Abstract
Potato tubers exhibiting necrotic rings/arcs were found in a winter potato crop ‘Favorita’ in the subtropical area Huidong county, Guangdong province, China, in 2012. When the symptomatic tubers were cut crosswise, light to dark brown necrotic arcs were observed in the tuber flesh. A mini-survey of the crop found 8% symptomatic tubers in an area of 10 meters2. To reveal the causal agent of the disease, reverse transcription–polymerase chain reactions (RT-PCR) targeting 9 common potato viruses including those viruses (e.g, Potato mop-top virus (PMTV), Tobacco rattle virus, Tomato spotted wilt virus, and Potato virus Y tuber necrosis strain) known to be capable of induction of tuber necrosis, were carried out. Except for PMTV, no other viruses were detected. Sequencing of the 460 bp amplicon revealed that the fragment exhibited a 99-100% sequence identify with most PMTV coat protein sequences deposited in GenBank, which was confirmed by a further phylogenetic analysis. Enzyme linked immunosorbent assay with PMTV antibody on the symptomatic tubers was also carried out, and a positive reading was observed. Together, these results demonstrate that PMTV was the causal agent for the spraing disease in potatoes in the winter crop in Guangdong. To our knowledge, this is the first report of PMTV in Guangdong province, China, and the first scientific confirmation of PMTV in China.
Résumé
On a trouvé, en 2012, dans la région sous-tropicale du comté de Huidong, dans la province du Guandong en Chine, des tubercules de la variété de pommes de terre d'hiver ‘Favorita’ qui affichaient des anneaux et des arcs nécrotiques. Quand on coupait les tubercules symptomatiques dans le sens de la largeur, on pouvait voir, dans leur chair, des arcs nécrotiques brun pâle à brun foncé. Un bref survol des plantes a permis de trouver 8 % de tubercules symptomatiques dans 10 m2 de culture. Afin d'identifier l'agent causal de la maladie, des analyses par RT-PCR ciblant neuf virus courants (p. ex., virus du sommet touffu de la pomme de terre [VSTPT], virus du bruissement du tabac, virus de la tache bronzée de la tomate et virus Y de la pomme de terre [souche causant la nécrose du tubercule]), connus pour induire la nécrose des tubercules, ontété menées. Aucun autre virus à part le VSTPT n'a été détecté. Le séquençage de l'amplicon de 460 bp a révélé que le fragment affichait une séquence identique (99 à 100 %) à la plupart des séquences des protéines de coques déposées dans la GenBank, ce qui a été confirmé par une analyse phylogénétique supplémentaire. Un test ELISA a également été mené avec des anticorps de VSTPT sur les tubercules symptomatiques, et l'on a obtenu un résultat positif. De concert, ces résultats démontrent que le VSTPT était l'agent causal de la tache en couronne chez les pommes de terre de la récolte d'hiver dans le Guandong. À notre connaissance, il s'agit de la première mention du VSTPT dans la province du Guandong et de sa première confirmation scientifique en Chine.
Introduction
Potato mop-top virus (PMTV, genus Pomovirus, family Virgaviridae) is an important potato pathogen that can cause potato spraing, a serious tuber disease whose symptoms include necrotic arcs and lines in infected tubers (Sandgren et al., Citation2002). The symptomatic tubers are rendered unmarketable, leading to great economic losses. PMTV can also induce a wide range of foliar symptoms, including stunting, mottling, chevrons, and yellow blotches or rings, depending on potato cultivars and environmental conditions (Salazar, Citation1996).
PMTV is transmitted by Spongospora subterranea, the causal agent of potato powdery scab disease. Nevertheless, there is no correlation between the occurrence of powdery scab and PMTV infection as PMTV-caused spraing disease is often found in tubers without powdery scab symptoms (Jones & Harrison, Citation1969). The virus occurs in many potato growing regions with cooler climates, such as northern Europe, Andean region of South America and Japan (Carnegie et al., Citation2012). In 2003, the virus was also detected in North America for the first time (Lambert et al., Citation2003), and since then, more PMTV cases have been reported in both the USA and Canada (Xu et al., Citation2004; David et al., 2010; Crosslin, Citation2011; Whitworth & Crosslin, Citation2013).
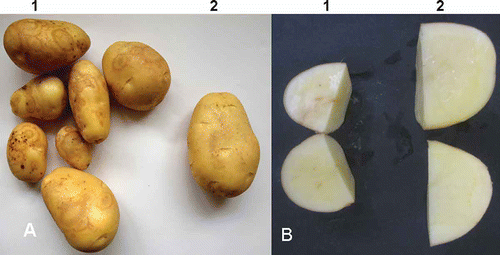
Despite the fact that several reviews (e.g., Salazar, Citation1996; Wang et al., Citation2011) have claimed the presence of PMTV in China, to date, the only experimental evidence to indicate the possible presence of PMTV is from a study by Zhang et al. (Citation2003), in which the “PMTV-like” viral particles were observed from potato plants in Yunnan province. However, the study by Zhang et al. did not perform any pathological, serological, or molecular experiments to validate the “PMTV-like” particles were indeed PMTV. In February 2012, potato tubers exhibiting necrotic arcs/rings (, B) were found in a winter potato crop ‘Favorita’ in the subtropical area of Huidong county, Guangdong province, China, thus prompting research to unveil the causal agent of the disease. Here, we report the detection of PMTV in the symptomatic potatoes using reverse transcription-polymerase chain reaction (RT-PCR), enzyme linked immunosorbent assay (ELISA), and sequence analysis. To our knowledge, this is the first report to confirm the presence of PMTV in China.
Fig. 1. Symptoms of Potato mop-top virus (PMTV) in potatoes collected in Huidong county in Guangdong province, China. A. Necrotic rings/arcs on tubers of potato ‘Favorita’ upon harvesting. 1, symptomatic tubers; 2, symptom-free (healthy-looking) tuber. B. Necrotic rings/arcs in flesh of tubers of potato ‘Favorita’ upon harvesting. 1, symptomatic tuber; 2, symptom-free (healthy-looking) tuber.
Materials and methods
Virus samples and symptom identification
In a field survey of potatoes in a winter potato crop ‘Favorita’ during the harvest in Huidong county in Guangdong province (22.31°N, 115.05°E) in February 2012, potato tubers exhibiting necrotic rings/arcs () were observed, and 23 tubers, approximately 8% of 288 tubers in an area of 10 m2 developed similar symptoms, but the foliar symptoms were difficult to distinguish because of late blight infection. Eight representative symptomatic tubers were brought back to the laboratory at Hunan Agricultural University, Changsha, for laboratory and greenhouse tests. Upon germination, one eye per tuber was cut out, planted in premix potting medium, and grown in a growth chamber at 20-22°C with a photoperiod of 16 h light: 8 h dark. The light intensity was 180 μmolm−2s−1, and the humidity was 70-80%. Foliar symptoms in the plants that developed were observed and recorded at various growth stages (i.e., sprout development, plant establishment, tuber initiation, tuber bulking, and tuber maturation stages).
Enzyme-linked immunosorbent assay (ELISA)
Triple-antibody sandwich ELISA with PMTV-specific monoclonal antibodies (Neogen Europe Ltd, Adgen Phytodiagnostics, Scotland, UK) was used to detect PMTV from potato tubers and leaves according to the manufacturer's guidelines. The absorbance at 405 nm (A405) was recorded using a Multiskan MK3 microplate reader (Thermo Electron Corporation, Waltham, MA, USA).
Reverse transcription-polymerase chain reaction, cloning and sequencing
Total RNA was extracted from 3 g of tuber tissues from the bud end or from potato leaves using the lithium chloride method (Mohapatra et al., Citation1987). Reverse transcription (RT)-PCR assays targeting nine potato viruses including Alfalfa mosaic virus (AlMV), Potato leafroll virus (PLRV), PMTV, Potato virus A (PVA), Potato virus S (PVS), Potato virus X (PVX), Potato virus Y (PVY), Tobacco rattle virus (TRV), and Tomato spotted wilt virus (TSWV) were carried out using primers and PCR conditions described by Crosslin & Hamlin (Citation2011). Upon completion of the above RT-PCR screening for different viruses, a duplex PCR targeting PMTV (primers: forward 5′- CATGAAGGCTGCCGTGAGGAAGT -3′; reverse 5′- CTATGCACCAGCCCAGCGTAACC-3’) (Xu et al., Citation2004) and the potato cytochrome c oxidase subunit I gene (COX1, a housekeeping gene; primers: forward 5’-GGTCGGACATACCCTGAAAC-3’, reverse 5’-CAAAAGTATGAAAAGCTGGAG-3’) (Nie & Singh, Citation2001) was performed using the PCR conditions described by Nie & Singh (Citation2001). The PMTV-like fragment was sequenced after being purified from agarose gel and inserted into a pGM-T cloning vector (TIANGEN Biotech, Beijing, China) according to the manufacturer's instructions. Identities between sequences obtained from GenBank were analyzed using BLAST (http://www.ncbi.nlm.nih.gov/BLAST). The 45 PMTV-Guangdong and other PMTV partial sequences (nt. 360 to nt. 819) of CP-encoding sequence in PMTV RNA3 were aligned using ClustalW2 (Larkin et al., Citation2007) and were subjected to phylogenetic analysis using MEGA version 4.2 (Tamura et al., Citation2007).
Results and discussion
Potato is susceptible to more than 35 different viruses or virus strains (Slack, Citation2001). Several viruses including PMTV, TRV, TSWV and PVY tuber necrotic strain (PVYNTN) are capable of inducing necrotic rings on potato tubers of sensitive cultivars (Xu et al., Citation2004). Therefore, when tubers exhibiting superficial necrotic rings/arcs () were found in a winter crop of ‘Favorita’, a popular Dutch potato cultivar, one or more of the above mentioned viruses were suspected to be the causal agent. When the symptomatic tubers were cut crosswise, light to dark brown necrotic arcs were observed in the tuber flesh (), resembling symptoms caused by PMTV (spraing) or TRV (corky ringspot) (Weingartner, Citation2001) and thus pointing to a possible infection with either PMTV or TRV or both. Clearly, laboratory tests with established procedures including RT-PCR and ELISA were needed for unambiguous diagnosis.
Firstly, nucleic acids extracted from 2 representative symptomatic tubers and one tuber produced from a virus-free tissue culture plantlet were subjected to reverse transcription (RT)-PCR assays. The first round of RT-PCR, as described by Crosslin & Hamlin (Citation2011), included nine separate reactions, each with specific primers (see Crosslin & Hamlin, Citation2011, for primer sequences), targeting one of nine common potato viruses, namely AlMV, PLRV, PMTV, PVA, PVS, PVX, PVY, TRV and TSWV. Except for PMTV, no other viruses were detected in the 2 symptomatic tubers (data not shown). To validate the results, a second round of RT-PCR with two primer pairs, one specific to PMTV (Xu et al., Citation2004) but different from that used in the first round RT-PCR, and the other specific to potato COX1, a housekeeping gene that served as an internal control (Nie & Singh, Citation2001), was performed. As expected, a PMTV-specific fragment of 460 bp was observed exclusively in the symptomatic tubers whereas the COX1 fragment of 332 bp was observed in both the diseased and the healthy samples (), consistent with the first round RT-PCR assays.
Fig. 2. Reverse transcription (RT)-PCR detection of Potato mop-top virus (PMTV) in potatoes collected in Guangdong province, China. A. Duplex RT-PCR for detection of PMTV and cytochrome c oxidase subunit one gene (COX1) in symptomatic tubers. B. Duplex RT-PCR for detection of PMTV and COX1 in progeny plants resulting from symptomatic tubers. The COX1 served as an internal indicator for the RT-PCR. The PMTV and COX1 primers were as reported in Xu et al. (Citation2004) and Nie & Singh (Citation2001), respectively. The fragment sizes were 460 bp for PMTV and 332 bp for COX1. Lanes 1 and 2, symptomatic tubers; lane 3, virus-free mini tuber (virus-free sample); lanes 4 and 5, progeny plants from symptomatic tubers; lane 6, PMTV-positive tuber, served as a positive control ; lane 7, virus-free plant.
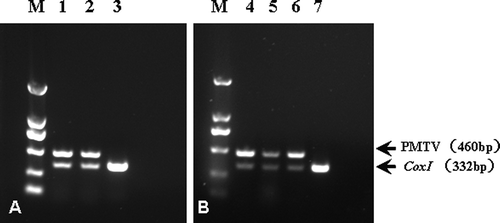
Despite the same results by both RT-PCRs, sequences would provide ultimate evidence to unveil whether indeed a PMTV-amplicon has been amplified. Thus, the PMTV-fragment from the second round RT-PCR was sequenced after being inserted into a pGM-T cloning vector. BLAST analysis demonstrated that the amplicon shared a 100% nucleotide sequence identity with 14 PMTV sequences and a 99% nucleotide sequence identity with another 30 PMTV sequences deposited in GenBank, thus unambiguously confirming its PMTV identity. It is noteworthy that a relatively low nucleotide sequence identity of 77% was found between the amplicon and two sequences (HQ171916 and HQ171917) reported from isolates in Colombia, suggesting a considerable evolutionary distance between these two Colombian isolates and other isolates (including this newly identified isolate, designed PMTV-Guangdong isolate, GenBank no. KC758592) reported to date. Phylogenetic analysis indeed grouped HQ171916/ HQ171917 in a cluster while the remaining isolates were grouped in another cluster ().
Fig. 3. Phylogenetic tree for Potato mop-top viurs (PMTV) isolates. The partial PMTV RNA3 sequence (nucleotides 360 to 819) of PMTV isolate “Guangdong” were analyzed with the sequences of PMTV retrieved from GenBank by employing MEGA version 4.2 with the neighbor-joining (NJ) method (Tamura et al., Citation2007). The numbers beside branches are bootstrap values of 100 replicates.

ELISA with PMTV-antibody was also carried out on sprouts from the symptomatic tubers. As shown in , a positive reading (A405 ≥ three times of the negative control) was obtained for these samples. Thereafter, the PMTV-positive tubers were planted in a growth chamber at 20-22°C, and the resulting plants were subjected to RT-PCR and ELISA analysis and symptom observation. As expected, PMTV was detected by RT-PCR in the plants readily (, lanes 4-5), indicating that the virus has been passed on to the progeny plants. However, a parallel ELISA on the same plants did not yield a positive reading (data not shown), suggesting that the virus titre might be below the threshold of ELISA. This is not unexpected as discrepancy between ELISA and RT-PCR tests on the virus has been observed by others in plants produced from infected tubers (Xu et al., Citation2004). Visual observation of the progeny plants did not identify any clear-cut symptoms (data not shown), similar to those reported by Xu et al. (Citation2004) for Canadian potatoes.
Table 1. Detection of Potato mop-top virus (PMTV) by triple antibody sandwich enzyme-linked immunosorbent assay (ELISA)
To our knowledge, this is the first report of PMTV in Guangdong province, a subtropical area of southern China. This finding also confirms the occurrence of PMTV in China. Further research is needed to determine the scope of occurrence of PMTV in potato growing areas in China and to develop strategies for control and management of the disease in the country.
Acknowledgments
This research was supported by the Natural Science Foundation of Hunan Province under the project #11JJ2018 and by the Scientific Research Fund of Hunan Provincial Education Department under the project #10A058 to X. Hu.
References
- Carnegie , S.F. , Cameron , A.M. and McCreath , M. 2010 . Foliar symptoms caused by Potato mop-top virus on potato plants during vegetative propagation in Scotland and their association with tuber yield, spraing and tuber infection . Potato Res , 53 : 83 – 93 .
- Carnegie , S.F. , Davey , T. and Saddler , G.S. 2012 . “ Prevalence and distribution of Potato mop-top virus in Scotland ” . In Plant Pathol., 61 623 – 631 .
- Crosslin , J.M. 2011 . “ First report of Potato mop-top virus on potatoes in Washington State ” . In Plant Dis Vol. 95 , 1483
- Crosslin , J.M. and Hamlin , L.L. 2011 . Standardized RT-PCR conditions for detection and identification of eleven viruses of potato and potato spindle tuber viroid . Amer. J. Potato Res , 88 : 333 – 338 .
- Jones , R.A.C. and Harrison , B.D. 1969 . The behaviour of potato mop-top virus in soil, and evidence for its transmission by Spongospora subterranea (Wallr.) Lagerh . Ann. Appl. Biol , 63 : 1 – 17 .
- Lambert , D.H. , Levy , L. , Mavrodieva , V.A. , Johnson , S.B. , Babcock , M.J. and Vayda , M.E. 2003 . First report of Potato mop-top virus on potato from the United States . Plant Dis , 87 : 872 – 872 .
- Larkin , M.A. , Blackshields , G. , Brown , N.P. , Chenna , R. , Mcgetigan , P.A. Mcwilliam , H. 2007 . ClustalW and ClustalX version 2 . Bioinformatics , 23 : 2947 – 2948 .
- Mohapatra , S.S. , Poole , R.J. and Dhindsa , R.S. 1987 . Changes in protein patterns and translatable messenger RNA populations during cold acclimation of alfalfa . Plant Physiol , 84 : 1172 – 1176 .
- Nie , X. and Singh , R.P. 2001 . Differential accumulation of Potato virus A and expression of pathogenesis-related genes in resistant potato cv. Shepody upon graft inoculation . Phytopathology , 91 : 197 – 203 .
- Rantanen , T. , Lehtinen , U. and Kurppa , A. 1999 . “ Development of a IC-RT-PCR test for the detection of potato mop-top virus ” . In In Proceedings of the14th Triennial Conference of the European Potato Association. Assessorato Agricoltura Regione Campina 547 – 548 . Sorrento , Italy
- Salazar , L.F. 1996 . Potato viruses and their control. International Potato Center, Lima, Peru 23–46 – 83–166 . Ppand
- Sandgren , M. , Plaisted , R.L. , Watanabe , K.N. , Olsson , S. and Valkonen , J.P.T. 2002 . Evaluation of some North and South American potato breeding lines for resistance to Potato mop-top virus in Sweden . Amer. J. Potato Res , 79 : 205 – 210 .
- Slack , S.A. and German , T.L. 2001 . “ Diseases caused by viruses and viroids ” . In Compendium of Potato Diseases , Second , Edited by: Stevenson , W.R. , Loria , R. , Franc , G.D. and Weingartner , D.P. 57 – 62 . St. Paul , MN : APS Press . InEds.Editionpp.
- Sokmen , M.A. , Barker , H. and Torrance , L. 1998 . Factors affecting the detection of mop-top virus in potato tubers and improvement of test procedures for more reliable assays . Ann. Appl. Biol , 133 : 55 – 63 .
- Tamura , K. , Dudley , J. , Nei , M. and Kumar , S. 2007 . MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0 . Mol. Biol. Evol , 24 : 1596 – 1599 .
- Torrance , L. , Cowan , G.H. and Pereira , L.G. 1993 . Monoclonal antibodies specific for potato mop-top virus and some properties of the coat protein . Ann. Appl. Biol , 122 : 311 – 322 .
- Wang , B. , Ma , Y. , Zhang , Z. , Wu , Y. , Wang , Q. and Li , M. 2011 . Potato viruses in China . Crop Prot , 30 : 1117 – 1123 .
- Weingartner , D.P. 2001 . “ Potato mop-top virus ” . In Compendium of Potato Diseases , Second , Edited by: Stevenson , W.R. , Loria , R. , Franc , G.D. and Weingartner , D.P. 54 – 65 . St. Paul , MN : APS Press . InEds.Editionpp.
- Whitworth , J.L. and Crosslin , J.M. 2013 . “ Detection of Potato mop-top virus (Furovirus) on potato in southeast Idaho ” . In Plant Dis Vol. 97 , 149
- Xu , H. , De Haan , T.-L. and De Boer , S.H. 2004 . Detection and confirmation of Potato mop-top virus in potatoes produced in the United States and Canada . Plant Dis , 88 : 363 – 367 .
- Zhang , Z. , Ding , M. , Fang , Q. , Zhang , L. , Peng , L. , Li , Z. and Li , T. 2003 . Virus species infected potato and screening system of virus-free seedling . Yunnan Agri. Sci. Tech., S(1) , : 121 – 131 .