Abstract
The occurrence of clubroot on canola (Brassica napus), caused by the soilborne protozoan Plasmodiophora brassicae, in western Canada is currently centred in a region of slightly acidic soils near Edmonton, AB. Warm temperatures and slightly acidic conditions are known to favour the development of clubroot. The current study was conducted as part of a larger project to assess the risk that P. brassicae will spread to other areas in the prairie region, e.g., where soil pH is neutral or alkaline. The interaction of temperature (10, 15, 20, 25, 30 °C) and pH (5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0) on root hair infection (RHI) and clubroot symptom development in canola roots was studied under optimum moisture conditions and moderate (1–5 × 106 resting spores per seedling) inoculum levels under controlled conditions. The highest levels of RHI (max = 61%) and clubroot incidence and severity (max. = 100%) developed at pH 5.0–6.5 × 20–25 °C. Clubroot levels were intermediate at pH 7.0–8.0 × 20–25 °C, and very low at 10 and 15 °C, regardless of pH. Surveys of clubroot-infested canola fields in Alberta demonstrated that there was only a weak correlation between soil pH and clubroot level (r = −0.30 for incidence and r = −0.33 for severity, based on 267 fields). The absence of a strong correlation supports the results of the controlled environment study. We conclude that moderate levels of clubroot can develop at pH levels well above its pH optimum when temperature and moisture are suitable. This may be the underlying cause of failures in clubroot control that occasionally occur in infested vegetable fields treated with lime. This study indicates that there is a substantial risk that moderate levels of clubroot will develop in regions where soils are neutral or slightly alkaline if other conditions (temperature, moisture, inoculum load) are favourable for disease development.
Résumé
L'incidence de la hernie chez le canola (Brassica napus), causée par le protozoaire terricole Plasmodiophora brassicae dans l'Ouest canadien, est principalement limitée à une région de sols légèrement acides, située près d'Edmonton en Alberta. Les températures chaudes et la faible acidité du sol s'avèrent propices au développement de la hernie. La présente étude a été menée dans le cadre d'un projet plus vaste visant à évaluer le risque de propagation de P. brassicae dans d'autres régions des Prairies où, par exemple, le pH du sol est neutre ou alcalin. Les effets de l'interaction entre la température (10, 15, 20, 25, 30 °C) et le pH (5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0) sur l'infection des radicelles et sur l'apparition des symptômes de la hernie sur les racines de canola ont été étudiés dans des conditions optimales d'humidité pour des doses modérées d'inoculum (1–5 × 106 cytosores par plantule) appliquées dans des conditions contrôlées. Les taux les plus élevés d'infection des radicelles (max. = 61 %) ainsi que d'incidence et de gravité de la hernie (max. = 100 %) sont apparus quand le pH variait de 5.0 à 6.5 et la température, de 20 à 25 °C. À un pH variant de 7.0 à 8.0 pour une température de 20 à 25 °C, le taux d'infection était modéré et très faible lorsque la température oscillait de 10 à 15 °C, et ce, indépendamment du pH. Les études menées dans 267 champs de canola infestés par la hernie en Alberta ont montré qu'il y avait qu'une faible corrélation entre le pH du sol et le taux d'infection (r = −0.30 pour l'incidence, et r = −0.33 pour la gravité). L'absence d'une forte corrélation valide les résultats de l’étude menée dans des conditions contrôlées. Nous concluons que la hernie peut provoquer des taux modérés d'infection à des pH qui dépassent celui qui lui est optimal quand la température et l'humidité sont propices. Cela peut aussi être la cause principale des échecs relatifs à la gestion de la hernie, qui surviennent occasionnellement dans des champs de légumes infestés traités avec de la chaux. Cette étude indique qu'il y a un risque élevé que la hernie sévisse modérément dans les régions où les sols sont neutres ou légèrement alcalins, si les autres conditions (température, humidité, charge d'inoculum) sont propices au développement de la maladie.
Introduction
Clubroot, caused by Plasmodiophora brassicae Woronin, was first identified on the Canadian prairies in commercial canola (Brassica napus L.) fields near Edmonton, Alberta in 2003 (Tewari et al., Citation2005). Since then, it has spread rapidly from the initial area of infestation to other regions in Alberta and was recently confirmed in Saskatchewan (Dokken-Bouchard et al., Citation2012; Strelkov et al., Citation2012) and Manitoba (S. Strelkov, unpublished). The canola industry in Canada generates more than $15 billion (Cdn) for the Canadian economy each year (Canola Council of Canada, Citation2010), so the potential for spread of this disease is of considerable concern to canola producers across the Prairie region.
Several factors have an important effect on infection and development of P. brassicae, including temperature (Gossen et al., Citation2012a ; Sharma et al., Citation2011a , Citation2011b ), pH (Donald & Porter, Citation2004), cation and micronutrient concentration (Hamilton & Crete, Citation1978; Webster & Dixon, Citation1991b ; Donald & Porter, Citation2004; Deora et al., Citation2011), moisture (Macfarlane, Citation1952; Gossen et al., Citation2012b ; Rastas et al., Citation2012) and inoculum concentration (Hwang et al., Citation2011). The influence of pH on clubroot development has been reported on many Brassica spp. For example, severe clubbing in broccoli was observed at pH values up to 7.2, even at a low inoculum concentration, but no clubbing occurred at pH 8.0 (Myers & Campbell, Citation1985). Application of cations to raise soil pH above 7.2 under greenhouse conditions reduced root hair infection and subsequent symptom development (Myers & Campbell, Citation1985; Webster & Dixon, Citation1991a , Citation1991b ; Donald & Porter, Citation2004). In contrast, clubbing developed irrespective of pH when spore density was high and moisture was optimum (Macfarlane, Citation1952), and infection of root hairs was not affected by high pH in the absence of calcium (Myers & Campbell, Citation1985). Raising the soil pH to 7.2 or above is a standard recommendation for clubroot management in vegetable crops (Colhoun, Citation1953; Hamilton & Crete, Citation1978; Fletcher et al., Citation1982), but this treatment is not always effective (McDonald et al., Citation2004). Even heavy liming may not prevent clubroot in cabbage if moisture and spore load are sufficiently high (Colhoun, Citation1953). The optimum temperature for development of P. brassicae lies between 20 and 26 °C, based on controlled environment (Karling, Citation1968; Sharma et al., Citation2011a , Citation2011b ; Gossen et al., Citation2012a ) and field studies (McDonald & Westerveld Citation2008; Gossen et al., Citation2012b ). However, no studies have examined the interaction of temperature and pH on clubroot development on canola.
The objectives of the current study were to: (i) assess the interaction of pH and temperature on the development of P. brassicae on canola under controlled conditions; (ii) determine the strength of the association between clubroot level and soil pH in infested fields in Alberta; and (iii) determine if the pattern of clubroot development observed under controlled conditions was consistent with these field results.
Materials and methods
Controlled environment studies
Clubroot development was assessed in the primary and secondary phases of the pathogen's life cycle in two separate but highly inter-connected studies: (i) infection and pathogen development in root hairs of canola seedlings (root hair infection study), and (ii) secondary infection and symptom development assessed in older plants (symptom development study). The times of seeding and subsequent transplanting for both studies were the same, and the environmental conditions, growth media, cultivar, pathogen source, and inoculation were identical to facilitate comparisons between the studies. Each study was arranged in a four-replicate split-plot design, with temperature (growth cabinet) as the main plot treatments and different pHs allocated to the subplots.
Seeds of the clubroot-susceptible canola 46A76 (Pioneer Hi-Bred, Chatham, ON, Canada) were sown in moist sand in Petri dishes at 20 °C with 16-h photoperiod. Seedlings for the root hair infection study were transplanted into pipette tips containing autoclaved, non-calcareous sand at pH 6.5. Seedlings for the symptom development study were transplanted into tall plastic pots (164 mL Conetainers, Stuewe & Sons Inc., Corvallis, OR). The transplanted seedlings were maintained at 20 °C for 5 days to allow them to establish prior to treatment. A 14-hr photoperiod with a light intensity of 200–250 μmol m−2 s−1 and 65% relative humidity was maintained throughout each study.
Resting spores of P. brassicae pathotype 6 were extracted from clubbed roots of cabbage collected from the Muck Crops Research Station, Holland Marsh, Ontario in 2008 and stored at −20 °C until required. The spore suspension was prepared using the standard method of Strelkov et al. (Citation2006). The inoculum was prepared on the day of inoculation. Each 10-day-old seedling was inoculated with 1 mL of a 1 × 106 spores mL−1 suspension in the root hair infection study and 5 mL of suspension in the symptom development study (to ensure uniform infection in the larger pot volume). After inoculation, the plants were moved to growth cabinets set at the respective temperature treatments.
In both studies, pH treatments of 6.0, 6.5, 7.0, 7.5 and 8.0 were assessed. The plants were watered with deionized water adjusted to the desired pH using 5% acetic acid or 10% sodium hydroxide. Fresh pH-adjusted water was prepared and applied every day. The pH meter (Hanna instruments, Woonsocket, RI) was calibrated before each use, using standard buffer solutions (Fisher Scientific, Nepean, ON). The temperature treatments assessed in both studies were 10, 15, 20, 25 and 30 °C, provided by standard growth cabinets. A HOBO temperature sensor (ProSeries Temp RH 2009 ONSET Computer Corporation, MA) was placed inside each growth cabinet to monitor the actual temperature at plant height. Temperatures were recorded at 5-min intervals and the daily mean temperature was calculated from the day after inoculation to the day before harvest. The plants were fertilized at weekly intervals by watering with dilute inorganic nutrient solution made by adding a 5-mL aliquot of stock solution (40 g of 15N : 15P : 18K and 20 g of ammonium sulphate in 1 L of water) to 1 L of deionized water adjusted to the treatment pH.
In the root hair infection (RHI) study, the seedlings were grown using a sand–liquid culture method (Donald & Porter, Citation2004). Each experimental unit consisted of three 5-mL pipette tips, each containing a single seedling, inside a 50-mL Falcon tube (Fisher Scientific, Markham, ON). Drainage holes were made at the bottom of each Falcon tube to avoid waterlogging. This encouraged plant growth and minimized algal growth, but the volume of water available for each plant was small and frequent watering was required. Plants were destructively harvested 12 days after inoculation to observe root hair infection. The root samples were rinsed in tap water and placed in a fixative solution of 50 mL glacial acetic acid and 50 mL ethyl alcohol until they were assessed (Donald & Porter, Citation2004). Each root was immersed in methylene blue stain for 10 min, then stained for 3 min in basic fuchsin stain (Buczacki & Ockendon, Citation1979), and mounted in glycerol on a glass slide. To estimate the percentage of root hair infection on each root, 10 fields of view (compound microscope, 10× objective) were selected at about 1 cm below the hypocotyls, and 10 root hairs were assessed per field (total, 100 root hairs). The developmental stages of the pathogen were identified as (i) undifferentiated plasmodia, (ii) differentiated plasmodia or zoosporangia, or (iii) dehisced sporangia. The undifferentiated plasmodia looked like a mass of cytoplasm, but stained dark pink. Differentiated plasmodia had prominent nuclei that stained dark blue, with surrounding protoplasm that stained light blue. The zoosporangia were spherical and stained blue. The dehisced sporangia looked like empty, round, transparent beads that stained pink.
In the symptom development study, individual canola seedlings were transplanted into tall plastic pots, with 10 plants per experimental unit. The plants were maintained in the same growth cabinets as those in the root hair infection study. The plants were destructively harvested for assessment of clubroot symptoms at 6 weeks after inoculation. Clubroot incidence and severity were assessed on a 0 to 3 scale, where: 0 = no clubbing, 1 = a few small clubs, 2 = moderate clubbing, and 3 = severe clubbing (Kuginuki et al., Citation1999). A disease severity index (DSI) was then calculated for each experimental unit using the formula of Horiuchi & Hori (Citation1980) as modified by Strelkov et al. (Citation2006):
Both studies were repeated, with the following modifications: treatments at pH 5.0 and 5.5 were added to assess clubroot development at lower pH values, and treatments at 10 and 30 °C were dropped because very low levels of clubroot had developed at 10 °C and many of the plants at 30 °C died before the experiments were completed. Finally, the plants were watered with deionized water adjusted to the desired pH using biological buffers (Myers & Campbell, Citation1985).
Field surveys of clubroot and soil pH
A total of 1626 commercial canola crops were surveyed in central Alberta for clubroot incidence and severity from 2005 to 2010. The crops were surveyed shortly after swathing by digging all of the plants within a 1-m2 area at each of 10 randomly selected points along the arms of a ‘W’ sampling pattern, and assessing each plant for clubroot symptom severity on the 0 to 3 scale described previously. In addition, about 1 kg of soil was collected from the top (0–15 cm) layer of soil at the sampling point nearest to the field entrance and taken back to the laboratory for pH analysis. Soil pH was measured according to the method of Peech (Citation1965) with some modifications. Briefly, 50 g of air-dried soil (soil particles < 1 mm) was suspended in 50 mL sterile deionized H2O and incubated for 1 h with occasional agitation. The soil particles were allowed to settle and the pH of the supernatant was determined with an Accumet AB15/15+ pH meter (Fisher Scientific, Ottawa, ON). Only soil samples collected from fields where clubroot had been identified were included in the analysis. None of the clubroot-positive fields were sampled more than once in the 2005–2010 surveys.
Statistical analysis
For the controlled environment studies, the data were analysed in a mixed model analysis of variance using SAS version 9.2 software (SAS Institute, Cary, NC). Temperature (main plot) and pH (sub-plot) were the fixed effects, and repetition and block were the random effects. Normality of residuals was tested with the Shapiro–Wilk statistic, which showed that each of the variables was normally distributed. Initially, the repetitions of each study were analysed separately, then the data from the initial study and the repetition were analysed together, using only those treatments in common. There was no repetition main effect or treatment by repetition interaction for RHI or disease severity index (DSI). For clubroot incidence, there was only a small treatment × repetition interaction (P = 0.04), so the data from the initial study and the repetition were pooled for subsequent analyses for all three variables. Means of RHI, CI and DSI were separated using Tukey's Multiple Mean Comparison Test.
The response to pH and temperature was assessed using single df contrasts in ANOVA to identify linear and quadratic relationships. Regression (first- and second-order design) was conducted to assess the contribution of temperature and pH to each response variable. The first derivative of each regression equation was solved to obtain the inflection points. Correlation analysis (PROC CORR, Pearson) was used to examine the relationship between RHI and clubroot level (CI and DSI). Comparison between the two studies was appropriate because both studies were performed at the same time and under the same conditions.
For the field study, correlation analyses were conducted between soil pH and clubroot level (CI and DSI). In all of the analyses, differences were significant at P = 0.05 unless stated explicitly.
Results
Controlled environment studies
In the root hair infection study, RHI occurred at each of the temperatures examined (10–25 °C), but there was a substantial temperature by pH interaction. Contrast analysis revealed a quadratic pattern of response to pH in certain temperature treatments, so regression analysis was used to assess the relative contribution of temperature and pH to RHI. The maximum RHI (61%) occurred at 25 °C × pH 6.0–6.5 and at 20 °C × pH 5.5–6.0; above this pH, RHI declined (). Zoospore release was almost finished by 12 DAI at 25 °C × pH 5.0–5.5 and at 20 °C × pH 5.0, leaving only a few differentiated plasmodia. In contrast, only the undifferentiated plasmodial stage was observed at 12 DAI at 20–25 °C × pH 8.0 (). Root hair infection was lower (26%) at pH 7.5 than at acidic pH of 5.0–6.5 (). At pH 7.5–8.0, only primary plasmodia were observed, irrespective of temperature. At 15 °C × pH 5.0–8.0, RHI was relatively low (range 5–33%), and very low (0–2%) at 10 °C, irrespective of pH (data not shown). Partially emptied zoosporangia were frequently observed at 25 °C × pH 6.0–6.5. Breaks in the walls of the root hairs were often observed adjacent to empty zoosporangia. These breaks likely indicated places where zoospores had been released through the root hair wall.
Fig. 2. Effect of temperature and pH on the mean proportion of primary plasmodia and sporangia of Plasmodiophora brassicae in root hairs of canola seedlings at 12 days after inoculation (repetition 2 only). Capped lines represent standard error.
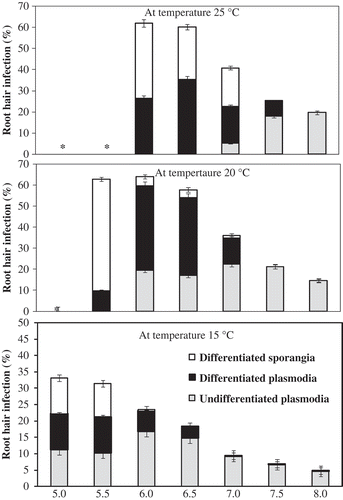
Fig. 1. The effect of temperature and pH on root hair infection and subsequent clubroot severity in canola inoculated with Plasmodiophora brassicae under controlled conditions. Only those treatments that were assessed in both repetitions of these studies are presented.
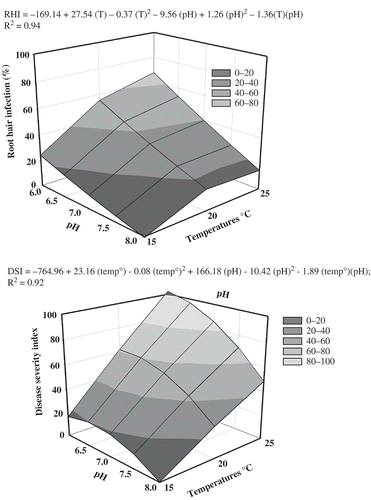
In the symptom development study, there was a temperature by pH interaction for clubroot incidence and severity (). There was a quadratic response to pH for CI and DSI at 20 and 25 °C, but no response to pH at 10 and 15 °C. Regression analysis indicated that 100% incidence occurred at 25 °C and pH 6.0. CI began to drop from 100% at 25 °C × pH > 6.5. At the optimum temperature of 25 °C, DSI was 100% at pH 6.0 (), which was the same as for CI. Above this pH, clubroot severity declined with increasing pH.
Fig. 3. Effect of pH and temperature on mean incidence (A) and severity (B) of clubroot in canola, and the best-fit regression lines at 20 and 25 °C across two repetitions of the study under controlled conditions. Very little clubroot developed at 15 °C, irrespective of pH.
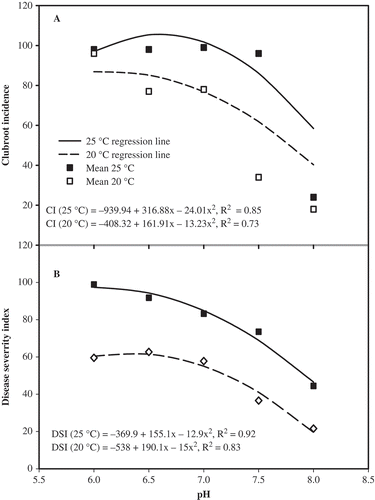
Clubroot development was highest at 25 °C × pH 5.0–6.5 ( and ). There were no differences in CI or DSI at 20–25 °C × pH 5.0–7.0. CI and DSI declined across temperatures at pH 7.0–8.0, but clubroot was not eliminated even at pH 8.0. DSI was low to moderate at 20–25 °C × pH 8.0 (44 DSI at 25 °C, 22 DSI at 20 °C). Low levels of clubroot were observed at 10–15 °C. At 10 °C, DSI was less than 10, irrespective of pH (data not shown).
RHI was positively correlated with subsequent clubroot incidence (r = 0.82; P = 0.0002) and severity (r = 0.86; P = 0.0001).
Field surveys of clubroot and soil pH
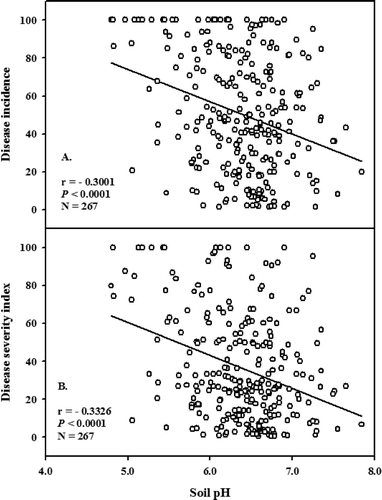
A total of 267 clubroot-infested canola crops were identified among the 1626 crops surveyed from 2005 to 2010. CI at the sampling point adjacent to the field entrance ranged from 1 to 100% and DSI ranged from 0.6 to 100. The soil pH at this sampling spot in clubroot-infested canola crops ranged from 4.8 to 7.8 (). Correlation analyses revealed that CI and DSI were negatively correlated with soil pH in 2005, 2006, 2007 and 2008, but not in 2009 or 2010 (). When the data from 2005 to 2010 were pooled (), clubroot levels were only weakly correlated with soil pH (r = −0.30, P < 0.0001 for CI, r = −0.33, P < 0.0001 for DSI).
Table 1. Correlation between clubroot incidence (%) and severity (based on the disease severity index, DSI) with soil pH in clubroot-infested commercial canola fields in Alberta, 2005–2010
Discussion
This is the first study to assess the interaction of a wide range of temperature and pH conditions on the development of clubroot on canola (or any other host) under controlled conditions. Soil pH above 7.0 reduced and delayed clubroot development, but moderately severe clubroot developed at pH 8.0 when temperatures were optimum for the pathogen (20–25 °C) and soil moisture was adequate. RHI, CI and DSI were all at the maximum at 25 °C × pH 5.0–6.0, but decreased as pH increased and temperature decreased. Similarly, soil pH was only weakly correlated with clubroot incidence and severity in 267 infested commercial canola fields in Alberta.
In the growth cabinet studies, the high RHI observed at 25 °C × pH 6.0 was consistent with the maximum levels of RHI reported in previous studies (Ayers, Citation1944; Samuel & Garrett, Citation1945; Macfarlane, Citation1952), and with a recent report on the effect of temperature on pathogen development in root hairs (Sharma et al., 2011a). However, RHI decreased with increasing pH and decreasing temperature. RHI was low (40%) at 20 °C × pH 7.5–8.0. The development of P. brassicae in root hairs was influenced by both temperature and pH. At 25 °C × pH 5.0–5.5 and 20 °C × pH 5.5, the primary infection phase (primary plasmodia, differentiated plasmodia, and zoosporangia) had been completed by 12 DAI, leaving only empty sporangia in the root hairs after the zoospores had dehisced. This supports the results of previous studies that showed that development in root hairs was rapid when temperature and pH were favourable ( Myers & Campbell, Citation1985; Donald & Porter, Citation2004). Development was much slower at 15 °C × pH 6.0–8.0, such that only primary plasmodia and a few zoosporangia were present when the study was terminated at 12 DAI. Development was even more inhibited at 10 °C, where only a few primary plasmodia developed at the optimum pH of 6.0–6.5 and no pathogen development was observed in the other pH treatments.
In the symptom development study, clubroot symptoms were not completely suppressed at slightly alkaline pH (7.5–8.0), although there was a substantial decline in severity. This is consistent with previous reports of lower levels of clubroot at alkaline pH (Macfarlane & Last, Citation1959; Palm, Citation1963; Myers & Campbell, Citation1985). The development of moderate levels of clubroot at 15 °C and acidic pH indicates that clubroot can develop under less-than-ideal conditions in slightly acidic soils, which may be part of the explanation of previous reports that acidic pH is required for clubroot development.
RHI was strongly correlated with subsequent clubroot incidence and severity. This is consistent with several previous reports of a strong correlation between primary infection and subsequent gall development under controlled conditions (Macfarlane, Citation1952; Sharma et al., 2011a). In contrast, there was no correlation of RHI with clubroot symptoms in a greenhouse study (Voorrips, Citation1992). This absence of correlation may have been due to the complex factors that influence infection success by secondary zoospores, including moisture level, temperature and pH.
The survey of commercial fields in central Alberta demonstrated that there was a small but highly significant negative correlation between clubroot levels (CI and DSI) and pH each year from 2005–2008. A weak negative correlation was also observed when the 2005–2008 and 2005–2010 data were pooled. However, there was no correlation in 2009 or 2010, when the surveys were extended outside of the main region of acidic soils. There were no substantial differences in the mean monthly temperature from May to September of the years 2005, 2006, 2007, 2008, 2009 and 2010, when calculated over the survey area (not shown). In contrast, the mean monthly rainfall from May to September was substantially higher in 2010 (77.1 mm) than in 2008 (36.3 mm) or 2009 (25.7 mm). However, this difference in rainfall did not have a consistent or substantial impact on the mean DSI in those years. This likely indicates that field-specific fluctuations in weather, which may not be reflected in regional means, had a large impact on clubroot levels.
The weak negative correlation observed between soil pH and clubroot levels (CI and DSI) supports the report of a weak correlation between soil pH and clubroot levels on oilseed and turnip rape (B. napus and B. rapa) in Finland (Rastas et al., Citation2012). These results indicate that other factors, such as inoculum load (Macfarlane, Citation1952; Hildebrand & McRae, Citation1998), moisture (Colhoun, Citation1952, Citation1953; Gossen et al., Citation2012b ; Kasinathan, Citation2012, Rastas et al., Citation2012), level of soluble calcium (Donald & Porter, Citation2009), temperature (Thuma et al., Citation1983; McDonald et al., Citation2004; McDonald & Westerveld, Citation2008; Gossen et al., Citation2012a , Citation2012b ), and cropping rotation (Rastas et al., Citation2012; S. Strelkov, unpublished) likely also influence clubroot levels. Despite the weak correlation between soil pH and clubroot levels, it is important to note that the results of the current study support previous reports that acidic soils favour the development of clubroot (Karling, Citation1968). This indicates that severe infestations of clubroot are likely to develop more quickly and more consistently on acidic soils than alkaline soils, other factors being equal. This pattern of response almost certainly applies equally to canola, to other Brassica field crops, and to vegetable Brassica crops (Gossen et al., Citation2013).
Although application of lime to raise the soil pH is a common practice for reducing clubroot in susceptible vegetable crops, it does not always result in an effective reduction in clubroot levels (McDonald et al., Citation2004). The results of the current study indicate that while raising soil pH may afford some protection against clubroot, it is unlikely to be enough on its own to prevent the development of clubroot when inoculum pressure is high and environmental conditions are favourable for the pathogen. This may be the underlying cause of failures in clubroot control that can occur in infested vegetable fields treated with lime. Based on these results, we conclude that there may be potential for clubroot to spread into regions of the Canadian prairies with neutral or slightly alkaline pH if large numbers of resting spores are introduced through human activities such as movement of contaminated equipment or via movement by wind and water.
Acknowledgements
We thank the Clubroot Risk Mitigation Initiative of Agriculture and Agri-Food Canada, the Canola Agronomic Research Program (Alberta Canola Producers Commission) and the Alberta Crop Industry Development Fund for partial funding of the project.
Notes
References
- Ayers , G.W. 1944 . Studies on the life history of the club root organism, Plasmodiophora brassicae . Can. J. Res , 22c : 143 – 149 .
- Buczacki , S.T. and Ockendon , J.G. 1979 . Preliminary observations on variation in susceptibility to clubroot among collections of some wild crucifers . Ann. Appl. Biol , 92 : 113 – 118 .
- Canola Council of Canada (2010). Canola in Canada. Available from12/2010 http://www.canolacouncil.org/canadian_canola_industry.aspx (http://www.canolacouncil.org/canadian_canola_industry.aspx)
- Colhoun , J. 1952 . Factors affecting the clubroot disease of Brassica . Nature , 169 : 21 – 22 .
- Colhoun , J. 1953 . A study of the epidemiology of club-root disease of Brassicae . Ann. Appl. Biol , 40 : 262 – 283 .
- Deora , A. , Gossen , B.D. , Walley , F. and McDonald , M.R. 2011 . Boron reduces development of clubroot in canola . Can. J. Plant Pathol. , 33 : 475 – 484 .
- Dokken-Bouchard , F.L. , Anderson , K. , Bassendowski , K.A. , Bouchard , A. , Brown , B. Cranston , R. 2012 . Survey of canola diseases in Saskatchewan in 2011 . Can. Plant Dis. Surv , 92 : 125 – 129 .
- Donald , E.C. and Porter , I.J. 2004 . A sand–solution culture technique used to observe the effect of calcium and pH on root hair and cortical stages of infection by Plasmodiophora brassicae . Australas. Plant Pathol , 33 : 585 – 589 .
- Donald , C. and Porter , I. 2009 . Integrated control of clubroot . J. Plant Growth Regul , 28 : 289 – 303 .
- Fletcher , J.T. , Hims , M.J. , Archer , F.C. and Brown , A. 1982 . Effects of adding calcium and sodium salts to field soils on the incidence of clubroot . Ann. Appl. Biol , 100 : 245 – 251 .
- Gossen , B.D. , Adhikari , K.K.C. and McDonald , M.R. 2012a . Effects of temperature on infection and subsequent development of clubroot under controlled conditions . Plant Pathol , 61 : 593 – 599 .
- Gossen , B.D. , Adhikari , K.K.C. and McDonald , M.R. 2012b . Effect of seeding date on development of clubroot in vegetable Brassica crops . Can. J. Plant Pathol , 34 : 516 – 523 .
- Gossen , B.D. , McDonald , M.R. , Hwang , S.F. , Strelkov , S.E. and Peng , G. 2013 . Comparison of clubroot (Plasmodiophora brassicae) development and management on canola and Brassica vegetables . Can. J. Plant Pathol , 35 : 175 – 191 .
- Hamilton , H.A. and Crete , R. 1978 . Influence of soil moisture, soil pH, and liming sources on the incidence of clubroot, the germination and growth of cabbage produced in mineral and organic soils under controlled conditions . Can. J. Plant Sci , 58 : 45 – 53 .
- Hildebrand , P.D. and McRae , K.B. 1998 . Control of clubroot caused by Plasmodiophora brassicae with nonionic surfactants . Can. J. Plant Sci , 20 : 1 – 11 .
- Horiuchi , S. and Hori , M. 1980 . A simple greenhouse technique for obtaining high levels of clubroot incidence . Chugoku Natl. Agric. Exp. Stn. E , 17 : 33 – 55 .
- Hwang , S.F. , Ahmed , H.U. , Strelkov , S.E. , Gossen , B.D. , Turnbull , G.D. , Peng , G. and Howard , R.J. 2011 . Seedling age and inoculum density affect clubroot severity and seed yield in canola . Can. J. Plant Sci , 91 : 183 – 190 .
- Karling , J.S. 1968 . The Plasmodiophorales , 2nd , New York : Hafner Publishing Company .
- Kasinathan , H. 2012 . Influence of pH, temperature, and biofungicides on clubroot of canola , Guelph , ON : M.Sc. Thesis, University of Guelph .
- Kuginuki , Y. , Yoshikawa , H. and Hirai , M. 1999 . Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot–resistant cultivars of Chinese cabbage (Brassica rapa L. ssp. pekinensis) . Eur. J. Plant Pathol , 105 : 327 – 332 .
- MacFarlane , I. 1952 . Factors affecting the survival of Plasmodiophora brassicae Wor. in the soil and its assessment by a host test . Ann. Appl. Biol , 39 : 239 – 256 .
- MacFarlane , I. and Last , F.T. 1959 . Some effects of Plasmodiophora brassicae Wor. on the growth of the young cabbage plant . Ann. Bot., N.S , 33 : 547 – 569 .
- McDonald , M.R. , Kornatowska , B. and McKeown , A.W. 2004 . Management of clubroot of Asian Brassica crops grown on organic soils . Acta Hort , 635 : 25 – 30 .
- McDonald , M.R. and Westerveld , S.M. 2008 . Temperature prior to harvest influences the incidence and severity of clubroot on two Asian Brassica vegetables . HortScience , 43 : 1509 – 1513 .
- Myers , D.F. and Campbell , R.N. 1985 . Lime and the control of clubroot of crucifers: effects of pH, calcium, magnesium, and their interactions . Phytopathology , 75 : 670 – 673 .
- Palm , E.T. 1963 . Effect of mineral nutrition on the invasion and response of turnip tissue to Plasmodiophora brassicae Wor . Contrib. Boyce Thompson Inst , 22 : 91 – 112 .
- Peech , M.H. 1965 . “ Hydrogen-ion activity ” . In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties , Edited by: Black , C.A. , Evans , D.D. , Ensminger , L.E. , White , J.L. , Clark , F.E. and Dinauer , C.F. 914 – 926 . Madison , WI : American Society of Agronomy .
- Rastas , M. , Latvala , S. and Hannukkala , A. 2012 . Occurrence of Plasmodiophora brassicae in Finnish turnip rape and oilseed rape fields . Agric. Food Sci , 21 : 141 – 158 .
- Samuel , G. and Garrett , S.D. 1945 . The infected root-hair count for estimating the activity of Plasmodiophora brassicae Woron. in the soil . Ann. Appl. Biol , 32 : 96 – 101 .
- Sharma , K. , Gossen , B.D. and Mcdonald , M.R. 2011a . Effect of temperature on primary infection by Plasmodiophora brassicae and initiation of clubroot symptoms . Plant Pathol , 60 : 830 – 838 .
- Sharma , K. , Gossen , B.D. and McDonald , M.R. 2011b . Effect of temperature on cortical infection by Plasmodiophora brassicae and clubroot severity . Phytopathology , 101 : 1424 – 1432 .
- Strelkov , S.E. , Tewari , J.P. and Degenhardt , S.E. 2006 . Characterization of Plasmodiophora brassicae populations from Alberta, Canada . Can. J. Plant Sci , 28 : 467 – 474 .
- Strelkov , S.E. , Manolii , V.P. , Liu , J. , Jurke , C. , Rennie , D.C. , Orchard , D. , Hwang , S.F. and Laflamme , P. 2012 . The occurrence of clubroot on canola in Alberta in 2011 . Can. Plant Dis. Surv , 92 : 122 – 124 .
- Tewari , J.P. , Strelkov , S.E. , Orchard , D. , Hartman , M. , Lange , R.M. and Turkington , T.K. 2005 . Identification of clubroot of crucifers on canola (Brassica napus) in Alberta . Plant Dis , 67 : 758 – 762 .
- Thuma , B.A. , Rowe , R.C. and Madden , L.V. 1983 . Relationships of soil temperature and moisture to clubroot (Plasmodiophora brassicae) severity on radish in organic soil . Plant Dis , 67 : 758 – 762 .
- Voorrips , R.E. 1992 . Plasmodiophora brassicae: aspects of pathogenesis and resistance in Brassica oleracea . Euphytica , 83 : 139 – 146 .
- Webster , M.A. and Dixon , G.R. 1991a . Calcium, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae . Mycol. Res , 95 : 64 – 73 .
- Webster , M.A. and Dixon , G.R. 1991b . Boron, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae . Mycol. Res , 95 : 74 – 79 .