Abstract
The antagonism of bean (Phaseolus vulgaris) root rot fungus Fusarium solani f. sp. phaseoli by the endophytic insect pathogenic fungus Metarhizium robertsii was investigated in vitro and in vivo. Dual cultures on Petri dishes showed antagonism of M. robertsii against F. solani. A relative inhibition of c. 60% of F. solani growth was observed in these assays. Cell-free culture filtrates of M. robertsii inhibited the germination of F. solani conidia by 83% and the inhibitory metabolite was heat stable. Beans plants colonized by M. robertsii, then exposed to F. solani, showed healthier plant growth and lower disease indices compared with plants not colonized by M. robertsii. These results suggest that the endophytic insect pathogenic fungus M. robertsii could also be utilized as a biocontrol agent against certain plant root pathogens.
Résumé
L'antagonisme du champignon entomopathogène Metarhizium robertsii à l'endroit du pourridié Fusarium solani f. sp. phaseoli du haricot commun (Phaseolus vugaris) a été étudié in vitro et in vivo. Des cultures en duel sur boîtes de Petri ont démontré l'antagonisme de M. robertsii à l’égard de F. solani. Au cours de ces tests, une inhibition relative d'environ 60 % de la croissance de F. solani a été observée. Des filtrats de cultures acellulaires de M. robertsii ont réduit de 83 % la germination des conidies de F. solani, et le métabolite inhibiteur était thermostable. Les plants de haricot colonisés par M. robertsii, puis exposés à F. solani, ont affiché une meilleure croissance et de plus faibles indices de la maladie que les plants qui n'avaient pas été colonisés par M. robertsii. Ces résultats suggèrent que le champignon entomopathogène M. robertsii pourrait également être utilisé comme agent de lutte biologique contre certains organismes pathogènes s'attaquant aux racines des plantes.
Keywords:
Introduction
Haricot beans (Phaseolus vulgaris L.) are highly susceptible to root rot disease which is caused by the soil-borne fungal pathogen Fusarium solani f. sp. phaseoli (Steadman et al., Citation1975; Graham, Citation1978; Tan & Tu, Citation1995; xCichy et al., Citation2007). Disease symptoms are initially characterized by narrow, long, red to brown lesions on stems extending down to the main taproot, which may decay and die. In some cases, the plant hypocotyl is also affected along with the development of lateral or adventitious roots above the damaged taproot. Infected plants show stunted growth when compared with healthy plants (Burke & Barker, Citation1966; Campbell, Citation1989; Schneider & Kelly, Citation2000; Cichy et al., Citation2007).
Generally, fungicides are used to control fusarium root rot (Campbell, Citation1989). Application of fungicides is not always successful and may enhance fungicide resistance, environmental contamination, harm to human health and production costs. In this context, biological control can be an effective alternative and a number of biological antagonists are currently available. For example, several species of Trichoderma (Grosch et al., Citation2006; Rojo et al., Citation2006; El-Kassas & Khairy, Citation2009), Pythium oligandrum Dreschler (Floch et al., Citation2005), and other fungal species are reportedly used as biocontrol agents of plant root diseases. In particular, several endophytic fungi have been shown to be antagonists of F. solani (Hassan Dar et al., Citation1997).
Metarhizium robertsii J.F. Bisch., Rehner & Humber sp. nov., a well-known insect pathogenic fungus, has recently been reported to be a plant endophyte and promotes plant root growth (Sasan & Bidochka, Citation2012). Metarhizium spp. have previously been shown to possess antifungal properties against Fusarium oxysporum Schlecht. emend. Snyder & Hansen, Botrytis cinerea (De Bary) Whetzel, and Alternaria solani Sorauer (Kang et al., Citation1996) as well as Ceratocystis ulmi (Buisman) Melin & Nannf. (Gemma et al., Citation1984). In this study, we explore the possibility that M. robertsii could be antagonistic to the bean root rot pathogen F. solani f. sp. phaseoli under in vitro and in vivo conditions. To our knowledge, this is the first report of biocontrol of a bean root pathogen by an endophytic, insect pathogenic fungus.
Materials and methods
Fungal isolates
Metarhizium robertsii (ARSEF 2575) was obtained from the United States Department of Agricultural Research Service Collection of Entomopathogenic Fungal Cultures, Ithaca, New York. Cultures were grown on potato dextrose agar (PDA; Difco Laboratories, Sparks, MD) at 27 °C at a 16/8 h day/night cycle for 10 days to obtain conidia. Fusarium solani f. sp. phaseoli (DAOM 170970) was obtained from the Canadian Culture Collections, Agriculture Canada, Ottawa. This strain was originally isolated from infected bean roots. Fusarium oxysporum Schlecht. emend. Snyder & Hansen, originally isolated from infected wheat roots, was obtained from Wards Natural Science Limited (St. Catharines, ON). Fusarium solani and F. oxysporum were grown on PDA for 14 days at 25 °C to obtain macroconidia. The conidial suspensions were filtered through glass wool to remove mycelia, and adjusted, using sterile distilled water containing 0.01% (v/v) Triton X-100, to 106 conidia mL−1 measured using a haemocytometer on a compound microscope.
Antagonism of M. robertsii to F. solani on Petri dishes
The antagonism of M. robertsii against F. solani was screened using dual culture Petri dish assays (Sobowale et al., Citation2010). Metarhizium robertsii and F. solani were inoculated 3 cm apart from the edge of the Petri dish (9 cm diameter) containing PDA. Metarhizium robertsii was inoculated first, allowed to grow for two days, before the introduction of F. solani. Controls contained M. robertsii or F. solani alone, in order to observe the growth in the absence of interactions. Additionally, controls with F. solani and F. oxysporum inoculated 3 cm apart, each 1.5 cm from the centre of the Petri dish, were utilized. Fungal growth was observed for 14 days at 25 °C. Growth of F. solani was measured as the average diameter of the colony size in four different directions. Fusarium solani growth on day 14, measured as the average diameter of the colony size in four different directions, was used for data analysis. The per cent inhibition of the radial colony growth was calculated as [(C-T)/C] ×100 (Mishra, Citation2010), where C is the radial growth in the control set and T is the radial growth in the treated set. There were 10 replicates per experiment and the experiment was repeated thrice.
The formation of clearing zones by M. robertsii in the presence of F. solani was investigated by introducing M. robertsii on a F. solani lawn culture that was grown for 5 days. These cultures were initiated by spreading a macoconidial suspension onto the surface of PDA plates. Metarhizium robertsii was inoculated (10 μL of 108 conidia mL−1) at four equidistant points on the F. solani lawn. Plates were incubated at 25 °C for 14 days at a 16/8 h day/night cycle and clearing zones due to M. robertsii were measured on days 7 and 14. There were 10 replicate dishes per experiment and the experiment was repeated thrice.
Antagonism of Metarhizium robertsii cell-free culture filtrate
The effect of cell-free culture filtrates of M. robertsii on spore germination and growth in liquid culture of F. solani was assessed. Metarhizium robertsii was inoculated into 100 mL of yeast extract broth (0.1% yeast extract in distilled water; YEB) and incubated at 25 °C. Culture filtrates were obtained on days 4 and 10 by vacuum filtration through Whatman filter paper no. 44 followed by filtration through 0.22 μm Millipore membrane. The culture filtrates were checked for the presence of M. robertsii by plating a sample on PDA. The cell-free culture filtrates were diluted in YEB, and 3 mL of 100%, 90%, 50% and 10% cell-free culture filtrate were inoculated with 300 μL of a conidial suspension of F. solani (108 conidia mL−1) in glass tubes. YEB was used as the control. The conidial suspensions were incubated at 25 °C. The number of F. solani conidia germinated per hundred cells was counted at 24 and 48 h. Three counts of 100 were taken from each treatment and the experiment was repeated thrice.
The effect of M. robertsii cell-free culture filtrates on F. solani growth was also observed for a 4-day period. Autoclaved, as well as non-autoclaved, M. robertsii cell-free culture filtrate was obtained after 4 days and 10 days of M. robertsii growth as described above. Fusarium solani conidial suspension (1.25 mL of 108 conidia mL−1) was added to 125 mL of M. robertsii 100% cell-free culture filtrate in glass flasks. For controls, YEB was used. Flasks were placed on a rotary shaker at 150 rpm at 25 °C for four days. The cultures were harvested by vacuum filtration through Whatman No. 44 filter paper and dried at 40 °C for 48 h and weighed. There were four replicate flasks per experiment and the experiment was repeated thrice.
Antagonism in host plants
Haricot bean seeds were obtained from OSC seeds (Waterloo, ON) and surface disinfected prior to use by a modification of the method of Miche & Balandreau (Citation2001). Seeds were soaked in sterile distilled water for 30 min and then immersed in 4% sodium hypochlorite thrice (10 min each) followed by a single 30 min wash with 30% hydrogen peroxide. Seeds were then washed with sterile distilled water and kept overnight at 4 °C to allow for synchronization of growth. Seeds were plated on PDA as well as nutrient agar to test for fungal and bacterial contamination.
The effect of pretreating the potting mixture (Schultz Potting Mixture, Brantford, ON) with M. robertsii as an antagonist of F. solani infection in bean plants was investigated in growth chamber experiments. Potting mixture was moistened with sterile distilled water and autoclaved in trays (3 cm × 19 cm × 28 cm) twice before use. Eight plugs (1 cm2 each) from an actively growing fungal colony were mixed in each tray and incubated for 10 days at 25 °C. The medium was mixed every alternate day to ensure uniform inoculation. Treatments for biocontrol assays were: (1) no treatment; (2) M. robertsii; (3) F. solani; and (4) M. robertsii and F. solani (1 : 1 ratio). Inoculum of F. solani consisted of 8 plugs (1 cm2) taken from a PDA colony. Bean seeds were individually placed at a 0.5 cm depth in each tray. The potting mixture was moistened with 10 mL half-strength MMN solution (0.05 g CaCl2, 0.025 g NaCl, 0.5 g KH2PO4, 0.5 g (NH4)2HPO4, 0.15 g MgSO4.7H2O, 1 mg FeCl3.6H2O, 100 μg thiamine hydrochloride, 5 g glucose monohydrate), 10 mL L−1 stock solution of trace elements (containing KCl 3.728 g, H3BO3 1.546 g, MnSO4.H2O 0.845 g, ZnSO4.7H2O 0.575 g, CuSO4.5H2O 0.125 g, (NH4)6Mo7O24.4H2O 0.018 g, per litre) (Kottke et al., Citation1987) once a week to promote plant growth and sterile distilled water was added daily to maintain soil moisture. Plants were maintained in a growth chamber (25/20 °C, 60/80% relative humidity and 16/8 h day/night cycle (Grosch et al., 2006) for four weeks. Plant growth parameters such as seedling emergence, shoot height, number of leaves, and any visible disease symptoms were assessed daily. After four weeks, whole plants were removed from the potting mixture and roots were rinsed with sterile water. Plants were individually rated for disease severity based on a root rot index and colour intensity according to Dar et al. (Citation1997). Individual plants were rated on a scale of 0–1, where 0 = no root rot symptoms; 0.10 = less than 10% root area rotted; 0.25 = 11–25% root area rotted; 0.50 = 26–50% root area rotted; 0.75 = 51–75% root area rotted; and 1.0 = more than 75% root area rotted. The individual ratings were converted to a mean disease index by taking the quotient of the sum of the individual plant rating values and the number of plants assessed. Plants were also rated for disease severity based on necrotic lesions on the roots and hypocotyl as described by Filion et al. (Citation2003). The ratings were based on a scale of 0–5, where 0 = no disease symptoms, 1 = slightly brown or <50% surface discolouration of the hypocotyl, firm upon pressure from thumb and forefinger, and slight root pruning; 2 = as 1 but >50% surface discolouration; 3 = discoloured hypocotyl and roots collapsing under pressure and extensive root pruning, 4 = darkly discoloured hypocotyl and roots completely collapsed or collapsing easily under pressure and severe root pruning; and 5 = dead or dying plant. Root and shoot fresh weights, height, number of leaves, and dry weights of entire plants were measured for all plants in each treatment. Each treatment included five plants and the experiment was repeated four times.
Results and discussion
Antagonism of M. robertsii to F. solani on Petri dishes
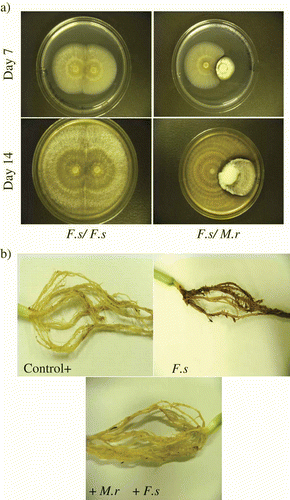
When F. solani and M. robertsii were co-cultured on Petri dishes, an inhibition clearing zone was observed on day 4 and this effect was observed up to day 14 (). Clearing zones were absent when F. oxysporum and F. solani were co-cultured (data not shown). Inhibition of F. solani by M. robertsii was also observed as a decrease in radial growth. A relative inhibition of 59.4% of F. solani growth occurred when co-cultured with M. robertsii. A zone of F. solani inhibition of 3.7 ± 0.5 mm and 5.9 ±1.1 mm on a lawn culture was observed at days 7 and 14, respectively, by M. robertsii. Furthermore, M. robertsii could be observed overgrowing F. solani in dual culture plate assays at day 14 and white mycelial growth of M. robertsii could be seen at the interface with F. solani. The darker zone of confluent growth could be indicative of melanization or necrosis of F. solani hyphae.
Fig. 1. Images of dual plate cultures of Metarhizium robertsii (M.r) and Fusarium solani (F.s) and biocontrol of F. solani by M. robertsii on bean root. (a) Dual plate assays showing antagonistic activity of M. robertsii on F. solani on day 7 and 14. In each photo, a zone of F. solani clearing is evident at the colony interface. (b) Images of bean root rot caused by F. solani after four weeks. Roots were obtained from control plants, F. solani treatment and M. robertsii + F. solani treatments.
Antagonism of Metarhizium robertsii cell-free culture filtrate
Fusarium solani conidia incubated in cell-free culture filtrates of M. robertsii showed delayed germination (). Fusarium solani conidia incubated in 100% M. robertsii cell-free culture extract collected on day 10 showed 83% and 66% germination inhibition, compared with the control, at 24 and 48 h, respectively (P<0.0001, t = 26.85, df = 6). At day 4, M. robertsii cell-free extract germination inhibition was 65% and 39% at 24 and 48 h, respectively (P<0.0001, t = 16.29, df = 6). These results suggest that M. robertsii secretes a compound that inhibits F. solani germination. Additionally, the amount of this compound in M. robertsii cultures increased with culture age.
Fig. 2. Inhibition of Fusarium solani in Metarhizium robertsii cell-free culture extracts. (a) Germination of F. solani conidia in M. robertsii cell-free culture extract. Fusarium solani conidia were suspended in different concentrations (100%, 90%, 50% and 10%) of 4-day-old and 10-day-old M. robertsii cell-free culture filtrates. Con = control, no cell-free extract. Germination of 100 F. solani conidia was counted (N = 4) after 24 h (light bars) and 48 h (dark bars). Each bar represents mean % germination ± S. E. (b) Fusarium solani biomass yield in M. robertsii cell-free culture filtrate (autoclaved – light bars and non-autoclaved – dark bars) after four days of growth. Data represents the mean ± S.E. of dry weights of F. solani grown under different concentrations (100%, 90% and 50%) of M. robertsii autoclaved and not autoclaved cell-free extract and the control (Con = control, no cell-free extract).
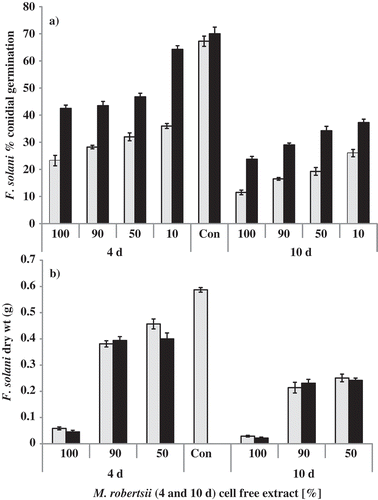
Metarhizium robertsii cell-free culture filtrates also decreased F. solani vegetative growth (). Autoclaving the cell-free culture filtrate did not reduce the inhibitory effect on F. solani. No significant differences were observed in F. solani biomass when grown in autoclaved compared with non-autoclaved M. robertsii cell-free culture filtrates (P>0.05, df = 8). This suggests that the inhibitory compound(s) are heat stable. Fusarium solani biomass obtained in control cultures was significantly higher (P<0.0001, df = 8) than without M. robertsii cell-free culture filtrate.
Many species of endophytic fungi show antagonism against plant pathogenic fungi. Furthermore, cell-free extracts from endophyte fungal cultures inhibited growth of various plant pathogenic fungi (Liu et al., Citation2001; Inácio et al., Citation2006; Kim et al., Citation2007). For example, treatment of wheat with the endophytic fungi Chaetomium sp. and Phoma sp. resulted in reduced severity of foliar disease caused by Puccinia and Pyrenophora spp. The same protective effect was observed when the endophytic fungal culture filtrates were applied to the plants (Dingle & McGee, Citation2003; Istifadah & McGee, Citation2006). Metarhizium spp. produce a number of metabolites, such as destruxins, swainsonine, serinocyclins and cytochalasins (Roberts & St Leger, 2004; Krasnoff et al., Citation2007). These compounds have insecticidal and phytotoxic activities; however, their antagonistic effects on plant pathogens have yet to be elucidated. Metarhizium spp. have previously been shown to have antifungal activity against F. oxysporum, Botrytis cinerea (De Bary) Whetzel, and Alternaria solani Sorauer (1896) (Kang et al., 1996). Other endophytic insect pathogenic fungi, including Beauveria bassiana (Bals.-Criv.) Vuill. and Lecanicillium spp., provide dual biological control properties (i.e. against insect pests and plant pathogens) (Ownley et al., 2010). Metarhizium anisopliae and B. bassiana were reported to show in-vitro inhibition of Ceratocystis ulmi (Buisman) Melin & Nannf. (1934) (Gemma et al., 1984). Beauveria bassiana, which is also an endophyte, provided plant protection against Rhizoctonia solani J.G. Kühn 1858 and Pythium myriotylum Drechsler, (1930) (Ownley et al., Citation2008). The mechanism by which Metarhizium provides plant protection against plant fungal pathogens is not known, but our results suggest that an extracellular, water-soluble metabolite is implicated. Earlier, it was suggested that M. anisopliae inhibition of Fusarium oxysporum f. sp. vasinfectum could also be due to competition for nutrition and space and antibiosis (Qi et al., Citation2010). However, multiple mechanisms may be involved. For example, it has been suggested that B. bassiana, Lecanicillium spp. and Trichoderma spp. induce systemic resistance in plants (Harman et al., Citation2004; Ownley et al., Citation2010).
Antagonism in host plants
Bean plant bioassays were performed in the presence of F. solani and M. robertsii. While all seeds germinated into healthy seedlings by day 11 in the control and F. solani + M. robertsii treatment, 60% of seeds emerged in the presence of the pathogen alone. After four weeks, roots were collected from all treatments and rated for growth and disease indices (). Roots obtained from the F. solani treatment were necrotic when compared with the control roots (without F. solani or M. robertsii) or the F. solani + M. robertsii treatment plants (). Roots infected with F. solani showed severe disease symptoms when compared with the F. solani + M. robertsii treatment (). Per cent root rot area rating for F. solani treatment was greater (0.69±0.03) than the control plants (0.25±0.03) (P<0.0001, t = 8.86, df = 14) and F. solani + M. robertsii treatments (0.34±0.03) (P<0.0001, t = 8.37, df = 14). There were no statistical differences in root rot rating between the control plants and F. solani + M. robertsii treatment (P > 0.05, t = 1.97, df = 14). Additionally, the disease index based on intensity of hypocotyl and root rot was also higher in the F. solani treatment (3.93±0.07) when compared with control plants (1.73±0.28) (P<0.0001, t = 9.89, df = 14) or F. solani + M. robertsii treatments (2.31±0.11) (P<0.0001, t = 12.22, df = 14).
Table 1. The effect of M. robertsii (M.r) as an antagonist of Fusarium solani (F.s) infection of bean plants
We have previously shown that M. robertsii is a plant endophyte (Sasan & Bidochka, Citation2012), and the present study describes antagonistic activity against a fungal plant root pathogen. A more complete understanding of the ecology of these endophytes is likely to aid in the development of more efficient insect and phytopathogen biocontrol programmes, and also provide beneficial traits such as increased yields (Kabaluk & Ericsson, Citation2007) through plant growth and root system promotion, and improved compatibility with other beneficial microorganisms (Sasan & Bidochka, Citation2012).
Acknowledgements
This research was conducted with funds provided by a Natural Sciences and Engineering Research Council of Canada Discovery Grant to M.J.B.
References
- Burke , D.W. and Barker , A.W. 1966 . Importance of lateral roots in Fusarium root rot of beans . Phytopathology , 56 : 292 – 294 .
- Campbell , R.E. 1989 . Biological Control of Microbial Plant Pathogens , Cambridge : Cambridge University Press .
- Cichy , K.A. , Snapp , S.S. and Kirk , W.W. 2007 . Fusarium root rot incidence and root system architecture in grafted common bean lines . Plant Soil , 300 : 233 – 244 .
- Dar , G.H.H. , Zargar , M.Y. and Beigh , G.M. 1997 . Biocontrol of Fusarium root rot in the common bean (Phaseolus vulgaris L.) by using symbiotic Glomus mosseae and Rhizobium leguminosarum . Microb. Ecol. , 34 : 74 – 80 .
- Dingle , J. and Mcgee , P.A. 2003 . Some endophytic fungi reduce the density of pustules of Puccinia recondita f.sp. tritici in wheat . Mycol. Res. , 107 : 310 – 316 .
- El-Kassas , H.Y. and Khairy , H.M. 2009 . A trial for biological control of a pathogenic fungus (Fusarium solani) by some marine microorganisms . Amer.-Euras. J. Agric. Environ. Sci. , 5 : 434 – 440 .
- Filion , M. , St-Arnaud , M. and Jabaji-Hare , S.H. 2003 . Quantification of Fusarium solani f. sp. phaseoli in mycorrhizal bean plants and surrounding mycorrhizosphere soil using real-time polymerase chain reaction and direct isolations on selective media . Phytopathology , 93 : 229 – 235 .
- Floch , G.L. , Benhamou , N. , Mamaca , E. , Salerno , M-I. , Tirilly , Y. and Rey , P. 2005 . Characterisation of the early events in atypical tomato root colonization by a biocontrol agent, Pythium oligandrum. Plant Physiol . Biochem. , 43 : 1 – 11 .
- Gemma , J.N. , Hartmann , G.C. and Wasti , S.S. 1984 . Inhibitory interactions between Ceratocystis ulmi and several species of entomopathogenous fungi . Mycologia , 76 : 256 – 260 .
- Graham , P.H. 1978 . Some problems and potentials of field beans (Phaseolus vulgaris L.) in Latin America . Field Crops Res. , 1 : 295 – 317 .
- Grosch , R. , Scherwinski , K. , Lottmann , J. and Berg , G. 2006 . Fungal antagonists of the plant pathogen Rhizoctonia solani: selection, control efficacy and influence on the indigenous microbial community . Mycol. Res. , 110 : 1464 – 1474 .
- Harman , G.E. , Howell , C.R. , Viterbo , A. , Chet , I. and Lorito , M. 2004 . Trichoderma species – opportunistic, avirulent plant symbionts . Nat. Rev. Microbiol. , 2 : 43 – 56 .
- Hassan Dar , G.H. , Zargar , M.Y. and Beigh , G.M. 1997 . Biocontrol of Fusarium root rot in the common bean (Phaseolus vulgaris L.) by using symbiotic Glomus mosseae and Rhizobium leguminosarum . Microb. Ecol. , 34 : 74 – 80 .
- InáCio , M.L. , Silva , G.H. , Teles , H.L. , Trevisan , H.C. , Cavalheiro , A.J. , Bolzani , V.S. , Young , M.C.M. , Pfenning , L.H. and Araujo , A.R. 2006 . Antifungal metabolites from Colletotrichum gloeosporioides, an endophytic fungus in Cryptocarya mandioccana Nees (Lauraceae) . Bioch. Syst. Ecol. , 34 : 822 – 824 .
- Istifadah , N. and Mcgee , P.A. 2006 . Endophytic Chaetomium globosum reduces development of tan spot in wheat caused by Pyrenophora tritici-repentis. Austral . Plant Pathol. , 35 : 411 – 418 .
- Kabaluk , J.T. and Ericsson , J.D. 2007 . Metarhizium anisopliae seed treatment increases yield of field corn when applied for wireworm control . Agron. J. , 99 : 1377 – 1381 .
- Kang , C.S. , Goo , B.Y. , Gyu , L.D. and Heon , K.Y. 1996 . Antifungal activities of Metarhizium anisopliae against Fusarium oxysporum, Botrytis cinerea and Alternaria solani . Kor. J. Mycol. , 24 : 49 – 55 .
- Kim , H.Y. , Choi , G.J. , Lee , H.B. , Lee , S.W. , Lim , H.K. , Jang , K.S. , Son , S.W. , Lee , S.O. , Sung , N.D. and Kim , J.C. 2007 . Some fungal endophytes from vegetable crops and their anti-oomycete activities against tomato late blight . Lett. Appl. Microbiol. , 44 : 332 – 337 .
- Kottke , I. , Guttenberger , M. , Hampp , R. and Oberwinkler , F . 1987 . An in vitro method for establishing mycorrhizae on coniferous tree seedlings . Trees , 1 : 191 – 194 .
- Krasnoff , S.B. , Keresztes , I. , Gillilan , R.E. , Szebenyi , D.M.E. , Donzelli , B.G.G. , Churchill , A.C.L. and Gibson , D.M . 2007 . Serinocyclins A and B, cyclic heptapeptides from Metarhizium anisopliae . J. Nat. Prod. , 70 : 1919 – 1924 .
- Liu , C.H. , Zou , W.X. , Lu , H. and Tan , R.X. 2001 . Antifungal activity of Artemisia annua endophyte cultures against phytopathogenic fungi . J. Biotech. , 88 : 277 – 282 .
- Miche , L. and Balandreau , J. 2001 . Effects of rice seed sterilization with hypochlorite on inoculated Burkholderia vietnamiensis . Appl. Environ. Microbiol. , 67 : 3046 – 3952 .
- Mishra , V.K. 2010 . In vitro antagonism of Trichoderma species against . Pythium aphanidermatum. J. Phytol. , 2 : 28 – 35 .
- Ownley , B.H. , Griffin , M.R. , Klingeman , W.E. , Gwinn , K.D. , Moulton , J.K. and Pereira , R.M. 2008 . Beauveria bassiana: endophytic colonization and plant disease control . J. Invert. Pathol. , 3 : 267 – 270 .
- Ownley , B.H. , Gwinn , K.D. and Vega , F.E. 2010 . Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution . BioControl , 55 : 113 – 128 .
- Qi , Y-X. , Chen , F-X. and Li , Z-Z. 2010 . Inhibitory mechanisms of Metarhizium anisopliae against the pathogens of Fusarium wilt of cotton . Cotton Sci. , 2 : 591 – 596 .
- Roberts , D. and St. Leger , R.J. 2004 . “ Metarhizium spp., cosmopolitan insect-pathogenic fungi: mycological aspects ” . In Advances in Applied Microbiology , Edited by: Laskin , A.I. , Bennet , J.W. and Gadd , G.M. 1 – 70 . Amsterdam: Elsevier Academic Press .
- Rojo , F.G. , Reynoso , M.M. , Ferez , M. , Chulze , S.N. and Torres , A.M. 2006 . Biological control by Trichoderma species of Fusarium solani causing peanut brown root rot under field conditions . Crop Prot. , 26 : 549 – 555 .
- Sasan , R.K. and Bidochka , M.J. 2012 . The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development . Am. J. Bot. , 99 : 101 – 107 .
- Schneider , K.A. and Kelly , J.D. 2000 . A greenhouse screening protocol for Fusarium root rot in bean . HortScience , 35 : 1095 – 1098 .
- Sobowale , A.A. , Odebode , A.C. , Cardwell , K.F. , Bandyopadhyay , R. and Jonathan , S.G. 2010 . Antagonistic potential of Trichoderma longibrachiatum and T. hamatum resident on maize (Zea mays) plant against Fusarium verticillioides (Nirenberg) isolated from rotting maize stem . Arch. Phytopathol. Plant Prot. , 43 : 744 – 753 .
- Steadman , J.R. , Kerr , E.D. and Mumm , R.F. 1975 . Root rot of bean in Nebraska: primary pathogen and yield loss appraisal . Plant Dis. Rep. , 59 : 305 – 308 .
- Tan , C.S. and Tu , J.C. 1995 . Tillage effect on root rot severity, growth and yield of beans . Can J. Plant Sci. , 75 : 183 – 186 .