Abstract
Post-harvest screening of potato ‘Kennebec’ revealed a Potato virus Y (PVY) incidence of 15.8%, a rate that is unusually high for a cultivar possessing a high level of field resistance to the virus. Randomly selected tubers were planted in a field plot and the resulting plants were monitored. Approximately 16% of plants developed symptoms ranging from mild mosaic symptoms to severe necrosis/rugosity/stunting. ELISA and RT–PCR analysis revealed that infections with Potato virus S (PVS), Potato virus X (PVX) and PVY, mostly in mixed-infections, occurred commonly in 14 sampled plants. Two strains, namely the common strain (PVY°) and the recombinant tuber necrotic strain (PVYNTN) were identified in the PVY-positive plants. In general, mild mosaic was associated with infections with PVX and PVS; intermediated mosaic was associated with PVS and PVYNTN infections; whereas severe leaf deformation/necrosis/drop symptoms were associated with PVYNTN and PVX co-infections, or with PVY° and either PVS or PVX co-infections. Virus-free plantlets of potato ‘Kennebec’ were mechanically inoculated with PVX, PVY°, and PVYNTN alone or with PVX+PVY° or PVX+PVYNTN combinations in the greenhouse. Single infections with PVY° or mixed-infections with PVX+PVY° or PVX+PVYNTN incited severe mosaic symptoms and systemic necrosis soon after inoculation; whereas single infections with PVX and PVYNTN induced mild to intermediate mosaic symptoms only. The most severe symptoms occurred in the mixed-inoculation with PVX+PVYNTN, demonstrating dramatic synergism between these strains. Similarly, profound PVX and PVYNTN synergism was also found in tobacco and Physalis floridana plants, suggesting that the strain of PVY plays an important role in the level of synergistic reactions between PVX and PVY on host plants.
Résumé
Le criblage consécutif à la récolte de la pomme de terre ‘Kennebec’ a révélé une incidence de 15,8 % du virus Y de la pomme de terre, soit un taux anormalement élevé pour un cultivar possédant un fort degré de résistance au virus en champ. Des tubercules choisis aléatoirement ont été plantés dans une parcelle de terrain et les plants qui en ont résulté ont été suivis de près. Environ 16 % des plants ont développé des symptômes variant des symptômes bénins de la mosaïque à ceux plus graves de la nécrose en passant par la rugosité ou le rabougrissement. Des tests ELISA et des analyses par RT-PCR ont révélé que des infections causées par les virus S (VSPT), X (VXPT) et Y (VYPT) de la pomme de terre, principalement sous forme d'infections mixtes, sont apparues couramment chez 14 plants échantillonnés. Deux souches, à savoir la souche ordinaire (VYPT°) et la souche recombinante causant la nécrose des tubercules (VYPTNTN), ont été identifiées chez les plants réceptifs au VYPT. En général, la mosaïque bénigne était associée aux infections causées par le VXPT et le VSPT; la mosaïque moyennement virulente, aux infections causées par le VSPT et le VYPTNTN, tandis que la déformation grave du feuillage, la nécrose et le flétrissement étaient associés au VYPTNTN et aux co-infections causées par le VXPT ou le VYPT° et à celles causées soit par le VSPT ou le VXPT. En serre, les plantules dépourvues de virus ont été inoculées avec le VXPT, le VYPT° et le VYPTNTN uniquement, ou avec une combinaison de VXPT + VYPT° ou de VXPT + VYPTNTN. Les infections causées par le VYPT° unique ou par les combinaisons mixtes de VXPT + VYPT° ou de VXPT + VYPTNTN ont engendré de graves symptômes de la mosaïque et de la nécrose systémique peu après l'inoculation, tandis que les infections uniques causées par le VXPT et le VYPTNTN ont engendré que des symptômes bénins à moyens de la mosaïque. Les symptômes les plus graves ont été causés par les inoculations mixtes de VXPT + VYPTNTN, ce qui démontre la forte synergie qui existe entre ces souches. De même, une importante synergie entre le VXPT et le VYPTNTN a été découverte chez les plants de tabac et de Physalis floridana, ce qui suggère que la souche VYPT joue un rôle important dans l'intensité des réactions synergiques entre le VXPT et le VYPT chez les plantes hôtes.
Introduction
As a vegetatively propagated crop, potato (Solanum tuberosum L.) is prone to virus infection and inoculum persistence from year to year. More than 35 different species of viruses can infect potato plants naturally and pass from one generation of plants to the next via infected tubers (Slack & German, Citation2001), thus threatening the potato crop and quality. To minimize the impact of virus diseases on potato production, stringent phytosanitary measures and seed certification programmes are employed by many countries in the world. In Canada, seven sequential classes of seed potatoes, starting from Pre-Elite (first generation seed potatoes in the field), to Elite 1 to 4, Foundation, and ending at Certified (seventh generation in the field), are categorized in a flush-through system (DeHaan, Citation1994). All lots that are intended for production of seed potatoes are subjected to a series of visual inspections during the growing season as well as postharvest laboratory testing, and only those seed lots that meet the minimum quality requirements for each class can be certified. Despite these efforts, virus incidence, especially incidence of ordinary viruses including Potato virus X (PVX, genus Potexvirus), Potato virus S (PVS, genus Carlavirus) and Potato virus Y (PVY, genus Potyvirus), occur commonly in seed potatoes grown in Canada as well as USA (Slack & Singh, Citation1998). In recent years, an increase in PVY incidence has been observed in potato crops in North America (Gray et al., Citation2010; Nanayakkara et al., Citation2012; Nie et al., Citation2013), causing significant concerns to the potato industry and growers.
Host responses to virus infection are determined by virus species/strains and host species/genotype, and are affected by both internal and external factors including plant age, physiological conditions and environmental conditions. Characteristic responses in a particular host species/genotype (or cultivar) to a specific virus/strain are the essential components of host plant-based bioassay for virus diagnostics (Salazar, Citation1996), as exemplified by tobacco-based differentiation of ordinary strain of PVY (PVY°) from PVY tobacco veinal necrotic strain (PVYN) using veinal/petiole/stem necrosis and mosaic symptoms as the characteristic responses (indicators) for PVYN and PVY°, respectively (Shukla et al., Citation1994). However, the presence of another unrelated virus may complicate symptom development. For example, PVX can aggravate potyvirus-induced symptoms in host plants (González-Jara et al. Citation2004; Pacheco et al., Citation2012), a phenomenon referred to as ‘synergism’. It has been long known that co-infection of potato plants with PVX and Potato virus A (PVA, genus Potyvirus) leads to crinkle disease (MacLachlan et al., Citation1954), a significantly more severe disease than that caused by single infections with either PVX or PVA. In tobacco, the symptoms caused by PVY° and PVX co-infection resemble those incited by PVYN to a certain extent (Goodman & Ross, Citation1974), potentially leading to strain misdiagnosis. Indeed, during the PVYN crisis in PEI, Canada, in the early 1990s, misdiagnosis occurred; and significant consequences and court cases were triggered subsequently (Turner, Citation2008). Despite the general understanding of synergism between PVX and PVY, little is known about the effects of co-infection with PVX and emerging strains of PVY such as the recombinant (or European) tuber necrotic strain (PVYNTN) on potato as well as other host species.
PVY is one of the most economically important viruses worldwide. Multiple strains and substrains of PVY have been unveiled according to their reactions to potato bearing different resistance genes and to tobacco plants (Singh et al., Citation2008). Initially, PVY° was the only known strain in North American potatoes until the end of the 1980s, when PVYN was detected in PEI potatoes (McDonald & Kristjansson, Citation1993); whereas in Europe, both PVY° and PVYN occurred commonly (Shukla et al., Citation1994). PVYNTN was discovered first in Hungary then in other European countries, and now occurs in most potato growing areas in the world (Singh et al., Citation2008). Recent surveys reveal that PVYNTN and the recombinant PVYN:O (or PVYN-wilga) have emerged as major strain groups in North America (Singh et al., Citation2003; Gray et al., Citation2010). In New Brunswick, despite the fact that PVY° remains the predominant strain, accounting for c. 60% of the overall PVY population, PVYNTN and PVYN:O have emerged, accounting for the remaining 40% of the virus population (Nanayakkara et al., Citation2012). The shift in population structure poses a particular challenge to the potato industry, not only because PVYNTN can cause potato tuber necrotic ringspot disease (PTNRD) in sensitive cultivars such as ‘Yukon Gold’, but also because PVYN:O and PVYNTN incite relatively milder symptoms than PVY° in most potato cultivars (Nie et al., Citation2012), thus potentially evading field inspections more easily. In addition, it is unknown whether the cultivars that possess field PVY resistance can hold their resistance to these emerging strains.
This study reveals the natural infections with PVY°, PVYNTN, PVX and PVS in potato plants originating from Foundation class seed potatoes of ‘Kennebec’, a cultivar that has been deemed field-resistant to PVY (likely PVY°, because it was the only PVY strain known to exist in Canadian potatoes when the studies were performed) as this cultivar is seldom infected with the virus in the field (Akeley et al., Citation1948; Bagnall & Tai, Citation1986). The synergistic interactions between PVX and PVY° or between PVX and PVYNTN in potato ‘Kennebec’, tobacco ‘Samsun’ and Physalis floridana are described. The results demonstrate that PVYNTN accounts for the majority (10/12) of PVY strains in the PVY-infected ‘Kennebec’ plants. Although synergism of PVX/PVY° or PVX/PVYNTN occurs, it is the PVX/PVYNTN co-infection that exhibits the most remarkable synergism, often lethal, in all tested plant species.
Materials and methods
Virus sample collection
During the post-harvest laboratory testing of 2010 seed potato crops, a seed lot of ‘Kennebec’ at the Foundation class was found to have a PVY infection rate of 15.8%, a rate that is significantly higher than normally observed for this cultivar because it possesses a high level of field resistance to PVY (Bagnall & Tai, Citation1986). To verify whether the rate was correct, and, moreover, to unveil the strain status of PVY in potatoes from the seed lot, randomly selected tubers were planted in a field plot at the Potato Research Centre, Agriculture and Agri-Food Canada, Fredericton, NB. As expected, approximately 16% developed varied degrees of symptoms ranging from mild mosaic to severe rugosity and leaf necrosis. Fourteen potato plants including one healthy-appearing plant and 13 symptomatic plants were flagged, and three leaves from each plant were collected and brought back to the laboratory for further analysis using ELISA, RT-PCR and biological assays.
Enzyme-linked immunosorbent assay (ELISA), reverse transcription–polymerase chain reaction (RT–PCR) and bioassay
ELISA was employed to detect common viruses including Potato leafroll virus (PLRV), Potato mop-top virus (PMTV), Potato virus A (PVA), Potato virus M (PVM), Potato virus S (PVS), Potato latent virus (PotLV), PVX and PVY in the field samples using virus-specific antibodies (Singh et al., Citation2013). In addition, ELISA with PVY°- and PVYN-serotype specific antibodies MAb2 (PVY°) and 1F5 (PVYN) (Phytodiagnostics, BC, Canada) was used to determine the PVY serotypes. The ELISA was carried out as described previously (Ellis et al., Citation1996, Citation1997) at the Agricultural Certification Services (ACS). For greenhouse experiments with potato, tobacco and P. floridana, leaves located above the inoculated leaves were sampled at 21 days post-inoculation (dpi) and used for ELISA test for PVX and PVY serotypes.
Multiplex RT–PCR targeting PLRV, PVA, PVS, PVX, PVA and Potato spindle tuber viroid (PSTVd) (Nie & Singh, Citation2001) was carried out on the leaf samples collected in the field. In addition, uniplex RT–PCR was also performed for detection of PMTV, PVM and PotLV using primers reported previously (Nie et al., Citation2008; Crosslin & Hamlin, Citation2011). Upon confirmation of PVY's presence in some of the samples, three sets of RT–PCR assays including the P1 gene-based RT–PCR (Nie & Singh, Citation2002), the RJ-based RT–PCR (Nie & Singh, Citation2003) and the multiplex RT–PCR described by Lorenzen et al. (Citation2006) were used to analyze the PVY genotype/strain type in the samples. These RT–PCR assays were also used to confirm the identity of virus inoculum prior to inoculation of virus-free plantlets/seedlings, and to assure the strain identity in the inoculated plants after inoculation as described previously (Nie et al., Citation2012). Total RNA from leaves was extracted using the sodium sulphite method (Singh et al., Citation2002), and RT–PCR assays were performed as described in the above-mentioned papers.
Tobacco and potato-based bioassays were used to assist in the determination of virus species and PVY strains as described previously (Hu et al., Citation2011; Nie et al., Citation2011). Specifically, three tobacco seedlings and three virus-free (VF) plantlets of each of the two potato cultivars ‘Jemseg’ and ‘Yukon Gold’ (obtained from Plant Propagation Centre (PPC), New Brunswick Department of Agriculture, Aquaculture and Fisheries) were mechanically inoculated with field samples (inocula) when the plants were at approximately six-leaf stage, and the resulting symptom development was monitored. Characteristic viral symptoms associated with a specific virus/virus strain on a particular indicator plant species/cultivar (e.g. tuber necrotic ringspots for PVYNTN in ‘Yukon Gold’, veinal necrosis for PVYN/NTN/N:O in tobacco) (Hu et al., Citation2011; Nie et al., Citation2011) were used as indicators for the bioassay. ELISA and RT-PCR were also used to verify the virus and strain identity in the bioassay plants three weeks after inoculation.
Virus isolates and inoculum purification
ELISA and RT-PCR analyses revealed that the field samples # 6, 8, 10 and 13 were infected with PVX+PVY°, PVS+PVX+PVYNTN, PVS+PVX, and PVX+ PVYNTN, respectively. After passing though tobacco ‘Samsun’ plants, PVS was removed due to its inability to infect ‘Samsun’ (De Bokx, Citation1970). The purified PVX isolate (PVX-K10 where ‘K’ represents the potato cultivar ‘Kennebec’, from which the virus isolate was obtained) was then maintained in tobacco plants, and was used as PVX inoculum for further experiments. To obtain pure PVY° (PVY°-K6) and PVYNTN (PVYNTN-K8 and -K13) isolates, the viruses were passed though potato ‘Jemseg’, which possesses extreme resistance (i.e. immunity) to PVX and PVS but is susceptible to PVY (Singh & Boiteau, Citation1984), and then maintained in tobacco plants as purified PVY inoculum for further experiments. The purity of the virus isolates and strain identity were verified by ELISA and RT-PCR assays. In addition to the newly collected virus isolates, several PVY isolates including PVY°-FL, PVY°-RB, PVYN:O-Mb58, PVYN-Jg and PVYNTN-Sl (Nie et al., Citation2011, Citation2012) were also used in this study for virus synergism. The viruses were maintained in tobacco hosts in the greenhouse at the Potato Research Centre, Agriculture and Agri-Food Canada (PRC-AAFC) as described previously (Nie et al., Citation2011, Citation2012).
Plant materials, virus inoculation and symptom observation for synergism studies
Virus-free tissue culture plantlets of potato cultivar ‘Kennebec’ were obtained from the Plant Propagation Centre (PPC), and transplanted to 6-inch (15.3 cm) pots containing premixed soil in the greenhouse. Seedlings of tobacco (Nicotiana tabacum L.) ‘Samsun’ and Physalis floridana were also transplanted as described above when the seedlings were c. 1 inch (2.5 cm) tall. The light/dark cycle was 16/8 h, and the ambient light was supplemented with artificial light or shading to give a light intensity of c. 90 um2 s−1. The temperature was 18–24 °C, and the humidity was 75%. Five plants each of ‘Kennebec’, ‘Samsun’ and P. floridana were mechanically inoculated with target virus combinations (i.e. mock, PVY°, PVYNTN, PVX, PVX+PVY°, PVX+PVYNTN) on each of the three uppermost leaves at the six-leaf stage as described previously (Nie et al., Citation2011), and grown in a greenhouse. The mock inoculation contained buffer (10 mM phosphate buffer, pH 7.5, with 32 mM sodium sulphite) only. Foliage symptoms were monitored daily after inoculation for the first three weeks, and every other day thereafter for up to 90 days when the potatoes were harvested. Potato tubers were also examined for possible symptoms, mainly necrotic ringspots, at harvest and monthly post-harvest for up to four months. The experiments were repeated twice.
Table 1. ELISA, RT-PCR and bioassay on potato ‘Kennebec’ leaf samples collected in a field plot in July 2011
Results
Viruses infecting ‘Kennebec’ plants in the field plot
ELISA and RT–PCR revealed that PVS was present in 12 of the 14 samples, including the one (#14) that did not show visible symptoms (; ), demonstrating the widespread occurrence of PVS in this seed lot. However, no visual symptoms appeared to be associated with the virus, suggesting that PVS alone does not induce visible symptoms in ‘Kennebec’ plants, consistent with previous observations (Manzer et al., Citation1978). PVX was found in four samples, all co-present with another virus species, either PVS (#10) or PVY (#6, #8 and #12). The co-infection with PVX and PVS (#10) led to visible mosaic symptoms on the plant (). PVY (PVY° and PVYNTN) was present in 12 of the 13 symptomatic samples (; ). The most severe symptoms that included leaf necrosis, severe mosaic and rugosity, and stunting occurred in plants infected with PVY° and PVS(#3), PVYNTN and PVX (#12), and PVYNTN and PVX and PVS (#8) (), suggesting a possible synergism between PVY and PVS/PVX. Interestingly, the plant (#6) that was co-infected with PVX and PVY° exhibited less symptom severity than the plant co-infected with PVS and PVY° (; ). Several other viruses including PLRV, PMTV, PVA and PVM, which are considered to be common in North American potatoes (Slack & Singh, Citation1998), were not detected in these samples by ELISA and RT-PCR.
Fig. 2. Reverse transcription–polymerase chain reaction (RT–PCR) detection and genotyping of Potato virus Y (PVY) in field samples. A. P1-gene based duplex RT–PCR for determination of PVY° and PVYN/N:O/NTN. B. Recombinant joint (RJ)-based multiplex RT–PCR for detection of recombinant PVYN:O and PVYNTN. C. Multiplex RT-PCR for detection of PVY°, PVYN:O, PVYNTN and PVYN strains. D. Schematic diagram of PVY genome structure and locations of P1 gene, and RJ1 to RJ3. The RT–PCR assays A and B were carried out as described in Nie & Singh (Citation2002, Citation2003), and assay C as described in Lorenzen et al. (Citation2006). The sizes of the amplicons produced by different PVY strains in assay C are: 689 + 267 bp for PVY°; 452 + 181 bp for recombinant PVYNTN (Lorenzen et al., Citation2006). Lane M, DNA Ladder (from top to bottom: 2000, 1200, 800, 400 and 200 bp); lanes 1 to 14, leaf samples collected from potato ‘Kennebec’ plants showing varied degrees of symptoms in the field.
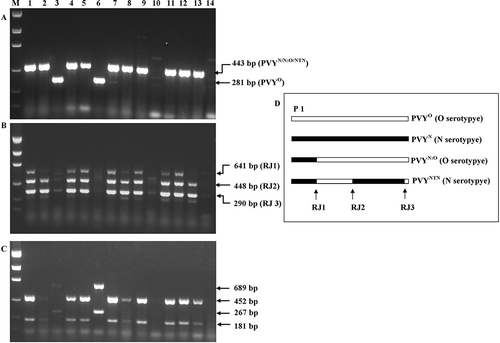
Fig. 1. Symptoms in potato ‘Kennebec’ in the field and tobacco- and potato ‘Yukon Gold’-based bioassay for unveiling symptom-causal agents in ‘Kennebec’ plants. A. Symptoms in ‘Kennebec’ in the field. B and C. Tobacco symptoms induced by the corresponding field samples (inoculum) shown in A at 7 (B) and 21 (C) days post inoculation. D. ‘Yukon Gold’ tuber symptoms induced by field PVY isolates obtained from the corresponding samples shown in A. One symptomless (# 14) and 13 symptomatic plants (# 1–13) were photographed and sampled in July 2011. The samples were used as inocula to sap-inoculate tobacco seedlings (B and C) or virus-free (VF) plantlets of potato ‘Jemseg’ (photos not shown), which in turn were used as inocula to inoculate VF ‘Yukon Gold’ plantlets. Tubers harvested from inoculated ‘Yukon Gold’ plants are shown in D.
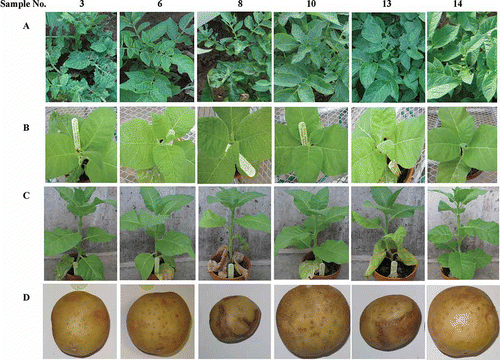
Varied symptoms were incited in tobacco plants upon sap-inoculation with the field samples. Inocula (samples) 1, 2, 4, 5, 7, 9, 11 and 13 induced veinal, petiole and stem necrosis; inoculum 3 induced mild mosaic; inoculum 6 incited severe vein clearing and netting on the first several leaves located above the inoculated leaves, which were accompanied with chlorotic spots; inoculum 10 induced chlorotic spots; inocula 8 and 12 induced the most severe symptoms including severe veinal, petiole and stem necrosis, systemic lesions on leaves along with severe leaf rugosity. Inoculum 14 did not cause any visible symptoms. ELISA with antibodies against PVS, PVX, PVY° and PVYN revealed that all tobacco plants were PVS free, indicating that PVS was unable to infect Nicotiana tabacum ‘Samsun’, consistent with previous observations (De Bokx, Citation1970). Other than that, the ELISA results on tobacco were identical to that on the field samples (inocula). Identical RT–PCR PVY genotyping/strain determination results were also obtained for the tobacco plants and the field samples. The more severe symptoms in the two-virus-infected plants over the single-virus-infected plants suggest synergism of PVX and PVY co-infection.
Potato plants-based bioassay analyses were carried out on cultivars ‘Jemseg’ and ‘Yukon Gold’. The first assay was performed using field samples as inocula on ‘Jemseg’ plants, which in turn served as inoculum for the second assay on ‘Yukon Gold’. ‘Jemseg’ plants responded to the field samples (inocula) with two types of reactions i.e. necrotic responses and mosaic responses. Since ‘Jemseg’ possesses extreme resistance (i.e. immunity) to PVX and PVS (Valkonen et al., Citation1994; Singh & Singh, Citation1995), any reactions would have likely resulted from PVY infection. Indeed, ELISA test confirmed that PVY was the only virus present in the symptomatic plants (i.e. plants inoculated with samples 1–9 and 11–13). The plants infected with inocula 3 and 6 developed severe symptoms including local and systemic necrosis, rugosity, and leaf drop; whereas the remaining (inocula 1, 2, 4, 5, 7–9, and 11–13) developed milder symptoms, mainly mosaic symptoms. As expected, the RT-PCR projected PVYNTN-positive inocula induced necrotic ringspots (i.e. PTNRD) on tubers of ‘Yukon Gold’ (), confirming their PVYNTN identity.
Response of ‘Kennebec’ to single and/or double infections with PVX and PVY°/PVYNTN
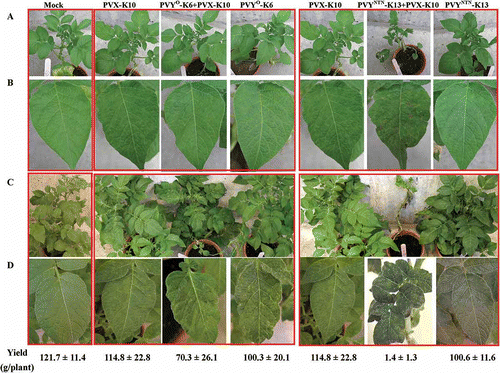
No localized symptoms were elicited on the inoculated leaves, regardless of the virus species, virus strains and the combinations. The first unambiguous symptoms occurred in the leaves located right above the inoculated leaves at 7 dpi in plants with PVX+ PVY° or PVX+PVYNTN. PVX-K10+PVY°-K6 induced mild mosaic symptoms whereas PVX-K10+PVYNTN-K8/-K13 incited severe mosaic, necrotic spots and leaf deformation (). By 8 dpi, unambiguous mosaic symptoms also occurred in plants inoculated with PVYNTN isolates including PVYNTN-K8, PVYNTN-K13 and PVYNTN-Sl. At 12 dpi, all virus-inoculated plants developed symptoms ranging from mosaic (PVX-10, PVY°-RB, PVY°-K6, PVX-K10+PVY°-K6, PVYNTN-Sl, PVYNTN-K8, PVYNTN-K13) to necrosis (PVY°-FL) and severe necrosis/leaf death (PVX-K10+PVYNTN-K13, PVX-K10+PVYNTN-K8) on leaves that emerged shortly after the inoculation (; Suppl. Fig. 1). ELISA tests on the plants at 12 dpi confirmed the infection with intended virus or virus combination (data not shown). No significant differences were observed in ELISA readings (A405) for PVX among PVX, PVX+PVY°, PVX+PVYNTN infection regimes or for PVY between PVY° and PVX+PVY° infections or for PVY between PVYNTN and PVX+PVYNTN treatments (Suppl. Fig. 2). At 21 dpi, mosaic remained as the main symptom in plants infected with PVX (PVX-K10) alone or PVYNTN (PVYNTN-K8, -K13 or -Sl) alone; whereas leaf death/drop occurred on the leaves that emerged shortly after the inoculation with PVY° (PVY°-FL, -RB or -K6) alone or PVY° in combination with PVX (PVX-K10+PVY°-K6). The most severe symptoms including extremely severe deformation, size reduction and necrosis on the upper leaves and leaf death/drop on the lower leaves were observed in plants doubly infected with PVX and PVYNTN (PVX-K10+PVYNTN-K13, PVX-K10+PVYNTN-K8) (). At 35 dpi, necrosis and leaf death/drop ceased to appear in later emerged leaves in PVY°- and PVX+PVY°-infected plants; whereas mosaic became the predominant symptoms in PVX-, PVY°-, PVYNTN- and PVX+PVY°-infected plants. In contrast,
Table 2. Response of potato ‘Kennebec’, tobacco ‘Samsun’ and Physalis floridana to PVX and/or PVY infections in the greenhouse
Fig. 3. Systemic symptoms caused by single or double infections with Potato virus X (PVX) and Potato virus Y common strain (PVY°) or PVY tuber necrotic strain (PVYNTN) on potato ‘Kennebec’ plants. The virus isolates were obtained from field ‘Kennebec’ plants and purified by passing through tobacco (for PVX) or potato ‘Jemseg’ (for PVY isolates). Virus-free plantlets of ‘Kennebec’ were sap-inoculated at six-leaf stage with the intended virus isolates or buffer (mock) and kept in the greenhouse. A. Plants at 12 days post-inoculation (dpi). B. Representative leaves from plants shown in A. C. Plants at 35 dpi. D. Representative leaves from plants shown in C. Potato yields (g/plant, mean ± sd, n = 5) from each treatment are shown correspondingly.
Response of tobacco to single and/or double infections with PVX and PVY°/PVYNTN in the greenhouse
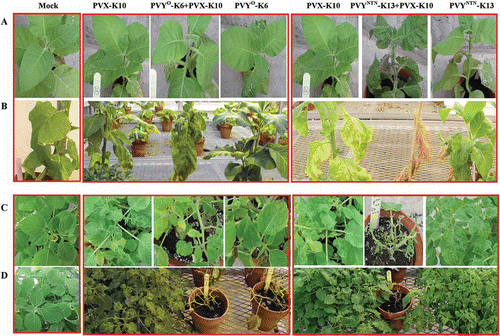
PVX-K10 incited chlorotic spots on the inoculated leaves at 7 dpi, and then on leaves that emerged after the inoculation (). All PVY° isolates (PVY°-FL, -RB, -K6) induced vein clearing at 7 dpi on the first three leaves that emerged after the inoculation and were located right above the inoculated leaves; and as it progressed, mosaic became the main symptom on these leaves as well as on the later emerging leaves. The double infection with PVY°-K6+ PVX-K10 induced more severe symptoms than PVX or PVY° infection alone, resulting in severe vein clearing, chlorotic spots and deformation at 7 dpi on the first three leaves that emerged after the inoculation and were located right above the inoculated leaves. As it progressed, the symptoms on these leaves remained severe. Nevertheless, milder symptoms, mainly mosaic and sporadic chlorotic spots, occurred on the later emerging leaves. All PVYNTN isolates (PVYNTN-Sl, -K8 and -K13) incited characteristic veinal, petiole and stem necrosis at 7 to 14 dpi, and thereafter, milder symptoms occurred on upper leaves (), consistent with previous observations (Nie, Citation2006). The double infections with PVX+ PVYNTN incited significantly more severe symptoms than either PVYNTN or PVX alone. The most striking difference between PVYNTN alone and PVX+PVYNTN combination is the severity and the duration of necrosis as well as leaf/stem/plant death associated with the necrosis (; , B), demonstrating strong synergism between the two strains on tobacco. As seen on potato, no significant differences were observed in ELISA readings at 12 dpi for PVX among PVX, PVX+PVY°, PVX+PVYNTN infection regimes or for PVY between PVY° and PVX+PVY° infections or for PVY between PVYNTN and PVX+PVYNTN treatments (Suppl. Fig. 2).
Fig. 4. Systemic symptoms caused by single or double infections with Potato virus X (PVX) and Potato virus Y common strain (PVY°) or PVY tuber necrotic strain (PVYNTN) on tobacco ‘Samsun’ and Physalis floridana plants. The virus isolates were obtained from field ‘Kennebec’ plants and purified by passing through tobacco (for PVX) or potato ‘Jemseg (for PVY isolates). Seedlings of tobacco and P. floridana were sap-inoculated at five-leaf stage with the intended virus isolates or buffer (Mock) and kept in the greenhouse. A. Tobacco plants at 14 days post-inoculation (dpi). B. Tobacco plants at 35 dpi. C. P. floridana plants at 14 dpi. D. P. floridana plants at 45 dpi.
Response of Physalis floridana to single and/or double infections with PVX and PVY°/PVYNTN in greenhouse
All PVY° isolates including PVY°-FL, -RB and -K6 induced necrotic responses, which were followed by leaf death/drop, on the inoculated leaves as well as on leaves that emerged after the inoculation, whereas PVYNTN isolates (PVYNTN-Sl, -K8 and -K13) induced only mosaic and leaf rugosity (; C, D). PVX infection incited necrotic responses including leaf death at early stages (6–13 dpi), but the necrotic reactions ceased after 13 dpi, and mosaic and leaf rugosity became the primary symptoms in the remaining lower leaves as well as the later emerging leaves in the upper part (; C, D). Double infections with PVX-K10+ PVY°-K6 led to symptoms similar to those of PVY°-K6 infection even though the symptoms (i.e. necrosis and leaf death/drop), like that in PVX infection, appeared earlier (6 dpi vs. 13 dpi) (). The most striking differences between single and double infections in P. floridana came from plants infected doubly with PVX and PVYNTN (PVX-K10+PVYNTN-K13/or -K8) versus those infected singly with PVX or PVYNTN (PVYNTN-K13, -K8 or -Sl). As shown in and , systemic and severe necrosis was elicited by the double infection as early as 6 dpi and continued until the plants were killed at c. 45 dpi. In contrast, plants infected singly with either PVX or PVYNTN did not lead to plant death even at 65 dpi, the very end of the symptom observation. Same as that in potato and tobacco, no significant differences were observed in ELISA readings (A405) at 12 dpi for PVX among PVX, PVX+PVY°, PVX+PVYNTN infection regimes or for PVY between PVY° and PVX+PVY° infections or for PVY between PVYNTN and PVX+PVYNTN treatments (Suppl. Fig. 2).
Discussion
‘Kennebec’ is regarded as a potato cultivar with a high degree of field resistance to PVY (likely PVY° because it was the only known strain to exist in Canadian potatoes when the studies were preformed) (Bagnall & Tai, Citation1986), and a low PVY infection rate has been anticipated and normally observed in Canadian potatoes, both in the field and at the post-harvest test (M. Singh, unpublished information). However, the resistance, like that in several other potato cultivars such as ‘Pentland Crown’ and ‘Desiree’ (Singh et al., Citation2008), might be PVY°-specific, conferred by a hypersensitive resistance (HR) gene such as Ny (Singh et al., Citation2008). Previous studies have demonstrated that ‘Jemseg’ and ‘Katahdin’, both exhibiting field resistance to PVY (Bagnall & Tai, Citation1986; Valkonen et al., Citation1994), do not show HR to PVY isolates belonging to PVYN, PVYN:O and PVYNTN strains (Nie et al., Citation2011, Citation2012). The presence of PVYNTN in 10 out of 12 PVY-positive ‘Kennebec’ plants suggests that the field resistance possessed by ‘Kennebec’ is indeed PVY°-specific, and with the emergence and subsequent spread of PVYN:O and PVYNTN in North American potatoes, more PVY incidences will likely occur in the cultivar as well as other cultivars with PVY°-specific HR. To our knowledge, this is the first report to show the breakdown of field resistance of ‘Kennbec’ to a new PVY strain. It is noteworthy that the field resistance or resistance conferred by N gene does not protect the plant from infection with the corresponding strain (Singh et al., Citation2008), and as such, the presence of PVY° in 2/12 of PVY-positive plants was not unexpected.
Symptom expression is the consequence of host–virus interactions and is determined by the genotypes of both the host and the virus. However, presence of other viruses can affect the otherwise characteristic symptoms associated with a particular virus strain (genotype) in a particular cultivar (genotype). In the field, multiple infections with different species of viruses (or different strains within a virus species) can occur in potatoes after one to several generations. The most common multiple infections in North America, where stringent certification programmes are applied on all seed potatoes, are PVS and PVX in combination with other viruses such as PVY, depending on potato cultivars and other factors such as aphid activity and abundance of virus inoculum. The widespread infection with PVS and PVX in potatoes is largely due to their effective transmission mode (i.e. mechanically transmitted by plant-to-plant contact or by machinery or human activities such as rogueing) and the relatively mild symptoms induced by them (Tavantzis & Southard, Citation1983; Barker & Dale, Citation2006; Nyalugwe et al., Citation2012). This is particularly true for PVS. The virus does not induce visible symptoms in most potato cultivars and, therefore, the infected plants cannot be effectively eradicated by rogueing, leading to further spread in the field and accumulation in the resulting seed potatoes. Indeed, of the 14 ‘Kennebec’ plants originating from the Foundation class sampled, 13 tested PVS positive by both ELISA and RT-PCR. PVX causes symptoms ranging from symptomless to severe mosaic, depending on virus strain and potato cultivar. In most potato cultivars and for most PVX strains, only mild mosaic is incited (Slack, Citation2001), which makes the visual symptom-based field inspection and rogueing difficult, and potentially leads to the spread in the field and buildup of virus incidence in lower class seed potatoes. The presence of PVX in 4 out of 13 symptomatic plants, all in combination with either PVS or PVY or both, is not unexpected. Unlike PVS and PVX, PVY relies on aphids for plant-to-plant transmission (Radcliffe & Ragsdale, Citation2002). Therefore, the abundance and activities of PVY-transmissible aphids and the availability of PVY-carrying plants (inoculum) are the key elements determining PVY spread in the field. The increase of PVY incidence in North America in recent years could be viewed as the consequence of inoculum build-up, especially the build-up of the non-traditional strains PVYN:O and PVYNTN (Gray et al., Citation2010; Nanayakkara et al., Citation2012).
The severe symptoms in field ‘Kennebec’ plants infected with PVX and PVYNTN were the result of synergistic reactions between PVX and PVYNTN, which was confirmed by the greenhouse inoculation experiments. Remarkable synergism also occurred in tobacco and P. floridana plants that were doubly-infected with PVX and PVYNTN. Despite the fact that synergistic reactions between PVX and PVY° occurred on potato ‘Kennebec’, tobacco ‘Samsun’ and P. floridana, the extent of PVX/PVY° synergism was not as significant as that of PVX/PVYNTN, suggesting that PVY strains play a critical role in determination of the symptom severity in host plants. The detrimental effects of PVX and PVYNTN double-infection on ‘Kennebec’ can greatly reduce the yield of the cultivar, affecting not only seed potato but also commercial production fields. It is unknown whether similar trends exist in other potato cultivars, and clearly more research is needed to establish this.
Acknowledgements
The authors thank Xiaowei Xue for technical assistance. This research was funded by New Brunswick Department of Agriculture and Aquaculture under the ‘Enabling Agricultural Research and Innovation Program’, and by Agriculture and Agri-Food Canada under the Peer-Reviewed Project #1389.
References
- Akeley , R.V. , Stevenson , F.J. and Schultz , E.S. 1948 . Kennebec: a new potato variety resistant to late blight, mild mosaic, and net necrosis . Am. Potato J , 25 : 351 – 361 .
- Bagnall , R.H. and Tai , G.C.C. 1986 . Field resistance to potato virus Y in potato assessed by cluster analysis . Plant Dis , 70 : 301 – 304 .
- Barker , H. and Dale , M.F.B. 2006 . “ Resistance to viruses in potato ” . In Natural resistance mechanisms of plants to viruses , Edited by: Loebenstein , G. and Carr , J.P. 341 – 366 . Dordrecht : Springer .
- Crosslin , J.M. and Hamlin , L.L. 2011 . Standardized RT-PCR conditions for detection and identification of eleven viruses of potato and Potato spindle tuber viroid . Am. J. Potato Res , 88 : 333 – 338 .
- De Bokx , J.A. 1970 . Reactions of various plant species to inoculation with potato virus S . Neth. J. Plant Pathol , 76 : 70 – 78 .
- Dehaan , T.L. 1994 . Seed potato certification and diagnostic testing . Can. J. Plant Pathol , 16 : 156 – 157 .
- Ellis , P. , Stace-Smith , R. , Gowler , G. and Mackenzie , D.J. 1996 . Production of monoclonal antibodies for detection and identification of strains of potato virus Y . Can. J. Plant Pathol , 18 : 64 – 70 .
- Ellis , P. , Stace-Smith , R. and De Villiers , G. 1997 . Identification and geographical distribution of serotypes of potato virus Y . Plant Dis , 81 : 481 – 484 .
- González-Jara , P. , Tenllado , F. , Martínez-García , B. , Atencio , F.A. , Barajas , D. , Vargas , M. , Díaz-Ruiz , J. and Díaz-Ruiz , J.R. 2004 . Host-dependent differences during synergistic infection by Potyviruses with potato virus X . Mol. Plant Pathol , 5 : 29 – 35 .
- Goodman , R.M. and Ross , A.F. 1974 . Enhancement by potato virus Y of potato virus X synthesis in doubly infected tobacco depends on the timing of invasion by the viruses . Virology , 58 : 263 – 271 .
- Gray , S. , De Boer , S. , Lorenzen , J. , Karasev , A. , Whitworth , J. , Nolte , P. , Singh , R. , Boucher , A. and Xu , H. 2010 . Potato virus Y: an evolving concern for potato crop in the United States and Canada . Plant Dis , 94 : 1384 – 1397 .
- Hu , X. , Nie , X. , He , C. and Xiong , X. 2011 . Differential pathogenicity of two different recombinant PVYNTN isolates in Physalis floridana is likely determined by the coat protein gene . Virol. J , 8 : 207
- Lorenzen , J.H. , Piche , L.M. , Gudmestad , N.C. , Meacham , T. and Shiel , P. 2006 . A multiplex PCR assay to characterize Potato virus Y isolates and identify strain mixtures . Plant Dis , 90 : 935 – 940 .
- Maclachlan , D.S. , Larson , R.H. and Walker , J.C. 1954 . Potato virus A . Am. Potato J , 31 : 67 – 72 .
- McDonald , J.G. and Kristjansson , G.T. 1993 . Properties of strains of potato virus PVYN in North America . Plant Dis , 77 : 87 – 89 .
- Manzer , F.E. , Merriam , D.C. and Helper , P.R. 1978 . Effects of potato virus 8 and two strains of potato virus X on yields of Russet Burbank, Kennebec, and Katahdin cultivars in Maine . Am. Potato J , 55 : 601 – 609 .
- Nanayakkara , U.N. , Singh , M. , Pelletier , Y. and Nie , X. 2012 . Investigation of Potato virus Y (PVY) strain status and variant population in potatoes in New Brunswick, Canada . Am. J. Potato Res , 89 : 232 – 239 .
- Nie , B. , Singh , M. , Sullivan , A. , Singh , R. P. , Xie , C. and Nie , X. 2011 . Recognition and molecular discrimination of severe and mild PVY° variants of Potato virus Y in potatoes in New Brunswick, Canada . Plant Dis , 95 : 113 – 119 .
- Nie , B. , Singh , M. , Murphy , A. , Sullivan , A. , Xie , C. and Nie , X. 2012 . Response of potato cultivars to five isolates belonging to four strains of Potato virus Y . Plant Dis , 96 : 1422 – 1429 .
- Nie , X. 2006 . Salicylic acid suppresses Potato virus Y isolate N:O-induced symptoms in tobacco plants . Phytopathology , 96 : 255 – 263 .
- Nie , X. , Bai , Y. , Molen , T.A. and Desjardins , D.C. 2008 . Development of universal primers for detection of potato carlaviruses by RT-PCR . J. Virol. Meth , 149 : 209 – 216 .
- Nie , X. and Singh , R.P. 2001 . A novel usage of random primers for multiplex RT-PCR detection of virus and viroid in aphids, leaves, and tubers . J. Virol. Meth , 91 : 37 – 49 .
- Nie , X. , Singh , M. , Pelletier , Y. and Mclaren , D. 2013 . Recent advances on Potato virus Y research in Canada . Am. J. Potato Res , 90 : 14 – 20 .
- Nie , X. and Singh , R.P. 2002 . A new approach for the simultaneous differentiation of biological and geographical strains of Potato virus Y by uniplex and multiplex RT-PCR . J. Virol. Meth , 104 : 40 – 54 .
- Nie , X. and Singh , R.P. 2003 . Specific differentiation of recombinant PVYN:O and PVYNTN strains by multiplex RT-PCR . J. Virol. Meth , 113 : 69 – 77 .
- Nyalugwe , E.P. , Wilson , C.R. , Coutts , B.A. and Jones , R.A.C. 2012 . Biological properties of Potato virus X in potato: effects of mixed infection with Potato virus S and resistance phenotypes in cultivars from three continents . Plant Dis , 96 : 43 – 54 .
- Pacheco , R. , García-Marcos , A. , Barajas , D. , Martiáñez , J. and Tenllado , F. 2012 . PVX-potyvirus synergistic infections differentially alter microRNA accumulation in Nicotiana benthamiana . Virus Res , 165 : 231 – 235 .
- Radcliffe , E.B. and Ragsdale , D.W. 2002 . Aphid-transmitted potato viruses: the importance of understanding vector biology . Am. J. Potato Res , 79 : 353 – 386 .
- Salazar , L.F. 1996 . Potato Viruses and their Control , Lima : CIP .
- Shukla , D.D , Ward , C.W. and Brunt , A.A. 1994 . The Potyviridae , Wallingford : CABI .
- Singh , M. and Singh , R.P. 1995 . Host dependent cross-protection between PVYN, PVY°, and PVA in potato cultivars and Solanum brachycarpum . Can. J. Plant Pathol , 17 : 82 – 86 .
- Singh , M. , Singh , R.P. , Fageria , M.S. , Nie , X. , Coffin , R. and Hawkins , G. 2013 . Optimization of a real-time RT-PCR assay and its comparison with ELISA, conventional RT-PCR and the grow-out test for large scale diagnosis of Potato virus Y in dormant potato tubers . Am. J. Potato Res , 90 : 43 – 50 .
- Singh , R.P. and Boiteau , G. 1984 . Necrotic lesion host for potato virus Y useful in field epidemiological studies . Plant Dis , 68 : 779 – 781 .
- Singh , R.P. , Nie , X. , Singh , M. , Coffin , R. and Duplessis , P. 2002 . Sodium sulphite inhibition of potato and cherry polyphenolics in nucleic acid extraction for virus detection by RT-PCR . J. Virol. Meth , 99 : 123 – 131 .
- Singh , R.P. , Mclaren , D.L. , Nie , X. and Singh , M. 2003 . Possible escape of a recombinant isolate of Potato virus Y by serological indexing and methods of its detection . Plant Dis , 87 : 679 – 685 .
- Singh , R.P. , Valkonen , J.P.T. , Gray , S.M. , Boonham , N. , Jones , R.A.C. , Kerlan , C. and Schubert , J. 2008 . Discussion paper: the naming of Potato virus Y strains infecting potato . Arch. Virol , 153 : 1 – 13 .
- Slack , S.A. 2001 . “ Potato virus X ” . In Compendium of Potato Diseases , 2nd , Edited by: Stevenson , W.R. , Loria , R. , Franc , G.D. and Weingartner , D.P. 69 St. Paul , MN : APS Press .
- Slack , S.A. and German , T.L. 2001 . “ Diseases caused by viruses and viroids ” . In Compendium of Potato Diseases , 2nd , Edited by: Stevenson , W.R. , Loria , R. , Franc , G.D. and Weingartner , D.P. 57 – 62 . St. Paul , MN : APS Press .
- Slack , S.A. and Singh , R.P. 1998 . “ Control of viruses affecting potatoes through seed potato certification programs ” . In Plant Virus Disease Control , Edited by: Hadidi , A. , Khetarpal , R.K. and Koganzawa , H. 249 – 260 . St. Paul , MN : APS Press .
- Tavantzis , S.M. and Southard , S.G. 1983 . Incidence of potato virus X in foundation and certified seed of seven cultivars . Plant Dis , 67 : 959 – 961 .
- Turner, R.S. (2008). Potato pathogens, international trade, and agricultural science: The challenges of regulatory self-sufficiency. Self-Sufficiency Conference Proceedings http://hdl.handle.net/1882/1071 (http://hdl.handle.net/1882/1071)
- Valkonen , J.P.T. , Slack , S.A. and Plaisted , R.L. 1994 . Use of the virus strain group concept to characterize the resistance to PVX and PVY° in the potato cv Allegany . Am. Potato J , 71 : 507 – 516 .