the invasive population that has recently spread across America and other wheat-growing areas of the world. Barley stripe rust was more diverse than that of Pst. As this is the first study of Psh distribution in Canada, further monitoring and research will be required to determine if the pathogen population is stable or subject to frequent changes. Overlapping virulence between Pst and Psh towards seedlings of both wheat and barley was demonstrated in the present study. These observations highlight that it is prudent to practice stripe rust management on both barley and wheat cultivars that are widely grown in central Alberta, despite the fact that the disease is more frequently observed on wheat than barley in this area.
Supplementary table 1
Download MS Word (320.5 KB)Acknowledgements
The authors wish to thank L. Langford and L. Vandermaar for technical assistance. The authors also wish to express gratitude to X.M. Chen and A. Navabi for providing barley and wheat differentials and B. McCallum and H. Booker for providing pathogen samples. The funding provided by Alberta Crop Industry Development Fund for this project is gratefully acknowledged.
References
- Anonymous . 2010 . Agriculture Statistics Year Book 2009 , Edmonton , AB : Alberta Agriculture and Rural Development, Statistics and Data Development Branch .
- Bahri , B. , Leconte , M. , Ouffroukh , A. , Vallavieille-Pope , C. and Enhalbert , J. 2009 . Geographic limits of a clonal population of wheat yellow rust in the Mediterranean region . Mol. Ecol. , 18 : 4165 – 4179 .
- Brown , W.M. , Jr , Hill , J.P. and Velasco , V.R. 2001 . Barley yellow rust in North America . Annu. Rev. Phytopathol. , 39 : 367 – 384 .
- Chagué , V. , Fahima , T. , Dahan , A. , Sun , G.L. , Korol , A.B. , Ronin , Y.I. , Grama , A. , Röder , M.S. and Nevo , E. 1999 . Isolation of microsatellite and RAPD markers flanking the Yr15 gene of wheat using NILs and bulked segregant analysis . Genome , 42 : 1050 – 1056 .
- Chen , X.M. 2005 . Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat . Can. J. Plant Pathol. , 27 : 314 – 337 .
- Chen , X.M. 2007 . Challenges and solutions for stripe rust control in the United States . Aust. J. Agri. Res. , 58 : 648 – 655 .
- Chen , X.M. 2008 . Races of Puccinia striiformis f. sp. hordei in the United States from 2004 to 2007 . Barley Newsletter , 51 : WABNL51
- Chen , X.M. and Line , R.F. 2002 . Identification of genes for resistance to Puccinia striiformis f. sp. hordei in 18 barley genotypes . Euphytica , 129 : 127 – 145 .
- Chen , X.M. , Line , R.F. and Leung , H. 1995 . Virulence and polymorphic DNA relationships of Puccinia striiformis f. sp. hordei to other rusts . Phytopathology , 85 : 1335 – 1342 .
- Chen , X.M. , Moore , M. , Milus , E.A. , Long , D.L. , Line , R.F. , Marshall , D. and Jackson , L. 2002 . Wheat stripe rust epidemics and races of Puccinia striiformis f. sp. tritici in the United States in 2000 . Plant Dis. , 86 : 39 – 46 .
- Chen , X.M. , Soria , M.A. , Yan , G.P. , Sun , J. and Dubcovsky , J. 2003 . Development of sequence tagged site and cleaved amplified polymorphic sequence markers for wheat stripe rust resistance gene . Yr5. Crop Sci. , 43 : 2058 – 2064 .
- Chen , X.M. , Penman , L. , Wan , A. and Cheng , P. 2010 . Virulence races of Puccinia striiformis f. sp. tritici in 2006 and 2007 and development of wheat stripe rust and distributions, dynamics, and evolutionary relationships of races from 2000 to 2007 in the United States . Can. J. Plant Pathol , 32 : 315 – 333 .
- Dawit , W. , Flath , K. , Weber , W.E. , Schumann , E. and Kosman , E. 2009 . Virulence and diversity of Puccinia striiformis f. sp. tritici in Ethiopia . Can. J. Plant Pathol. , 31 : 211 – 219 .
- Dubin , H.J. and Stubbs , R.W. 1986 . Epidemic spread of barley stripe rust in South America . Plant Dis. , 70 : 141 – 144 .
- Fraser , W.P. and Conners , I.L. 1925 . The Uredinales of the prairie provinces of western Canada . Trans. R. Soc. Can. , 19 : 275 – 308 .
- Hovmøller , M.S. 2001 . Disease severity and pathotype dynamics of Puccinia striiformis f. sp. tritici in Denmark . Plant Pathol. , 50 : 181 – 189 .
- Hovmøller , M.S. and Justesen , A. 2007 . Appearance of atypical Puccinia striiformis f. sp. tritici phenotypes in north-western Europe . Aus. J. Agri. Res. , 58 : 518 – 524 .
- Hovmøller , M.S. , Justesen , A.F. and Brown , J.K.M. 2002 . Clonality and long-distance migration of Puccinia striiformis f. sp. tritici in north-west Europe . Plant Pathol. , 51 : 24 – 32 .
- Hovmøller , M.S. , Yahyaoui , A.H. , Milus , E.A. and Justesen , A.F. 2008 . Rapid global spread of two aggressive strains of a wheat rust fungus . Mol. Ecol. , 17 : 3818 – 3826 .
- Li , F.Q. , Li , Z.F. , Yang , W.Y. , Zhang , Y. , He , Z.H. , Xu , S.C. , Singh , R.P. , Qu , Y.Y. and Xia , X.C. 2006 . Molecular mapping of stripe rust resistance gene YrCH42 in Chinese wheat cultivar Chuanmai 42 and its allelism with Yr24 and Yr26 . Theor. Appl. Genet. , 112 : 1434 – 1440 .
- Line , R.F. and Qayoum , A. 1992 . Virulence, aggressiveness, evolution, and distribution of races of Puccinia striiformis (the cause of stripe rust of wheat) in North America, 1968–87 . USDA-ARS, Technical Bulletin no. , 1788 : 44
- Manners , J.G. 1950 . Studies on the physiologic specialization of yellow rust (Puccinia glumarum (Schm.) Erikss. & Henn.) in Great Britain . Ann. Appl. Biol. , 37 : 187 – 214 .
- Markell , S.G. and Milus , E.A. 2008 . Emergence of a novel population of Puccinia striiformis f. sp. tritici in eastern United States . Phytopathology , 98 : 632 – 639 .
- Marshall , D. and Sutton , R.L. 1995 . Epidemiology of stripe rust, virulence of Puccinia striiformis f. sp. hordei, and yield loss in barley . Plant Dis. , 79 : 732 – 737 .
- McCallum , B. , Fetch , T. Jr. , Seto-Goh , P. , Xi , K. and Turkington , T.K. 2006 . Stripe rust of wheat and barley in Manitoba, Saskatchewan and Alberta in 2005 . Can. Plant Dis. Surv. , 86 : 50
- McDonald , D.B. , McIntosh , R.A. , Wellings , C.R. , Singh , R.P. and Nelson , J.C. 2004 . Cytogenetical studies in wheat XIX. Location and linkage studies on gene Yr27 for resistance to stripe (yellow) rust . Euphytica , 136 : 239 – 248 .
- McIntosh , R.A. , Wellings , C.R. and Park , R.F. 1995 . Wheat Rusts: An Atlas of Resistance Genes , Australia : Commonwealth Scientific and Industrial Research Organization, and Dordrecht: Kluwer Academic .
- Milus , E.A. , Kristensen , K. and Hovmøller , M.S. 2009 . Evidence for increased aggressiveness in a recent widspread strain of Puccinia striiformis f. sp. tritici causing stripe rust of wheat . Phytopathology , 99 : 89 – 94 .
- Newton , M. and Johnson , T. 1936 . Stripe rust, Puccinia glumarum, in Canada . Can. J. Res. C. , 14 : 89 – 109 .
- Ordoñez , M.E. and Kolmer , J.A. 2009 . Differentiation of molecular genotypes and virulence phenotypes of Puccinia triticina from common wheat in North America . Phytopathology , 99 : 750 – 758 .
- Peng , J.H. , Fahima , T. , Roder , M.S. , Huang , Q.Y. , Dahan , A. , Li , Y.C. , Grama , A. and Nevo , E. 2000 . High-density molecular map of chromosome region harboring stripe-rust resistance genes YrH52 and Yr15 derived from wild emmer wheat, Triticum dicoccoides . Genetica , 109 : 199 – 210 .
- Purdy , L.H. and Allan , R.E. 1963 . Seedling and mature plant reaction of wheat to stripe rust . Plant Dis. Rep. , 47 : 797 – 799 .
- Rohlf , F. 2000 . NTSYS-pc Numerical Taxonomy and Multivariate Analysis System Version 2.10z Manual , New York : Applied Biostatistics .
- Rossi , C. , Cuesta-Marcos , A. , Vales , I. , Gomez-Pando , L. , Orjeda , G. , Wise , R. , Sato , K. , Hori , K. , Capettini , F. , Vivar , H. , Chen , X. and Hayes , P. 2006 . Mapping multiple disease resistance genes using a barley mapping population evaluated in Peru, Mexico, and the USA . Mol. Breed. , 18 : 355 – 366 .
- Sneath , P.A. and Sokal , R.R. 1973 . Numerical Taxonomy , San Francisco , CA : W. H. Freeman Co .
- Su , H. , Conner , R.L. , Graf , R.J. and Kuzyk , A.D. 2003 . Virulence of Puccinia striiformis f. sp. tritici, cause of stripe rust on wheat, in western Canada from 1984 to 2002 . Can. J. Plant Pathol. , 25 : 312 – 319 .
- Wan , A.M. and Chen , X.M. 2012 . Virulence, frequency, and distribution of races of Puccinia striiformis f. sp. tritici and P. striiformis f. sp. hordei identified in the United States in 2008 and 2009 . Plant Dis. , 96 : 67 – 74 .
- Wan , A. , Zhao , Z. , Chen , X. , He , Z. , Jin , S. , Jia , Q. , Yao , G. , Yang , J. , Wang , B. , Li , G. , Bi , Y. and Yuan , Z. 2004 . Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002 . Plant Dis. , 88 : 896 – 904 .
- Wellings , C.R. 2007 . Puccinia striiformis in Australia: a review of the incursion, evolution, and adaptation of stripe rust in the period 1979–2006 . Aust. J. Agric. Res. , 58 : 567 – 575 .
- Wellings , C.R. and McIntosh , R.A. 1990 . Puccinia striiformis f. sp. tritici in Australasia: pathogenic changes during the first 10 years . Plant Pathol. , 39 : 316 – 325 .
- Wellings, C.R., Singh, R.P., McIntosh, R.A., & Pretorius, Z.A. (2004). The development and application of near isogenic lines for the stripe (yellow) rust pathosystem. In Proceedings of the 11th International Cereal Rusts and Powdery Mildew Conference. Norwich, UK. Abstract 1.39, Cereal Rusts and Powdery Mildews Bulletin. www.crpmb.org/icrpmc11/abstracts.htm (http://www.crpmb.org/icrpmc11/abstracts.htm)
- Wellings , C.R. , Singh , R.P. , Yahyaoui , A. , Nazari , K. and McIntosh , R.A. 2009 . “ The development and application of near-isogenic lines for monitoring cereal rust pathogens ” . In Proceedings Borlaug Global Rust Initiative Technical Workshop , 77 – 87 . BGRI Cd Obregon, Mexico .
- Wen , W.E. , Zi , G.Q. , He , Z.H. , Yang , W.Y. , Xu , M.L. and Xia , X.C. 2008 . Development of an STS marker tightly linked to Yr26 against wheat stripe rust using the resistance gene-analog polymorphism (RGAP) technique . Mol. Breed. , 22 : 507 – 515 .
- Yan , G.P. , Chen , X.M. , Line , R.F. and Wellings , C.R. 2003 . Resistance gene analog polymorphism markers co-segregating with the Yr5 gene for resistance to wheat stripe rust . Theor. Appl. Genet. , 106 : 636 – 643 .
- Zadoks , J.C. 1961 . Yellow rust on wheat. Studies in epidemiology and physiologic specialization . Tijdschr. Plantenzek. , 67 : 69 – 256 .
- Zeybek , A. and Yigit , F. 2004 . Determination of virulence genes frequencies in wheat stripe rust (Puccinia striiformis f. sp. tritici) populations during natural epidemics in the regions of southern Aegean and western Mediterranean in Turkey . Pak. J. Biol. Sci. , 7 : 1967 – 1971 .