Abstract
Foliage side trimming opens the carrot canopy, permitting greater sunlight penetration and airflow. This prevents moisture build-up and creates unfavourable conditions for the development of common carrot pathogens such as Sclerotinia sclerotiorum (Lib.) de Bary. This study was carried out at the Agriculture and Agri-Food Canada Harrington Research Farm in the growing seasons of 2007, 2008 and 2009, to examine cultivar yield response to foliage side trimming and suppression of Sclerotinia rot of carrot (SRC). All plots were assessed for disease incidence at the time of trimming and again at harvest. As well, carrots were placed into storage at 10 °C for three months to assess the incidence of infected roots. Cultivars varied in yield and other biological parameters, including susceptibility to SRC. Across cultivars, side foliage trimming significantly reduced total and marketable yield by approximately 7.5 and 9.1%, respectively. Overall, side foliage trimming significantly reduced the number of petioles with SRC at both the September and harvest assessments. The incidence of stored roots with SRC was also significantly reduced by side foliage trimming in one year of the study. Disease management strategies for SRC could include selection of cultivars with low disease susceptibility and use of side foliage trimming to reduce disease incidence in susceptible cultivars that are grown for specific agronomic traits.
Résumé
La taille latérale du feuillage ouvre la couverture des carottes offerte par les fanes, ce qui permet une meilleure pénétration de la lumière ainsi qu'une meilleure circulation de l'air. Cela prévient l'accumulation d'humidité et établit des conditions impropres au développement d'agents pathogènes courants de la carotte comme Sclerotinia sclerotiorum (Lib.) de Bary. Cette étude a été menée à la Ferme expérimentale de Harrington d'Agriculture et Agroalimentaire Canada durant les saisons de croissance 2007, 2008 et 2009 afin d’évaluer le rendement des cultivars en fonction de leurs réactions à la taille latérale du feuillage et à la suppression de la pourriture sclérotique de la carotte (PCC). Toutes les parcelles ont été évaluées quant à l'incidence de la maladie lors de la taille et de nouveau lors de la récolte. De même, les carottes ont été entreposées à 10 °C durant trois mois pour évaluer l'incidence de racines infectées. Chez les cultivars, nous avons noté des différences dans le rendement ainsi qu'en ce qui a trait à d'autres paramètres biologiques, y compris la réceptivité à la PCC. Chez tous les cultivars, la taille latérale du feuillage a réduit significativement le rendement total et le rendement de la valeur marchande d'environ 7,5 % et de 9,1 %, respectivement. Aux évaluations de septembre et de la récolte, en général, la taille latérale avait significativement réduit le nombre de pétioles infectés. L'incidence de racines entreposées infectées avait également été significativement réduite par la taille au cours d'une année en particulier. Les stratégies de gestion de la PCC pourraient inclure la sélection de cultivars faiblement réceptifs à la maladie et la taille latérale du feuillage pour en réduire l'incidence chez les cultivars sensibles qui sont cultivés en fonction de propriétés agronomiques particulières.
Introduction
Carrot (Daucus carota L. subsp. sativus) is one of the most widely grown commercial vegetable crops in Canada. A total of 8347 hectares (359 041 metric tons) of carrots were harvested for processing and sale on the fresh market in Canada in 2009 (Statistics Canada, Citation2010). The most common carrot pathogens in Canada include Sclerotinia sclerotiorum (Lib.) de Bary, Alternaria dauci (Kühn) Groves & Skolko, and Cercospora carotae (Pass.) Solheim (Howard et al., Citation1994). The diseases caused by these three pathogens are sclerotinia rot of carrot (SRC), alternaria leaf blight (ALB), and cercospora leaf blight (CLB), respectively. SRC is especially prevalent in Canada, where carrots are kept in storage for up to 8 months, and where storage losses have been reported to be as high as 50% (Kora et al., Citation2005b ; Monaghan, Citation2008). In particular, SRC is an annual issue for carrot producers in the cool, moist Maritime region of Canada. Pre-harvest field infections, which often go unseen during grading, can develop into lesions in storage with external mycelial growth that can spread quickly from one diseased carrot in a storage container to adjacent roots, joining numerous contiguous roots together into cottony, white masses (Kora et al., Citation2005b ; Monaghan, Citation2008).
Symptoms of SRC in the field first appear at a point in the growing season referred to as ‘row closure’. At this time, the thick carrot foliage overlaps with foliage from adjacent rows, which closes over the open area above the furrow. This closure causes the understory of the carrot canopy to have reduced sunlight and airflow (Kora et al., Citation2005a , Citation2008; Monaghan, Citation2008). This moist, shaded environment is ideal for development of apothecia, spore release and germination leading to subsequent development of sclerotinia rot, which affects both aboveground and belowground portions of the carrot plant (Kora et al., Citation2003). In Prince Edward Island (PE), row closure generally occurs from mid-July through August.
Researchers are continually seeking improved methods of integrated pest management (IPM) for these common carrot diseases (Davis & Nuñez, Citation2007). Globally, there are no management strategies using chemical treatments for control of SRC (Davis & Nuñez, Citation2007). Host resistance is often a cornerstone of IPM programmes. Nelson (Citation2002) reported that susceptibility to sclerotinia white mould varied among soybean cultivars. However, SRC-resistant carrot cultivars are currently unavailable, although the range of susceptibility to this disease may vary among cultivars and growing locations (Gugino et al., Citation2007; Monaghan, Citation2008). While there are no approved chemical treatments for effective in-field control of sclerotinia rot of carrots in Canada, two biopesticides, Contans (Coniothyrium minitans W.A. Campb.) and Serenade (Bacillus subtilis (Ehrenberg) Cohn) are registered in Canada for sclerotinia rot control. These products are expensive and not broadly used by the carrot industry. Even with cultivar selection for reduced disease susceptibility, locally grown carrots are highly vulnerable to pathogen growth and disease development. The climate of PE is humid, with a cool boreal temperature regime and a relatively short growing season that extends from May to October. Annual precipitation ranges from 800–1100 mm with approximately 50% being received during the growing season, which often leads to numerous periods of extended foliage wetness.
Canopy-modifying techniques such as increasing row spacing, decreasing planting density, and using cultivars with more porous canopies have helped to lower the incidence of S. sclerotiorum in experimental carrot plots in Ontario (Kora et al., Citation2005a ). In PE, previous research has demonstrated similar strategies for reducing SRC in the field through cultivar selection, lowering nitrogen rates, and decreasing plant density (authors, unpublished data). Cultural control methods such as 2, 3, and 4-year crop rotations with non-hosts of carrot pathogens (Kora et al., Citation2008), cultivation on raised beds to lower soil moisture, nitrogen fertilization to reduce the need for fungicides (Westerveld et al., Citation2008), and, more recently, lateral clipping of the carrot canopy (Kora et al., Citation2005a ; Sanderson & Peters, Citation2007; Monaghan, Citation2008) have also been implemented to reduce SRC in the field.
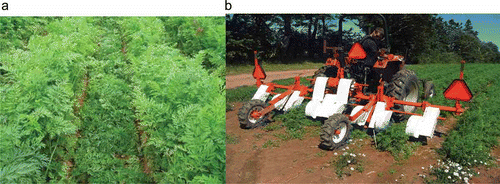
Foliage side trimming (trimming) of the carrot canopy () has become common practice for disease control in most major commercial carrot production areas throughout North America, the UK, and Europe. Carrot producers in the western USA have reported reasonable control of S. sclerotiorum after manually trimming lateral carrot foliage several times throughout the growing season (Kora et al., Citation2003). Researchers in Ontario showed that manually trimmed experimental plots had 75% fewer apothecia (i.e. reproductive bodies containing sexual spores of S. sclerotiorum) than untrimmed plots. These apothecia are localized mostly on top of the carrot beds, underneath the shaded canopy, with some occasionally being found in debris lying in the furrow of trimmed plots. In one study, apothecia in untrimmed plots were found throughout the foliage, on bed tops, slopes and in the depressions between rows (Kora et al., Citation2005a ). Lateral foliage trimming in PE showed varying levels of suppression of sclerotinia rot among different carrot cultivars (Monaghan, Citation2008). Early results indicated that the practice of lateral side foliage trimming did not significantly reduce root yield (Sanderson & Peters, Citation2007; Kora et al., Citation2008; Monaghan, Citation2008). In 2006, a prototype mechanical carrot foliage side trimmer (CFT) was designed and manufactured at Agriculture and Agri-Food Canada in PE to demonstrate the effects of lateral trimming on a commercial field scale ().
Fig. 1. a, A view between rows in a carrot field following trimming of the foliage, showing the cuts left by the trimmer coulters, and the detached foliage in the furrow. b, Prototype carrot foliage trimmer (CFT) designed and built by Agriculture and Agri-Food Canada, Charlottetown, PE.
In 2006, trials performed with the mechanical carrot foliage side trimmer in PE showed an 80% reduction of sclerotinia rot in foliage and subsequently in storage (Sanderson & Peters, Citation2008). In 2007, foliage side trimming again was correlated with a highly significant reduction of SRC in the field and storage (Monaghan, Citation2008).
It is important to quantify the impact of this new trimming technology on carrot cultivar response to SRC suppression and on carrot yield. The objective of this study was to investigate the impact of foliage side trimming on SRC affecting carrot cultivars in PE.
Materials and methods
Experimental design
Experiments were conducted in 2007, 2008 and 2009 at the Agriculture and Agri-Food Canada, Crops and Livestock Research Centre in Harrington (46°22′N, 63°14′W), PE. Carrots were grown at different sites in each year of the study. Soils at these sites were classified as Charlottetown Soil, Orthic Humo-Ferric Podzol (Orthic Podzol in F.A.O. system) with sandy loam to loamy sand textures (MacDougall et al., Citation1988). Clay (< 2 μm), silt (2–50 μm), and fine sand (50–250 μm) content ranged from 5 to 12, 10 to 25, and 25 to 28%, respectively. Soil pH and organic matter ranged from 5.6–6.3 and 3.1–3.4%, respectively. The previous crop in all years was soybeans. The experimental design was a split block with three replicates, with trimming as the main block and cultivar as subplots. Treatments consisted of six cultivars in 2007 and 10 cultivars in 2008 and 2009 with two foliage side trimming regimens: not trimmed and trimmed. The six cultivars in 2007 were ‘Arrowhead’ (Peto Seed), ‘HMX 3297’ (Harris Moran Seed Co.), ‘Maverick’ (Sunseeds – Nunhems), ‘Neptune’ (Vesey's Seed Ltd.), ‘Nevada’ (Bejo Seeds Inc.) and ‘Pronto’ (Noresco). The cultivars ‘Dominion’ (Seminis Vegetable Seeds Inc.), ‘Sugarsnax’ (Nunhems – Nunhems), ‘SVR 9225’ (Noresco), and ‘Uppercut’ (Sunseeds – Nunhems) were added in 2008 and 2009. All sites were fertilized with 5N-20P-20K-0.2B at 1000 kg ha−1. Plots were 10 m long and 4 rows wide. Between row spacing was 91 cm in 2007 and 86 cm in 2008 and 2009. All fertilizers were broadcast and incorporated by harrowing to a depth of approximately 10 cm before hill formation. A bed shaper was used to make a hill approximately 28 cm across the top and 35 cm in height. Carrots were seeded using a Stanhay Singulaire 785 air-seeder in 3-lines per hill. The seeding density was 108 seeds m−1 for all cultivars in all years. The crop was seeded on 1 June, 30 May and 26 May in 2007, 2008 and 2009, respectively. Trimming was performed at row closure. In 2007, ‘Arrowhead’, ‘Maverick’, and ‘Nevada’ were trimmed on 3 August and HMX 3297, ‘Neptune’, and ‘Pronto’ were trimmed on 5 September. In 2008, all cultivars were trimmed on 13 August, except for ‘Maverick’ and ‘Sugarsnax’ which were trimmed on 3 September. In 2009, all cultivars were trimmed on 20 August, except for ‘Dominion’, ‘HMX 3297’, ‘Pronto’ and ‘Sugarsnax’, which were trimmed on 25 August. Plots were mechanically trimmed with a prototype (CFT), designed and manufactured at the Crops and Livestock Research Centre in Harrington (). The CFT opened the canopy by trimming the foliage between the rows, leaving 44 cm of canopy over the carrot row. Weeds were controlled with Linuron applied pre-emergence at 0.6 kg ai ha−1 and post-emergence at 1.2 kg ai ha−1. All plots were managed using agronomic practices similar to those recommended by the Atlantic Provinces Advisory Committee on Vegetable Crops (Anonymous, 1989), and were not irrigated.
Data collection
Soil samples were taken with a hand-held soil probe to a depth of 15 cm prior to each experiment. A composite of 10–12 cores was generated for each experimental site. Samples were air dried and passed through a 2-mm sieve prior to analysis. Organic matter was determined using a LECO CNS analyzer (LECO Corp., St. Joseph, MI), and pH was determined using a 1:1 soil:water ratio.
A 3 m section of one of the centre two rows was assessed for the presence of sclerotinia rot in mid-September and again just prior to harvest. SRC was rated by counting the number of diseased petioles per 3 m plot (Monaghan, Citation2008). The same 3 m section was used as the harvest area. At harvest, a 1 m section of the same row was sampled to determine dry weight (DW) of the carrot tops. All foliage was removed at the soil level and dried at 80 °C for 72 h. Canopy width was measured at the widest point of the canopy with measurements taken just prior to trimming and again just prior to harvest.
In all years, the crop was hand-harvested between 18 and 29 October. Yield data from a 3 m length were recorded from one of the two centre rows as described above. Roots were sized based on crown diameter, according to Canada No. 1 size standards (small = 19 to 32 mm; medium = 32 to 44 mm; large = > 44 mm). Carrots not meeting Canada No. 1 standards (small and misshapen) were classified as unmarketable. Mean root weight, mean root diameter, and mean root length were calculated from the weighted average of roots from the small, medium and large yield categories. Summation of marketable and unmarketable yield was equivalent to total yield.
Forty-two marketable carrots from each plot were washed with water to remove any soil and placed in open-ended plastic cones (Ray Leach ‘Cone-tainer™ Single Cell System’, ID Code SC10, Stuewe & Sons, Inc., Tangent, OR) with the crown facing upwards, and then placed in corresponding storage trays (RL98 storage tray, Stuewe & Sons, Inc.). Carrots were incubated at 10 °C and 95% RH for a period of two months. Stored carrots were evaluated at 30 days and 60 days by counting the number of stored carrots showing rot due to Sclerotinia. Due to technical difficulties with refrigeration, no storage data were collected in 2007.
Statistical analysis
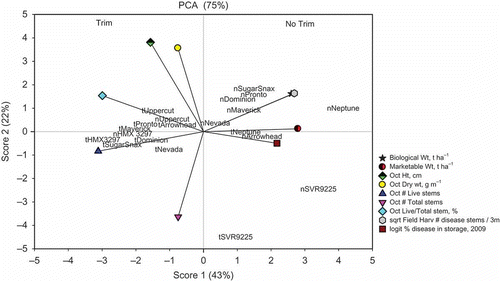
The data were statistically analysed in a split plot model with trimming (no trim, trim) as the main plot factor and cultivars (‘Neptune’, ‘Maverick’, ‘Nevada’, ‘Pronto’, ‘HMX 3297’, ‘Arrowhead’, ‘Sugarsnax’, ‘Uppercut’, ‘Dominion’, ‘SVR 9225’) as the sub-plot factor. Statistical analysis was completed using the ANOVA directive of the statistical programming language GenStat® (VSN International, Citation2008) with significant differences evaluated at P = 0.05. Statistical analysis of the data for yield, morphological plant data, and disease over the three years were analysed as broad types of replication (Steel & Torrie, 1960). The data for number of diseased stems in the field were transformed by obtaining the square root. The data from the stored, coned carrots was transformed by obtaining the logit [logit (P) = log (P/1−P)] (Cramer, Citation2003) of the per cent of Sclerotinia development (% SRC) from the 42 carrots that were stored from each plot. In both instances this was required to achieve normality of the data for analysis within the ANOVA model. To compare and evaluate the relationships between the treatment combinations based on measured variables, principal component analysis (PCA) was applied to the significant datasets. The PCA provided an estimate of variation explained by the significant datasets along with the spatial analysis of the treatment combinations. The bi-plot superimposes the data used in the PCA to provide a graphical representation of the relationship between datasets and treatment combinations. The relationships are evaluated as score 1 and 2 and outline the relationship among treatment combinations, their relation to various datasets and the correlations between datasets.
Results
Cultivar yield
Within individual years, total yield and marketable yield was not affected by trimming (data not shown). The increased precision provided by pooling data across years revealed that total and marketable yield were significantly affected by trimming and by cultivar selection (). Across cultivars, total and marketable yield ranged from 75.6 to 97.8 t ha−1 (P < 0.001) and 56.4 to 80.2 t ha−1 (P < 0.001), respectively. The cultivars ‘Neptune’ and ‘Pronto’ provided the highest total and marketable yield, whereas ‘Sugarsnax’ and ‘HMX 3297’ were among the lowest yielding cultivars. Trimming reduced total (P = 0.03) and marketable (P = 0.044) yield by 7.5 and 8.5%, respectively, but did not affect unmarketable yield. Unmarketable yield was significantly different among cultivars. There was no significant interaction between cultivar and trimming with respect to unmarketable yield. The number of roots m−1 at harvest was not affected by trimming. Across cultivars, the number of roots m−1 at harvest ranged from 45.3 to 75.1 roots m−1 (P < 0.001).
Table 1. Effect of cultivar and side foliage trimming on yield of carrots (2007–2009)
Morphological assessments
Several morphological measurements were assessed pre-harvest and differences were recorded (). Across treatments, mean canopy height was 45.6 ± 0.85 cm. Trimming did not significantly affect canopy height, which ranged from 38.3 to 47.9 cm. Canopy height varied among cultivars. Canopy spread and the number of healthy petioles per root was significantly affected by trimming and cultivar. Across cultivars, canopy spread was 84.1 ± 2.38 cm. Overall, trimming performed in August reduced canopy spread from 89.2 to 78.9 cm at the pre-harvest assessment. The number of healthy petioles ranged from 5.2 to 7.4 petioles per root and varied among cultivars. Across cultivars, trimming significantly increased the number of healthy petioles at the harvest assessment from 6.1 to 6.7 per root. Trimming did not affect the total number of petioles per root. Across treatments, the total number of petioles per root ranged from 8.5 to 10.7 and was significantly affected by cultivar selection. There was a significant interaction of trimming with cultivar for the variables of canopy spread, healthy petioles and total petioles. Foliage side trimming significantly increased the ratio of healthy petioles to total number of petioles per root (healthy/total). Across cultivars, the healthy/total petiole ratio was increased from 65.0 to 70.8% (SEM = 1.16) in non-trimmed vs. trimmed plots. At pre-harvest, the total DW of tops per m was significantly affected by foliage side trimming and cultivar. Across cultivars, foliage side trimming reduced the DW of tops by 23 g m−1.
SRC response to trimming
The number of diseased petioles or stems per 3 m was significantly affected by cultivar and trimming at both the September and pre-harvest assessments (). There was a significant interaction between cultivar and trimming factors. Some cultivars were much more prone to disease than others, with ‘Neptune’, ‘Arrowhead’, ‘Maverick’ and ‘Sugarsnax’ showing high susceptibility to disease, ‘Pronto’, ‘SVR 9225’, ‘Nevada’ and ‘Uppercut’ showing an intermediate response and ‘HMX 3297’ expressing the least disease. ‘Neptune’ had in excess of 140 diseased petioles/ 3m of row at harvest compared with approximately 20 diseased petioles for ‘HMX 3297’. Trimming reduced the number of diseased petioles at the two assessments by 72 and 74%, respectively (). The number of diseased petioles increased from the September to harvest assessment, irrespective of trimming regime. The number of diseased petioles per 3 m ranged from 2.6 to 63.1 and 12.1 to 142.2 for the September and pre-harvest assessments, respectively. Across cultivars, the number of diseased petioles per 3 m increased from 33 to 90 in the untrimmed plots compared with 9 to 23 in the trimmed plots from the September to the pre-harvest rating (). Trimming reduced SRC incidence across all cultivars.
Table 2. Effect of cultivar and side foliage trimming on morphological characteristics of carrots taken pre-harvest (2007–2009)
Table 3. Effect of cultivar and foliage side trimming on the incidence of sclerotinia rot of carrot in the field and storage (2007–2009)
SRC incidence in storage was low in 2008, so that treatment differences were not discernible (data not shown). However, in 2009, cultivars were shown to vary significantly in their response to SRC (). Interestingly, although some cultivars with a high incidence of petiole disease also had high levels of storage rot, the converse was also found. For example, ‘HMX 3297’ had the lowest incidence of diseased petioles among the cultivars examined, yet expressed a relatively high incidence of storage rot (). Across cultivars, trimming significantly reduced the incidence of SRC in storage. Trimming reduced storage disease incidence by over 80% for some cultivars ().
Principal component analysis
Principal component analysis (PCA) showed that the first two components explained 75% of the variation between samples with the first component alone accounting for 43% of the variation (). Score 1 is the contrast of high yield on the positive score 1 and a high number of healthy stems on the negative score 1. Cultivars that were not trimmed yielded more but had fewer healthy stems at harvest. A higher number of diseased stems in the field at harvest was positively correlated with total yield. The trimming treatments (trim or no trim) were also split across the score 1 values, indicating that most cultivars responded similarly to trimming. However, there were a few cultivars such as ‘Uppercut’ and ‘HMX 3279’ that did not follow the split. Trimming reduced the yield of ‘Neptune’ and ‘SVR9225’, but not to the magnitude of that found in the other cultivars. Score 2 is the contrast of higher total stem number on the negative score 2 and the canopy height and canopy dry weight. The healthy/total stem ration is in contrast with the 2009 disease in storage. The cultivar ‘SVR9225’ had more total stems with trimming and behaved differently from the remaining cultivars, which had lower canopy height and less canopy dry weight. The amount of disease in storage and marketable yield was positively correlated. Cultivars without trimming may yield more but also exhibit a greater amount of disease in storage and in the field.
Discussion
The susceptibility of carrots to SRC can vary among cultivars as reported by Gugino et al. (Citation2007) and Monaghan (Citation2008). The carrot cultivars employed in this study were at least partly chosen for their current and/or potential use in commercial production areas of Atlantic Canada. These cultivars varied widely in biological properties of yield, canopy architecture and SRC susceptibility. Differences in canopy architecture, including density of foliage and foliage habit (upright vs lateral fronds) can affect microclimates beneath canopies, and therefore impact on conditions conducive to SRC development. For example, we have often observed that the heavy, dense foliage associated with ‘Maverick’ (highest DW of cultivars tested – ) creates conditions conducive for SRC development. Cultural practices that affect carrot canopy architecture, including increasing plant spacing and cultivation on raised beds, have been shown to improve disease control (Rubatzky et al., Citation1999). Similarly, application of lower rates of nitrogen (compared with standard commercial controls) was reported to be associated with significant reduction of canopy size and density, less lodging, and lower levels of SRC (Couper, Citation2001). An open canopy architecture has been directly associated with a lower incidence of white mould in bean (Schwartz & Steadman, Citation1978; Schwartz et al., Citation1978) or sclerotinia stem rot in soybean (Boland & Hall, Citation1987) and disease control in beans has been improved by breeding for traits such as an upright growth habit and lodging resistance (Fuller et al., Citation1984; Park Citation1993; Saindon et al., Citation1993). Our observations in this study concur with these findings, such that carrot cultivars with a less dense, more open growth habit were much less prone to SRC development.
Carrot roots of the various cultivars differed significantly in their incidence of SRC in storage. Finlayson et al. (Citation1989) also found differences in SRC levels among field-grown carrot cultivars in long-term storage. They suggested that varying degrees of susceptibility to SRC was due to variations in integrity and permeability of root cell membranes and hence, differences in quantitative resistance of root tissues to cellular disruption by S. sclerotiorum. In some cultivars (‘Neptune’, ‘Arrowhead’, ‘Maverick’), a high incidence of disease in petioles was correlated with high incidence of disease in storage. It stands to reason that a higher incidence of infected petioles would provide greater opportunities for the fungus to progress down the stem to infect the crown of the carrot root, leading to an increased incidence of root disease. However, ‘HMX 3297’ was the most resistant cultivar (of those tested) to SRC development in the field, and yet developed a high incidence of root disease (). ‘HMX 3297’ may have had a higher rate of infected petioles in the field that were asymptomatic; this cultivar had a tall canopy with the greatest number of healthy petioles per root at the pre-harvest assessment.
Carrots show differences in disease resistance between the roots and shoots. The polyacetylenic compound falcarindiol, which has strong antifungal properties and is concentrated in root periderm and pericyclic parenchyma (Lewis & Garrod, Citation1983), was associated with the physiological resistance of carrot roots to SRC in storage (Olsson & Svensson, Citation1996). Foster et al. (Citation2008) also noted a thick layer of cells that stained red with safranin O and a zone of leaf abscission at the base of infected petioles, both of which were associated with restricted disease progress. Thus, differences in physiological susceptibility of carrot root tissue to infection by S. sclerotiorum may account for some of the variation in response of different cultivars to SRC that we observed, regardless of their foliar susceptibility. Indeed, it is commonly noted in the potato–Phytophthora infestans (Mont.) de Bary interaction that foliar and tuber susceptibility to disease may differ dramatically within a cultivar (Peters et al., Citation1999). As such, carrot breeding programmes targeting SRC resistance need to consider both foliar and root resistance to infection and disease development.
Canopy trimming has been shown to reduce the development of apothecia of S. sclerotiorum in the field (Kora et al., Citation2005a ; McDonald et al., Citation2008) and provide SRC suppression in field and storage (Kora et al., Citation2005a ; Monaghan, Citation2008). The major mechanism of action of this cultural control is to open the canopy, increase airflow and sunlight penetration, which consequently reduces moisture and increases temperature within the canopy. This alteration of canopy microclimate helps to deter pathogen infection and disease development (Kora et al., Citation2005a ). In this study, canopy trimming provided significant suppression of foliar disease across cultivars and was associated with lower severity of SRC in storage. Disease could even be managed with trimming in cultivars, such as ‘Maverick’, which were highly susceptible to SRC development. Growers select and grow cultivars which are adapted to their production region and have acceptable qualities for fresh and/or processing markets. For example, ‘Neptune’ has been a mainstay of carrot production in PE for many years, and is highly susceptible to SRC development. Losses due to SRC in storage with this cultivar have been as high as 0.5 million dollars in PE in recent years (D. Read, PEI Vegetable Growers Co-Operative, Charlottetown, PE, personal communication). In the past, growers have discarded cultivars due to their susceptibility to SRC. However, by implementing trimming as a standard production practice, a greater number of cultivars, including ‘Neptune’, can be grown with a reduced risk of SRC development. Recently, post-harvest application of fludioxonil (Scholar® – emergency use registration for carrots in 2012) to carrots has also been shown to be an effective means of managing SRC in storage and can be considered as a component of the integrated management of this disease (Monaghan, Citation2008).
Although a slight yield loss due to trimming was found across the three years of this study, this trend was not evident in all years and was more than offset by the concomitant increases in root quality that trimming provided. In addition, canopy trimming is environmentally friendly and may allow for fewer fungicide applications (A. Ells, Oxford Frozen Foods Inc., personal communication) and can be incorporated into both conventional and organic production systems. Future directions for this new technology will be aimed at developing trimming protocols for specific carrot cultivars and processing types.
Acknowledgements
This study was conducted with the collaboration of Oxford Frozen Foods Inc. The technical assistance of Sylvia Wyand, Basil Dickson, Kathy Drake and Ian MacDonald is gratefully acknowledged.
References
- Anonymous. 1999. Atlantic provinces vegetable crops guide to pest management 1999–2000. Atlantic Vegetable Committee. Publ. no.1400A. Adgex no. 250, 74 pp.
- Boland , G.J. and Hall , R. 1987 . Evaluating soybean cultivars for resistance to Sclerotinia sclerotiorum under field conditions . Plant Dis. , 71 : 934 – 936 .
- Couper , G. 2001 . The biology, epidemiology and control of Sclerotinia sclerotiorum on carrots in North East Scotland , Aberdeen, Scotland , UK : Ph.D. thesis. University of Aberdeen .
- Cramer , J.S. 2003 . “ The origins and development of the logit model ” . In Logit models from economics and other fields , Cambridge : Cambridge University Press .
- Davis , R.M. and Nuñez , J. 2007 . “ Integrated approaches for carrot pests and disease management ” . In General Concepts in Integrated Pest and Disease Management , Edited by: Ciancio , A. and Mukerji , K. G. 149 – 188 . Dordrecht : Springer .
- Diseases and Pests of Vegetable Crops in Canada Howard, R.J., Garland, J.A., & Seaman, W.L. (Eds.) (1994). Ottawa, ON: The Canadian Phytopathological Society and the Entomological Society of Canada.
- Finlayson , J.E. , Pritchard , M.K. and Rimmer , S.R. 1989 . Electrolyte leakage and storage decay of five carrot cultivars in response to infection by Sclerotinia sclerotiorum . Can. J. Plant Pathol. , 11 : 313 – 316 .
- Foster , A.J. , McDonald , M.R. and Boland , G.J. 2008 . Disease progression of sclerotinia rot of carrot, caused by Sclerotinia sclerotiorum, from shoot to root before and after harvest . Can. J. Plant Pathol. , 30 : 206 – 213 .
- Fuller , P.A. , Steadman , J.R. and Coyne , D.P. 1984 . Enhancement of white mold avoidance in dry beans by canopy elevation . HortScience , 19 : 78 – 79 .
- Gugino , B.K. , Carroll , J.E. , Widmer , T.L. , Chen , P. and Abawi , G.S. 2007 . Field evaluation of carrot cultivars for susceptibility to fungal leaf blight diseases in New York . Crop Prot. , 26 : 709 – 714 .
- Kora , C. , McDonald , M.R. and Boland , G.J. 2003 . Sclerotinia rot of carrot: an example of phenological adaptation and bicyclic development by Sclerotinia sclerotiorum . Plant Dis. , 87 : 456 – 470 .
- Kora , C. , McDonald , M.R. and Boland , G.J. 2005a . Lateral clipping of canopy influences the microclimate and development of apothecia of Sclerotinia sclerotiorum in carrots . Plant Dis. , 89 : 549 – 557 .
- Kora , C. , McDonald , M.R. and Boland , G.J. 2005b . Occurrence of fungal pathogens of carrots on wooden boxes used for storage . Plant Pathol. , 54 : 665 – 670 .
- Kora , C. , McDonald , M.R. and Boland , G.J. 2008 . “ New progress in the integrated management of sclerotinia rot of carrot ” . In Integrated management of plant pests and diseases: Integrated Management of Diseases caused by Fungi, Phytoplasma and Bacteria , Edited by: Ciancio , A. and Mukerji , K.G. 243 – 270 . Dordrecht : Springer .
- Lewis , B.G. and Garrod , B. 1983 . “ Carrots ” . In Post-Harvest Pathology of Fruits and Vegetables , Edited by: Dennis , C. 103 – 124 . London : Academic Press .
- MacDougall , J.I. , Veer , C. and Wilson , F. 1988 . Soils of Prince Edward Island: Prince Edward Island Soil Survey , 210 Ottawa , ON : Agriculture Canada . LRRC Contrib. No. 84–54
- McDonald , M.R. , Vander Kooi , K.D. and Westerveld , S.M. 2008 . Effect of foliar trimming and fungicides on apothecial number of Sclerotinia sclerotiorum, leaf blight severity, yield, and canopy microclimate in carrot . Plant Dis. , 92 : 132 – 136 .
- Monaghan , P. 2008 . Management of Sclerotinia rot achieved with pre-harvest and post-harvest measures , Charlottetown, PE , Canada : Undergraduate Thesis, Department of Biology, University of Prince Edward Island .
- Nelson, B. D. (2002). Sclerotinia Stem Rot (White Mold). Department of Plant Pathology, North Dakota State University, Fargo, ND http://www.ndsu.edu/pubweb/~bernelso/soydiseases/sclerotinia.shtml (http://www.ndsu.edu/pubweb/~bernelso/soydiseases/sclerotinia.shtml)
- Olsson , K. and Svensson , R. 1996 . The influence of polyacetylenes on the susceptibility of carrots to storage diseases . J. Phytopathol. , 144 : 441 – 447 .
- Park , S.J. 1993 . Response of bush and upright plant type selections to white mold and seed yield of common beans grown in various row widths in southern Ontario . Can. J. Plant Sci. , 73 : 265 – 272 .
- Peters , R.D. , Platt , H.W. , Hall , R. and Medina , M. 1999 . Variation in aggressiveness of Canadian isolates of Phytophthora infestans as indicated by their relative abilities to cause potato tuber rot . Plant Dis. , 83 : 652 – 661 .
- Rubatzky , V.E. , Quiros , C.F. and Simon , P.W. 1999 . Carrots and Related Vegetable Umbelliferae , New York : CABI Publishing .
- Saindon , G. , Huang , H.C. , Kozub , G.C. , Mundel , H.H. and Kemp , G.A. 1993 . Incidence of white mold and yield of upright bean grown in different planting patterns . J. Phytopathol. , 137 : 118 – 124 .
- Sanderson, K.R., & Peters, R.D. (2007). Carrot foliage trimmer reduces storage rot. Agriculture and Agri-Food Canada, Charlottetown, PE, Canada. Available from: <accessed 13 June 2008 http://www4.agr.gc.ca/resources/prod/doc/pmc/pdf/carr_e.pdf (http://www4.agr.gc.ca/resources/prod/doc/pmc/pdf/carr_e.pdf)
- Sanderson , K.R. and Peters , R.D. 2008 . “ Side trimming carrot canopies expected to become the standard practice ” . In Carrot Country, Summer 2008 issue , Sparta , MI : Columbia Publishing .
- Schwartz , H.F. and Steadman , J.R. 1978 . Factors affecting sclerotia populations of, and apothecium production by, Sclerotinia sclerotiorum . Phytopathology , 68 : 383 – 388 .
- Schwartz , H.F. , Steadman , J.R. and Coyne , D.P. 1978 . Influence of Phaseolis vulgaris blossoming characteristics and canopy structure upon resistance to Sclerotinia sclerotiorum . Phytopathology , 68 : 465 – 470 .
- Statistics Canada . 2010 . “ Fruit and vegetable production: February 2010 ” . In Statistics Canada Catalogue no. 22-003-X , Ottawa , ON : Statistics Canada .
- 1960 . The principles and procedures of statistics. McGraw-Hill Book Company New York Steel, R.G.B. and Torrie, J.H. NY.
- VSN International . 2008 . GenStat for Windows 11th Edition , VSN International, Hemel Hempstead, UK. Web page: GenStat.co.uk .
- Westerveld , S.M. , McKeown , A.W. and McDonald , M.R. 2008 . Relationship between nitrogen fertilization and Cercospora leaf spot and Alternaria leaf blight of carrot . HortScience , 43 : 1522 – 1527 .