Abstract
An outbreak of Dooks needle blight (Lophophacidium dooksii) in a five-needle pine genetic archive containing nine five-needle pine species and their interspecific hybrids provided an opportunity to observe the differential susceptibility of these pine species to the disease. Survey results indicated that four of the nine species in the archive were susceptible, including new hosts western white pine (Pinus monticola) and Himalayan blue pine (P. wallichiana), as well as previously reported hosts eastern white pine (P. strobus) and Macedonian pine (P. peuce). Japanese white pine (P. parviflora) and Korean pine (P. koraiensis) were seemingly not affected by the pathogen. While P. peuce and its interspecific hybrids showed the highest susceptibility and the heaviest needle damage, P. parviflora and its interspecific hybrids, including those with P. peuce, were rarely infected, indicating a potential strong and dominant genetic resistance to the pathogen. Pinus monticola and P. strobus showed similar levels of susceptibility and needle damage. Implications of these results to forest health in North America and the Balkans are discussed.
Résumé
Une éclosion de brûlure des aiguilles de Dook (Lophophacidium dooksii) dans un réservoir génétique de pins à cinq aiguilles contenant neuf espèces et leurs hybrides interspécifiques a fourni l'occasion d'observer la susceptibilité différentielle de ces espèces à la maladie. Les résultats de l'étude indiquent que quatre des neuf espèces du réservoir étaient réceptives à l'égard de la maladie, y compris de nouveaux hôtes comme le pin argenté (P. monticola) et le pin pleureur de l'Himalaya, et des hôtes préalablement signalés comme le pin blanc (P. strobus) et le pin de Macédoine (P. peuce). Le pin blanc du Japon (P. parviflora) et le pin de la Corée (P. Koraiensis) n'étaient apparemment pas touchés par l'agent pathogène. Tandis que P. peuce et ses hybrides interspécifiques affichaient le plus haut degré de réceptivité et les dommages les plus lourds causés aux aiguilles, P. parviflora et ses hybrides interspécifiques, y compris ceux créés avec P. peuce, étaient rarement infectés, indiquant une résistance génétique potentielle forte et dominante à l'agent pathogène. Pinus monticola et P. strobus ont affiché des degrés de réceptivité et des dommages aux aiguilles similaires. Nous discutons les implications de ces résultats sur la santé des forêts d'Amérique du Nord et des Balkans.
Introduction
Since the early 1900s, needle browning of eastern white pine (Pinus strobus L.) has been observed in eastern Canada and the northeastern USA (Clinton, Citation1908; Faull, Citation1920); however, a causal agent was not described until more recently. In 1984, Lophophacidium dooksii Corlett & Shoemaker was newly described from symptomatic P. strobus collected in Ontario and New Brunswick (Corlett & Shoemaker, Citation1984). The later description of Merrill et al. (Citation1996) of Canavirgella banfieldii W. Merr. N.G. Wenner & Driesbach, found on P. strobus in Pennsylvania, New Hampshire, Vermont, and Maine, and on Macedonian pine (Pinus peuce Grisebach) in Vermont, closely resembles that of L. dooksii, suggesting that they are descriptions of the same fungus (Laflamme et al., Citation2011; Dr. Robert Shoemaker, pers. comm.). Cultural descriptions of L. dooksii and C. bandfieldii are lacking because the pathogen cannot be grown on artificial media. Recently completed sequencing of the Internal Transcribed Spacer region of DNA samples obtained from needles diagnosed as being infected with either L. dooksii or C. bandfieldii collected from several locations in eastern Canada and the USA has provided evidence that L. dooksii and C. bandfieldii are synonyms for the same species (Gaston Laflamme, pers. comm.). Henceforth, the name L. dooksii should take precedence because its publication predates that of C. bandfieldii.
Incidence of needle blight on P. strobus was initially reported as uncommon in Ontario (Corlett & Shoemaker, Citation1984) and sporadic with only light needle damage in the northeastern USA (Merrill et al., Citation1996). Since then, the disease has been increasingly reported on P. strobus in New Brunswick, Quebec, and Ontario in Canada (Gouv. du Québec, Citation2006; Carter et al., Citation2010; Laflamme et al., Citation2011; Scarr et al., Citation2012), as well as in the USA, including new reports in New England, Michigan, Connecticut and West Virginia (Merrill et al., Citation1997; Douglas, Citation2010; Ewing, Citation2011; Munck et al., Citation2011).
Infection occurs on current-year needles during the period of needle elongation in early summer. Infected needles turn reddish-brown, becoming greyish-tan by the autumn, although their bases remain green (Sinclair & Lyon, Citation2005). Needles within a fascicle are variably affected; one or more needles often remain completely green while the others exhibit varying amounts of browning (Merrill et al., Citation1996). During the autumn and winter, hysterothecia develop on the needles. Ascospores which develop and mature within the hysterothecia are released the following spring and early summer, coinciding with new needle elongation. Although not usually fatal to P. strobus, repeated years of severe needle browning will compromise tree health and may eventually lead to death (Douglas, 2010).
To date, needle blight has been reported only on P. strobus and P. peuce (Corlett & Shoemaker, Citation1984; Merrill et al., Citation1996). Our 2011 survey of a needle disease outbreak in a five-needle pine genetic archive indicates that two other five-needle pine species and their interspecific hybrids can also become infected, and that these pines have differential susceptibility to the disease. In this note, we present the results of our survey and discuss implications for five-needle pine tree improvement programmes.
Materials and methods
The five-needle pine genetic archive
The five-needle pine genetic archive, located in the arboretum of the Ontario Forest Research Institute in Sault Ste. Marie, Ontario (UTM 16 T 695561 5157893; 46° 32′ 46″ N, 84° 26′ 57″ W) was established for a breeding programme used to develop genetic resistance to white pine blister rust (caused by Cronartium ribicola J.C. Fisch.) using an introgressive hybridization approach (Lu & Derbowka, Citation2009). The archive, consisting of nine five-needle pine species from Eurasia and North America and a number of interspecific hybrids among 11 five-needle pine species, comprised 457 trees at 3 × 3 m spacing. Planted in 1995, most of the trees are five-needle pine species grafted onto P. strobus root stock with 105 clones having one ramet, 82 clones having two ramets and six clones having more than five ramets. Where original grafts died after planting, younger and newer generations of interspecific hybrid trees were planted into the gaps. The site is flat and has loamy, well-drained soil. lists the five-needle pine species and interspecific hybrids that were present in the archive.
Table 1. Infection rate and severity of Lophophacidium dooksii damage on five-needle pine species and hybrids in the genetic archive in Sault Ste. Marie, ON
The needle disease
Browning of pine needles in the genetic archive was first recorded in spring 2010. Periodic sampling of three P. peuce trees occurred throughout 2010 and 2011. All samples were examined microscopically in the Ontario Ministry of Natural Resources forest pathology laboratory at the Ontario Forest Research Institute (OFRI). Development and characteristics of sexual and asexual fruiting structures and their spores concurred with the original descriptions of L. dooksii and C. banfieldii. Secondary infections observed on brown needles, especially the needle tips, included Sclerophoma pythiophila (Corda) Höhn, Hendersonia pinicola Wehm, Pestalotiopsis funerea (Desm.) Steyaert, and Lophodermium pinastri (Schrad.) Chevall. Secondary fungal infections are common on needles attacked by C. banfieldii (Merrill et al., Citation1996).
Survey of needle damage
To determine species susceptibility to Lophophacidium dooksii and to rate the severity of damage, a detailed survey of the archive was conducted in late September 2011. Because needle browning was more severe on lower branches, each tree was divided into an upper (> 3.0 m) and a lower (≤ 3.0 m) portion. Trees were visually assessed to estimate the percentage of needle browning in the upper and lower crown by viewing the tree from each of the four cardinal directions. The amount of needle tissue browning was estimated in percentage intervals (0% – no damage, and 1–10%, 11–20% … 91–100%). From every tree with visible needle browning, representative shoot tip samples (approximately 15–20 cm long) bearing current-year needles were collected from the upper and lower parts of the crown and from each of the four cardinal directions (= eight samples per tree). Samples were brought to the OFRI forest pathology laboratory to assess individual needle damage. For each tree, the four shoot samples from the upper crown were pooled, and all needles were stripped from the branches and placed in a bag. The sample was hand mixed, and 150 needles were randomly drawn from the bag. Each needle was assessed to determine the proportion of needle length that was damaged. Damage was recorded in one of five classes: 0 – no browning, and 1 (< 25%), 2 (25–49%), 3 (50–75%) and 4 (> 75%) of needle brown. The same procedure was used to assess needles from the lower crown.
Data analysis was based on clone means. Statistical analysis was performed with a one-way classification to detect differences among tree species and their interspecific hybrids. SAS Glimmix procedure (SAS Institute Citation2011) with the logit link function was used to analyse the categorical data.
Results and discussion
Overall infection
Trees with light to heavy infection were scattered throughout the genetic archive. Overall infection on the site was 26% and 35% among the clones for the upper and lower tree crowns, respectively. Based on field visual estimates, the average percentage of browning of needles on infected trees was 38.5% in the upper crown and 44.6% in the lower crown. Percentage of needle infection and heavier needle damage were greater in the lower than the upper crown. As with many needle diseases, infection might be influenced by humidity and temperature, which are generally higher closer to the ground and in the lower crowns of young pine trees.
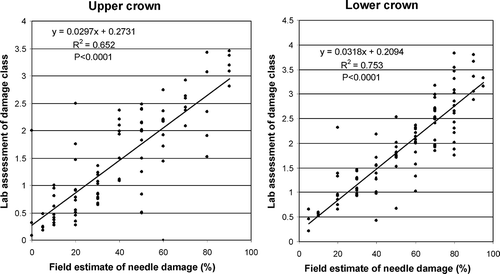
Individual needle assessments in the laboratory confirmed needle damage estimates made in the field, with correlation coefficients of 0.84 between the field and lab assessments for upper tree crown and 0.88 for the lower tree crown, which were highly statistically significant (P < 0.0001) ().
Fig. 1. Correlations between visual estimates of needle damage of five-needle pine species infected with Lophophacidium dooksii in the upper (> 3.0 m) and lower (3.0 m) portions of the crown. Field estimates were in 10% increments and lab estimates in five damage classes: 0 – no browning, and 1 (< 25%), 2 (25–49%), 3 (50–75%) and 4 (> 75%) of needle brown.
Differential susceptibility among five-needle pine species
Four of the nine pine species in the archive exhibited needle blight, including western white pine (Pinus monticola Douglas ex D. Don), P. peuce, P. strobus and Himalayan blue pine (P. wallichiana A.B. Jacks.). Species not affected were Swiss stone pine (P. cembra L.), limber pine (P. flexilis James), Korean pine (P. koraiensis Siebold & Zucc.), Japanese white pine (P. parviflora Siebold & Zucc.) and dwarf stone pine (P. pumila Scrub) (). The observed disease ratings among the five-needle pine species could have been influenced to some degree by the spatial distributions of these species in the archive, since this is a naturally infected site with possible non-uniform pathogen spore distribution and varying micro-environmental conditions, which could cause escapes. Specifically, the possibility that P. cembra, P. flexilis and P. pumila escaped infection cannot be ruled out as they had only one to three trees growing in the archive; thus, their resistance to L. dooksii could have been over-estimated. The reliability of our assessment was higher for P. koraiensis and P. parviflora as eight and nine trees, respectively, were growing in the archive and their hybrid progeny had mimicked their parents. Our confidence was high in the observed infection rates for species with larger number of clones and ramets as they were scattered in the archive and the infected trees appeared throughout the archive. Our confidence was strengthened by the observation that ramets of a susceptible clone of P. monticola located between two other P. monticola clones were all infected while the ramets of adjacent clones were not. Nevertheless, these results need to be confirmed with further observations and possibly inoculation experiments.
The four pine species that were affected by the disease showed considerable differences in the amount of needle browning per tree and the severity of damage on individual needles (). Eighty-five per cent of the P. peuce, 50% of the P. wallichiana, 21% of the P. strobus and 9% of the P. monticola clones were affected by the disease. Only seven P. wallichiana trees from six clones were in the archive; thus, the infection estimate for this species was less reliable than those for the other three species. The average percentage of needle browning on affected trees was relatively low for P. wallichiana (mean = 17%; range: 10–30%), P. strobus (mean = 13%; range: 5–40%) and P. monticola (mean = 18%; range: 5–40%), and high (mean = 57%; range: 10–95%) for P. peuce. Severe needle browning of P. peuce was especially striking (), confirming a previous report of the high susceptibility of this species to the disease (Merrill et al., Citation1996). The needle browning observed on P. monticola and P. strobus was similar, both in the proportion of trees affected and the severity of needle damage. Since this needle blight has not been reported on P. monticola or P. wallichiana, these observations should be of particular interest to forest managers in western North America.
Statistically, P. cembra, P. flexilis, P. pumila, P. monticola and P. strobus were not significantly different from each other in disease incidence (), although the infected and uninfected species may be categorically different. The relatively small sample size of clones within some of these species reduced the statistical power to detect the differences among them. The infection rate of P. wallichiana was significantly higher than these mentioned above. The infection rates of P. peuce and several of its interspecific hybrids were significantly higher than those of P. cembra, P. flexilis, P. pumila, P. monticola, P. strobus and P. wallichiana. A similar trend was evident for the severity of needle damage on infected trees ().
Table 2. Statistical significance test among five-needle pine species and their interspecific hybrids in Lophophacidium dooksii infection rate (upper diagonal) and the severity of needle damage on infected trees at the lower tree crown (lower diagonal)
Except for P. koraiensis and P. pumila, all five-needle pine species are biologically mutually compatible, and produce viable seeds from either controlled- or open-pollination (Critchfield, Citation1986). Pinus peuce is often planted as an ornamental tree worldwide (Lines, Citation1985; Merrill et al., Citation1996; Mortenson & Mack, Citation2006). This pine hybridizes easily with P. strobus and P. monticola and usually produces abundant viable seed (Lu & Derbowka, Citation2009); therefore, it has been used in genetic trials to provide blister rust resistance to these white pine species (Kriebel, Citation1983; Blada, Citation2000; Sniezko et al., Citation2008). Few diseases and little related damage are reported for P. peuce in its native range (Lines, Citation1985); however, this pine from the Balkans appears highly susceptible to infection and damage from this needle blight. Steps should be taken to prevent this disease from spreading into the native range of P. peuce as it could have devastating effects on forest health in those areas. Continued planting of P. peuce in North America may also lead to genetic contamination of the gene pools of our native white pine species, thereby potentially increasing their disease susceptibility, as demonstrated by the susceptibility of our interspecific hybrids between P. peuce and P. strobus or P. monticola ().
Despite the relatively high rate of infection, the severity of needle damage on infected P. wallichiana trees was light (7% and 17% for upper and lower crown). Unlike the browning crown of infected P. peuce, at the time of the survey infected P. wallichiana trees looked green from a distance. Pinus wallichiana is capable of producing viable hybrid seeds with P. strobus or P. monticola, although it is less successful than P. peuce (Critchfield, Citation1986). Because of proven strong resistance to white pine blister rust in some of its interspecific hybrids with P. strobus in the past (Heimburger, Citation1972; Lu et al., Citation2005), P. wallichiana trees were selected and used as major resistance gene donors to P. strobus (Lu & Derbowka, Citation2009). Interestingly, a number of selected individuals in the genetic archive that stemmed from backcrosses of P. strobus with P. wallichiana at various degrees of introgression to P. strobus did not show symptoms of this needle blight. This result was unexpected, as pure P. wallichiana were susceptible to the disease. There is a possibility that the genes conferring genetic resistance to white pine blister rust may also be correlated to resistance to the needle blight, but this requires further study.
Pinus parviflora and P. flexilis are compatible with P. strobus and P. monticola and produce viable seeds (Critchfield, Citation1986), although hybridization between P. strobus and P. flexilis is less successful than other crosses. Strong resistance to white pine blister rust was found in interspecific hybrids between P. strobus and P. parviflora (Lu & Derbowka, Citation2009). Because this species was not infected, using P. parviflora as a major blister rust resistance gene donor may also enhance resistance to needle blight.
Korean pine (P. koraiensis) and its interspecific hybrids with sugar pine (P. lambertiana Dougl.) showed no symptoms of needle infection. Resistance of P. koraiensis may not be relevant to P. strobus and P. monticola because the former is not biologically compatible with these species, making the integration of resistance using traditional breeding approaches more difficult.
Within a pine species, not all trees are equally susceptible to white pine needle blight (Corlett & Shoemaker, Citation1984; Merrill et al., Citation1996). In our survey, almost all of the P. peuce trees and its hybrids were severely affected by needle browning; however, a considerable proportion of P. strobus and P. monticola were not infected (). An infected tree was often located adjacent to an uninfected tree, suggesting substantial genetic variability in disease resistance among individual trees within a species. A selection of putatively resistant P. monticola or P. strobus could build a breeding population with genetic resistance to the needle blight. Before an effective breeding strategy can be developed, more information is needed on gene reaction modes and the inheritance of resistance.
Evidence of inheritable susceptibility to Dooks needle blight
A comparison of needle infection between pure five-needle pine species and their F1 progeny of interspecific hybrids shows that susceptibility to the needle blight may be inheritable. Although data from this survey were not sufficient to estimate genetic parameters controlling this trait, the data did show a correlation between the performances of five-needle pine species and their F1 progeny (; ). None of the seven P. parviflora clones were infected and only one clone of its interspecific hybrids between P. parviflora and other species was lightly infected by the disease ( and ). In contrast, 85% of the 46 P. peuce clones were infected, as were 83% of its F1 interspecific hybrids with other species. The correlation could have been stronger had the F1 data been more balanced with regard to the mating partners of the species encountered (). For example, average infection for P. peuce F1 interspecific hybrid progeny was lowered by its uninfected progeny with P. parviflora.
Table 3. Comparison of needle infection by Lophophacidium dooksii on five-needle pine species and their F1 progeny of interspecific hybrids with other species
Fig. 3. Correlation between disease incidence on lower crown of mother tree species and F1 hybrid progeny susceptibility to Dooks needle blight (see for details).
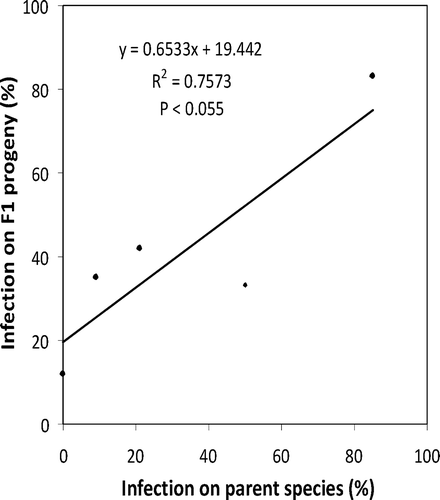
The F1 progeny from resistant P. parviflora mated with susceptible five-needle pine species almost uniformly remained uninfected. Except for one lightly infected progeny of P. strobus × P. parviflora, all F1 progeny between P. parviflora and P. peuce, P. strobus, P. monticola and P. wallichiana were not infected, whether P. parviflora was used as male or female parent (). This consistent result suggests that resistance could have been conferred by dominant major genes which, if confirmed, could be the basis for developing genetic resistance to needle blight.
Conclusions
The occurrence of Dooks needle blight is increasingly common on white pines growing in eastern Canada and the northeastern USA. Although not reported in the native range of P. monticola, this species is susceptible to this needle blight. Action may be needed to prevent spread of the disease to the native range of western white pine. Because P. peuce is highly susceptible to the disease and readily hybridizes with native white pine, planting this species in North America increases the risk of contaminating the P. strobus gene pool with more susceptible alleles. Differential disease susceptibility of five-needle pines and of individuals within a species indicates that genetic resistance, possibly conferred by dominant alleles, exists within and among white pine species. Tree improvement programmes can use this information to enhance genetic resistance to disease. Japanese white pine (P. parviflora) seems a good candidate gene donor to use in developing resistance to Dooks needle blight in P. strobus and P. peuce.
Acknowledgements
The authors thank Mike Francis for assistance with the field survey; Lisa Buse and Trudy Vaittinen for help in editing the manuscript; Robert Shoemaker and Michael Corlett for their advice regarding the taxonomy of the pathogen, Gaston Laflamme for sharing the results of his molecular assessment of the synonymy of L. dooksii and C. bandfieldii, and anonymous reviewers for their helpful and constructive comments.
References
- Blada , I. 2000 . Genetic variation in blister rust resistance and growth traits in Pinus strobus H P. peuce hybrid at age 17 (experiment 1) . For. Genet. , 7 : 109 – 120 .
- Carter, N., Hartling, L., Lavigne, D., Gullison, J., O'Shea, D., Proude, J., et al. (2010). Preliminary summary of forest pest conditions in New Brunswick in 2009 and outlook for 2010. In Proceedings of the Forest Pest Management Forum, 2009. Gatineau, Québec (pp. 106–113) http://publications.gc.ca/collections/collection_2010/nrcan/Fo121-1-2009.pdf (http://publications.gc.ca/collections/collection_2010/nrcan/Fo121-1-2009.pdf) (Accessed: 1 April 2012 ).
- Clinton , G.P. 1908 . “ Pine blight ” . In Rept. of the Botanist for 1907 , 353 – 355 . New Haven , CT : Conn. Agric. Exp. Stn .
- Corlett , M. and Shoemaker , R.A. 1984 . Lophophacidium dooksii n. sp., a phacidiaceous fungus on needles of white pine . Can. J. Bot. , 62 : 1836 – 1840 .
- Critchfield , W.B. 1986 . Hybridization and classification of the white pines (Pinus section strobus) . Taxon , 35 : 647 – 656 .
- Douglas, S.M. (2010). Canavirgella Needlecast of White Pine. New Haven, CT: Conn. Agric. Exp. Stn http://www.ct.gov/caes/lib/caes/pdio/documents/canavirgella_needlecast_of_white_pine_04-10r.pdf (http://www.ct.gov/caes/lib/caes/pdio/documents/canavirgella_needlecast_of_white_pine_04-10r.pdf) (Accessed: 1 April 2012 ).
- Ewing, E.W. (2011). New Threat to Eastern White Pine. The Market Bulletin http://www.wvagriculture.org/market_bulletin/Past_Issues/PDF_Pages/2011/4-11-pg4.pdf (http://www.wvagriculture.org/market_bulletin/Past_Issues/PDF_Pages/2011/4-11-pg4.pdf) (Accessed: 1 April 2012 ).
- Faull , J.H. 1920 . “ ‘Needle blight’ of white pine ” . In Rept. of the Minister of Lands, Forests and Mines of the Prov. of Ont. for the year ending 31st October 1919 , 120 – 122 . Toronto , ON : A.T. Wilgress .
- Gouvernement du Québec (2006). Insectes, maladies et feux dans les forêts québécoises en 2005. Ministère des Ressources naturelles et de la Faune http://www.mrn.gouv.qc.ca/publications/forets/fimaq/insectes/bilan2005.pdf (http://www.mrn.gouv.qc.ca/publications/forets/fimaq/insectes/bilan2005.pdf) (Accessed: 1 April 2012 ).
- Heimburger , C. 1972 . “ Relative blister rust resistance of native and introduced white pines in North America. In R.T. Bingham (dir.) ” . In Biology of Rust Resistance in Forest Trees: Proceedings of a NATO-IUFRO Advanced Study Institute August 17–24, 1969 , 257 – 269 . Washington , D.C : Misc. Publ. 1221 . USDA For. Serv
- Kriebel , H.B. 1983 . Breeding eastern white pine: a world-wide perspective . For. Ecol. Manage. , 6 : 263 – 279 .
- Laflamme , G. , Côté , C. and Innes , L. 2011 . “ White pine needle diseases in eastern Canada ” . In Global Change and Forest Diseases: New Threats, New Strategies , Edited by: Diez , J.J. , Martínez-Álvarez , P. and Romeralo , C. 54 Cantabria , , Spain : Universidad de Valladolid, Spain. IUFRO 2011 WP 7.02.02 . Montesclaros Monastery, May 23–28 2011
- Lines , R. 1985 . The Macedonian Pine (Pinus peuce Grisebach) in the Balkans and Great Britain . Forestry , 58 : 27 – 40 .
- Lu , P. and Derbowka , D. 2009 . Breeding eastern white pine for blister rust resistance: a review of progress in Ontario . For. Chron. , 85 : 745 – 755 .
- Lu , P. , Sinclair , R.W. , Boult , T.J. and Blake , S.G. 2005 . Seedling survival of Pinus strobus and its interspecific hybrids after artificial inoculation of Cronartium ribicola . For. Ecol. Manage. , 214 : 344 – 357 .
- Merrill , W. , Wenner , N.G. and Dreisbach , T.A. 1996 . Canavirgella banfieldii gen. and sp. nov.: a needlecast fungus on pine . Can. J. Bot. , 74 : 1476 – 1481 .
- Merrill , W. , Wenner , N.G. and O'Donnell , J. 1997 . Canavirgella banfieldii needlecast of Pinus strobus in Michigan . Plant Dis. , 81 : 231
- Mortenson , S.G. and Mack , R.N. 2006 . The fate of alien conifers in long-term plantings in the USA . Diversity Distrib. , 12 : 456 – 466 .
- Munck, I.A., Ostrofsky, W.D., & Burns, B. (2011). Pest Alert: Eastern White Pine Needle Damage. USDA For Serv. Northeastern Area State and Private Forestry NA–PR–01–11 http://na.fs.fed.us/pubs/palerts/white_pine/eastern_white_pine.pdf (http://na.fs.fed.us/pubs/palerts/white_pine/eastern_white_pine.pdf) (Accessed: 1 April 2012 ).
- Institute , SAS . 2011 . SAS/STAT 9.2 User's Guide , 2nd , Cary , NC : SAS Institute Inc .
- Scarr , T.A. , Ryall , K.L. and Hodge , P. 2012 . Forest Health Conditions in Ontario, 2011 , Toronto , ON : Ontario Ministry of Natural Resources, Forestry Branch .
- Sinclair , W.A. and Lyon , H.H. 2005 . Diseases of Trees and Shrubs , 2nd , 660 Ithaca , NY : Cornell University Press .
- Sniezko , R.A. , Kegley , A.J. and Danchok , R. 2008 . White pine blister rust resistance in North American, Asian and European species – results from artificial inoculation trials in Oregon . Ann. For. Res. , 51 : 53 – 66 .
