Abstract
Monilinia fructicola, the causal agent of brown rot, was recovered from decayed stone fruits (peach, plum and nectarine) in 11 West Virginia orchards. There was significant variation among these isolates with respect to colony morphology, growth rate, sporulation level, sensitivity to fenbuconazole, vegetative compatibility and virulence. Species identification was confirmed using ITS sequences from the nuclear ribosomal RNA gene. The cultural phenotypes on potato dextrose agar (PDA) ranged from white to dark, melanized colonies. The growth rate of the isolates on PDA ranged from 0.3 to 3.2 mm day−1 at 4 °C, from 2.9 to 7.6 mm day−1 at 10 °C, and from 4.8 to 19 mm day−1 at 24 °C. There was a statistically significant relationship between the growth of the isolates on PDA and their aggressiveness on nectarines at 24 °C, especially at higher inoculum concentrations. Sporulation of 3-day-old cultures on peach agar at 24 °C varied from profuse to no sporulation, with some isolates sporulating only sparsely after 10 days. The EC50 for fenbuconazole ranged from 0.003 to 0.129 μg μL−1 and for two reference isolates was 0.020 and 0.016 μg μL−1. Only a few vegetative compatibility groups were identified among isolates within orchards, reflecting the lack of sexual recombination in this region.
Résumé
Monilinia fructicola, l'agent causal de la pourriture brune, a été collecté sur des drupes pourries (pêches, prunes et nectarines) dans 11 vergers de la Virginie-Occidentale. Il y avait des variations notables parmi les isolats quant à la morphologie de la colonie, au taux de croissance, au degré de sporulation, à la sensibilité au fenbunoconazole, à la compatibilité végétative et à la virulence. Les espèces ont été identifiées en se basant sur les séquences ITS de l'ARN ribosomique du gène nucléaire. Les phénotypes culturaux qui se sont développés sur la gélose dextrosée à la pomme de terre ont formé des colonies mélanisée variant de blanc à foncé. Le taux de croissance des isolats se développant sur la gélose variait de 0,3 à 3,2 mm/jour à 4 °C, de 2,9 à 7,6 mm/jour à 10 °C et de 4,8 à 19 mm/jour à 24 °C. Il y avait une relation statistiquement significative entre la croissance des isolats sur la gélose et leur virulence à l’égard des nectarines à 24 °C, particulièrement à de très fortes concentrations d'inoculum. La sporulation de cultures vieilles de 3 jours sur de la dextrose à la pêche à 24 °C variait d'abondante à nulle, avec quelques isolats sporulant très légèrement au bout de 10 jours. La CE50 pour le fenbunoconazole variait de 0,003 à 0,129 μg/μl et, pour deux isolats de référence, elle variait de 0,020 à 0,016 μg/μl. Seulement quelques groupes de compatibilité végétative ont été identifiés parmi les isolats provenant des vergers, soulignant l'absence de recombinaison sexuée dans cette région.
Introduction
Brown rot caused by Monilinia fructicola (Wint.) Honey is the most destructive pre- and postharvest disease in most stone fruit-growing regions of the United States, including eastern West Virginia. Next to frost damage, which may totally destroy a stone fruit crop in some years, brown rot management is the most challenging aspect of stone fruit production for growers in West Virginia. A warm and humid environment during the summer months is conducive to infection of both immature and ripe fruit. Infection of immature fruit may result in latent infections, which may not be apparent during harvest but can cause decay as the fruit matures during storage.
On peach, nectarine, plum and cherry fruit, latent infections can resume invasion and colonization as fruits approach maturity (Northover & Cerkauskas, Citation1994; Emery et al., Citation2000; Förster & Adaskaveg, Citation2000; Lee & Bostock, Citation2006). Fungicides have been used to manage brown rot in the field and after harvest. This often requires spraying trees at regular intervals from flowering to harvest, and treating fruit after harvest. Sterol demethylation inhibitor (DMI) fungicides have been widely used to control brown rot in the orchard for over two decades. However, the control levels are not always satisfactory and result in fungicide failures (Schnabel et al., Citation2004). Postharvest application of biocontrol agents may reduce brown rot of stone fruits originating from wound infections, but not from latent infections (Pusey & Wilson, Citation1984; Lee & Bostock, Citation2006). Results from pilot studies with a biocontrol agent Bacillus subtillis (B3) were promising but variable (Pusey et al., Citation1988) and none of the biocontrol agents developed against brown rot of stone fruits are available commercially (Pusey & Wilson, Citation1984; Smilanick et al., Citation1993; Spotts et al., Citation2002; Schnabel & Mercier, Citation2006).
The main objectives of this study were to: (i) identify Monilinia sp. isolates from decayed fruit in eastern West Virginia orchards to species level based on sequences of the amplified ITS region developed to differentiate Monilinia laxa (Aderh. & Ruhland) Honey, Monilinia fructigena Honey and M. fructicola (Van Brouwershaven et al., Citation2010); and (ii) characterize isolates with regard to colony morphology, amount of sporulation, growth and aggressiveness in causing fruit decay at different temperatures, sensitivity to fenbuconazole, and vegetative compatibility.
Materials and methods
Sample collection, isolation and purification of fungal isolates
Isolates of Monilinia species were obtained from decayed stone fruits (peaches, nectarines and plums) from 11 orchards in Jefferson, Berkeley and Hampshire counties of eastern West Virginia. Decayed fruits with apparent brown rot symptoms were placed inside glass jars lined with water-soaked paper towels and covered with Parafilm to retain moisture, and incubated at 24 °C. After several days, when new hyphae and conidia began to appear on the fruit surface, the conidia and mycelia were removed with forceps, placed onto acidified potato dextrose agar (APDA) and covered with sterile coverslips that were pressed gently into the medium to force growth of the fungal hyphae through the agar. Petri dishes were incubated at 24 °C until mycelium grew out from underneath the cover slip and hyphal tips were then transferred to PDA.
Isolates that produced conidia were purified via single conidia isolation while those that did not sporulate well were grown on canned peach halves. Before inoculation, the syrup from the fruit halves was washed off with sterile water, they were placed in jars, and microwaved for 45 s to reduce contamination, and inoculated with mycelial plugs. After the isolates sporulated on the fruit, single conidia were selected and stored on PDA slants. Three cultures, WV-Mf 2, WV-Mf 16 and WV-Mf 38, that did not sporulate readily using this fruit inoculation method, were purified by hyphal tip isolation on PDA medium. For long-term preservation of the isolates, conidia were produced on peach halves as described above, collected with a hand-held vacuum powered cyclone spore collector (Geoff Harms, Physics Laboratory, University of Minnesota, St. Paul, MN), suspended in 20% glycerol with 8% skim milk, and stored at -80 °C. For conducting fungicide sensitivity experiments, the collected conidia were suspended in water and were either used immediately or frozen at −20 °C and used within seven days.
Growth characteristics
Cultures were grown on PDA for two consecutive transfers before a plug (5 mm diameter) was removed from the edge of an actively growing colony and placed in the centre of a 9 cm Petri dish containing PDA. The dishes were sealed with Parafilm and placed in incubators set at 4, 10 or 24 °C. The diameter of the colonies was measured daily until the most rapidly growing isolates reached the edge of the dish for dishes incubated at 10 °C and 24 °C, and up to 16 days for those incubated at 4 °C. Then, based on the final colony diameter, the growth rate in mm day−1 was calculated. There was one dish per each of 38 isolates for each temperature and the experiment was conducted three times. The results are presented as the average of the three experiments.
To determine cultural and morphological characteristics, the isolates were grown on PDA and on peach agar (PA; 900 g of blended peach halves from heavy syrup, 20 g of Bacto Agar, 1 L water) at 24 °C in 10 h light and 14 h dark cycles, and the Parafilm seal around the dishes was perforated to increase gas exchange. Cultures were visually examined for intensity of sporulation, morphology and pigmentation of both the top and reverse sides of the dish. A ‘typical’ M. fructicola culture was described as having a grey colony colour, showing concentric rings, and smooth non-lobed colony margins, generally with a growth rate more than 9 mm day−1 (Lane, Citation2002).
Sensitivity to fenbuconazole
Fifty percent effective concentration (EC50) values for mycelial growth were determined using the modified spiral gradient dilution method described by Förster et al. (Citation2004). Briefly, 50 mL of PDA was poured into 15-cm Petri dishes and allowed to harden overnight. Before the dishes were used, they were examined for the presence of free moisture on the agar surface and only those without free moisture were used. Fifty μL of fungicide stock solution was applied to each dish using an automated spiral plater (Autoplate 4000, Spiral Biotech, Bethesda, MD) set in the exponential mode. After the fungicide was absorbed by the agar (˜ 20 to 30 min), an agar disk 23 mm in diameter was removed from the centre of the dish with a sterile cork borer, where no fungicide was deposited, to avoid diffusion of the fungicide to that area (). The dishes were incubated for 3–4 h, to establish a fungicide gradient throughout the agar, before they were inoculated with the fungus.
Fig. 1. Inhibition of growth of Monilinia fructicola isolates on PDA amended with fenbuconazole deposited by spiral gradient dilution (left) and a control dish without fungicide (right). The concentration of the fungicide decreases toward the edge of the dish (see text for details).
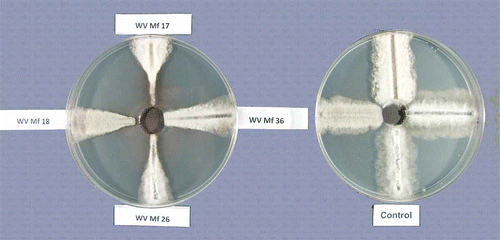
The fenbuconazole fungicide stock solutions were prepared by dissolving 2.4 mg of Indar 75 WSP (Dow AgroSciences LLC, Indianapolis, IN) in 100 μL of acetone and then diluting to 50 mL with water (final concentration 36 mg a.i. L−1), the ‘balanced’ concentration was calculated on the basis of the molecular weight by the Spiral Gradient Endpoint (SGE) program (Spiral Biotech). The final concentration of fenbuconazole in the PDA after deposition with the spiral plater ranged from 0.001 to 0.279 mg L−1. The amount of acetone used was determined using the Pesticide Property Database (PPDB) (Anonymous, 2011). Control dishes were set up with no fungicide to determine the effect of the acetone carrier on the growth of M. fructicola.
Autoclaved cellophane strips, 3–4 mm wide and 55 mm long, were placed on PDA media in 10-cm Petri dishes and 75 μL of a conidial suspension (2 × 105 to 5 × 106 conidia mL−1) of the M. fructicola isolates was evenly spread over each strip. The dishes were incubated for 48 h at 24 °C before they were used in the fungicide assay. For isolates that did not sporulate (WV-Mf 2, WV-Mf 16, WV-Mf 38), strips of PDA with the actively growing culture were placed next to the cellophane strips and incubated for 72 h to allow strips to be colonized. The strips were transferred onto fungicide-amended 15-cm dishes and placed radially from the centre to the edge of the dish, following the fungicide gradient (). Four strips were placed on each dish, each inoculated with a different isolate. After 72 h of incubation at 24 °C, the width of the fungal growth was measured at its widest point on control dishes (growth width was even throughout the strips for all isolates) and half of that measurement was marked for isolates grown on dishes with fungicides. The distance from the centre of the dish to that mark was measured and recorded in the SGE program as ER (ending radius). The concentration of the fungicide at this location was automatically calculated by the program. Isolates previously unexposed to fenbuconazole (deposited to ATCC before registration of the fungicide), ATCC 16103 and ATCC 46606, were used as reference isolates. The experiment was conducted three times. At each time, strips of different isolates placed on individual dishes were selected randomly. The data from the experiments were pooled to calculate the standard error of the means (). The reference isolates were analysed for the presence of the ‘Mona’ element upstream from the MfCYP51 gene using the procedures described by Luo et al. (Citation2008), except for minor changes in the extraction of DNA, as described below. This was done to confirm the presence or absence of the ‘Mona’ element, that was found in M. fructicola isolates resistant to fenbuconazole (Luo et al., Citation2008), in our reference isolates that were not exposed to this fungicide.
Genetic identification
The identity of the isolates was determined using sequences of the ITS region of the nuclear ribosomal RNA gene repeat (Van Brouwershaven et al., Citation2010). We assumed that the isolates were M. fructicola and therefore used M. laxa (three isolates obtained from Dr T. Michailides, University of California, Davis, CA) as control strains. For DNA extraction, a 125 mL flask containing 50 mL of potato dextrose broth was inoculated with five plugs (5 mm in diameter) of fungal mycelium from actively growing PDA cultures. Liquid cultures were grown at 25 °C for seven days with shaking at 150 rpm in an incubator. The liquid cultures were transferred to 50 mL plastic conical tubes and mycelium was harvested by centrifugation at 10000 g for 30 min. The supernatant was discarded and the mycelium was flash-frozen in liquid nitrogen. Approximately 100 mg of frozen mycelium was then used to extract genomic DNA using the Wizard Genomic DNA Extraction Kit (Invitrogen, Carlsbad, CA). 100 ng was used for PCR amplification. The set of ITS primers Mon139-F (CACCCT GTGTATYATTACTTTGTTGCTT) and Mon139-R (CAAGAGATCCGTTGTTGAAAGTTTTAA) were used to obtain amplicons for sequence analysis (Van Brouwershaven et al., Citation2010 ).
Cycling parameters for DNA amplification were: 1 min at 95 °C, followed by 95 °C for 1 min, 60 °C for 1 min and 72 °C for 1 min which was cycled 30 times, and a final extension at 72 °C for 10 min. Amplicons were purified using the Wizard PCR Clean-up (Invitrogen). PCR products were then sequenced (MacrogenUSA, Rockville, MD). Forward and reverse sequences were aligned in the CodonCode Aligner (CodonCode Corp., Dedham, MA), evaluated for the quality of the sequencing reactions, and trimmed appropriately. The 21 nucleotide sequences corresponding to the species-specific probe for M. fructicola, were annotated and compared with those reported in the literature for M. fructicola, M. laxa and M. fructigena (Aderhold and Ruhland) Honey (Van Brouwershaven et al., Citation2010). In addition, the entire ITS amplicon sequences were subjected to a BLAST search to further affirm their identity (Altschul et al., Citation1990).
Vegetative compatibility grouping
Mycelial interaction zones were studied among monoconidial isolates from individual orchards by pairing isolates on oatmeal agar (OMA) dishes. This medium was selected for its superiority in forming barrage reactions between unlike isolates of M. fructicola (Sonoda et al., Citation1982). Up to six different isolates from an individual orchard were paired on a single OMA dish. Also, for each monoconidial isolate, subcultures of two single-conidial clones were made and paired as control treatments. The interaction between these clones served as the reference for the comparison of interactions with the other isolates.
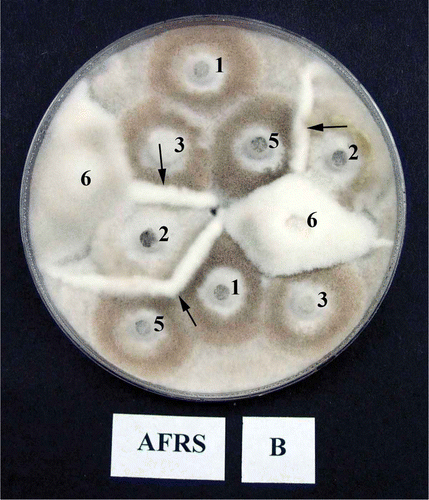
For studying interactions among isolates, mycelial discs (5 mm in diameter) taken from the edge of actively growing colonies on PDA were transferred to OMA. The mycelia plugs were arranged to maximize the number of interactions in the plate (). The dishes were incubated for up to 10 days at 22 ± 2 °C, and during that time they were observed for the formation of barrage zones between isolates.
Fig. 3. An example of vegetative compatibility groups (VCG) of Monilinia fructicola isolates. Numbers correspond to the isolate number e.g. 1 = WV-Mf 1 and designation B following the location (AFRS) corresponds to one of the two (A and B) single-spore clones of the same isolates, and only interactions between B clones are presented. Note the strong barrage formation between isolates (arrows). There was no interaction between clones A and B of the isolates in control treatments.
Aggressiveness of isolates
Investigation of isolate aggressiveness was carried out on nectarine fruit. Ripe fruit were harvested from the Appalachian Fruit Research Station (AFRS) orchard, washed, allowed to dry, and placed on fruit pack trays in plastic boxes. Fruit were wounded by removing a cylinder of tissue (3 mm in diameter and 4 mm deep) mid-way between the blossom and stem end. The wounds were inoculated with 25 μL of an aqueous conidial suspension of the M. fructicola isolates at 103, 104 and 105 conidia mL−1. These concentrations were selected because they produced a broad range of decays and very rapid development of decay at the highest concentration on ripe fruit. The inoculum was produced by inoculating peach halves as described earlier. Only five isolates did not produce conidia and in those cases the mycelium produced on the peach halves was used to inoculate the nectarine wounds. There were two sets of five fruit for each inoculum concentration of the pathogen. One set was incubated at 24 °C and the other at 4 °C. The diameter of brown rot decay was measured after 2 and 15 days for fruit incubated at 24 °C and 4 °C, respectively, and the experiment was conducted twice.
Statistical analysis
All in vitro and in situ studies were set up in a completely randomized design. The regression of the isolate growth per day on PDA against lesion diameter on nectarines, the standard error of the means for isolate aggressiveness on fruit, and for EC50 for fenbuconazole were calculated using SigmaPlot 12 software (Systat Software Inc., San Jose, CA).
Results
Cultural characteristics
Most of the decayed fruit samples collected in the various orchards produced conidia after incubation in jars with moisture. The isolates of M. fructicola varied greatly with respect to culture appearance, ability to sporulate, growth habit and sensitivity to fenbuconazole. One isolate, WV-Mf 7, sporulated profusely on peach agar (PA) medium after only 3 days, and two other isolates, WV-Mf 1 and WV-Mf 4, also sporulated well during that time (). Most of the isolates sporulated after 10 days on PA, some produced only mycelia and sporulated later at the edge of ageing cultures, and several did not sporulate at all. With the exception of WV-Mf 2 and WV-Mf 38, all cultures sporulated on peach halves after an extended incubation of 14 days. The colony appearance on PDA varied from irregular, undulate with concentric layers or distinctly imbricate to filamentous and almost powdery-like. The colour also varied from white on the top and reverse of the colony to grey or dark grey brownish, especially on sporulating cultures, to very dark or almost black on the top and on the reverse of the plate, especially on some older cultures. A few isolates stained the medium a rusty brown colour (Supplement ). Isolates cultured on peach halves also differed greatly in morphology and after four days of growth they produced typical grey brown profusely sporulating colonies, or non-sporulating cultures with abundant aerial white mycelium, or greyish mycelium with some sporulation, and profusely sporulating cultures with greyish-brown mycelium producing a strong brown discolouration of the fruit tissue (Supplement ).
Table 1. List of Monilinia fructicola isolates used in this study and visual appearance of the colonies on agar media
Table 2. Growth rate per day (mm) on PDA medium of Monilinia fructicola isolates at three temperatures
Fungal growth at various temperatures
The ability to grow at 4 °C on PDA varied greatly and ranged from 0.3 (isolate WV-Mf 44) to 3.0 mm (isolate WV-Mf 34) per day (). At 10 °C, the growth ranged from 1.8 (isolate WV-Mf 30) to 7.1 mm (isolate WV-Mf 26) per day. Growth at 24 °C ranged from 4.8 (isolate WV-Mf 32) to 19.0 mm (isolate WV-Mf 38) per day. The WV-Mf 44 isolate was the slowest growing (0.3 mm/day) at 4 °C and one of the slowest (6.8 mm/day) at 24 °C, while another slow-growing isolate, WV-Mf 38, at 4 °C (0.4 mm/day), was the fastest-growing isolate at 24 °C.
Sensitivity to fenbuconazole
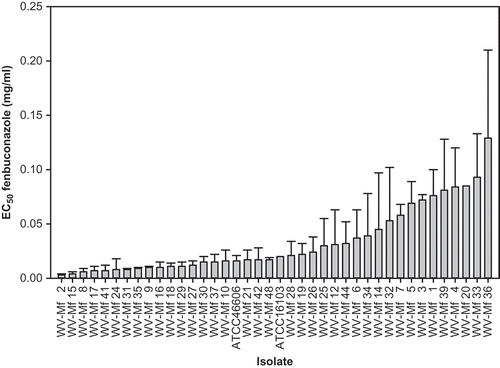
The EC50 of fenbuconazole among isolates ranged from 0.003 to 0.129 μg mL−1 and for the two reference isolates, ATCC 16103 and ATCC 46606, it was 0.020 and 0.016 μg μL−1, respectively (). The spiral-plate method used in this study confirmed its usefulness in determining EC50 values, as first described by Förster et al. (Citation2004). Also, this method provided additional confidence to the relative resistance among isolates when tested on the same plate (four per plate in our study). Sequences of the PCR products for the ‘Mona’ element generated from reference isolates yielded a 311 bp fragment (Luo et al., Citation2008), which did not contain the restriction site for BsrBI (CCG↓CTC) that indicates a phenotype resistant to fenbuconazole.
Vegetative compatibility groups
In the VCG tests, some isolate pairings produced distinct barrage zones, indicating the presence of different VCG groups in individual orchards (). However, in some orchards, especially those with a fewer number of isolates tested, only a single VCG was observed. There were only two VCGs among six isolates from the orchard at AFRS, one VCG between two isolates at CK, two VCG among three isolates at LB, one VCG between two isolates at BM, three VCG among seven isolates at FE, two VCG among six isolates at MA, one VCG among five isolates at RU, and two VCG among three isolates at the SL orchard.
Molecular genetic identification
The 21 nucleotide sequences in all our isolates matched perfectly with the same area used for developing probes specific for M. fructicola (Van Brouwershaven et al., Citation2010). The sequences from the three M. laxa isolates obtained from California matched perfectly with the sequence used for developing a probe specific for M. laxa. In addition, a BLAST search of the ITS amplicons revealed that all isolates from West Virginia were M. fructicola, while the three isolates from California were M. laxa. The e values were close to zero, indicating a very low probability of finding the same sequence by random chance in GenBank.
Aggressiveness of the isolates
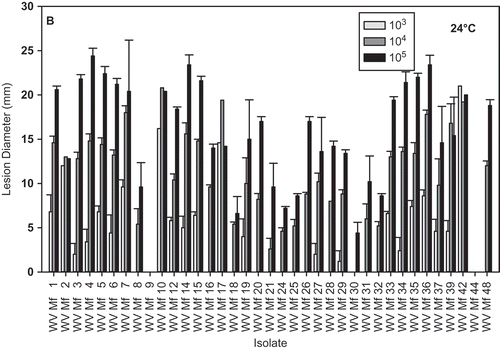
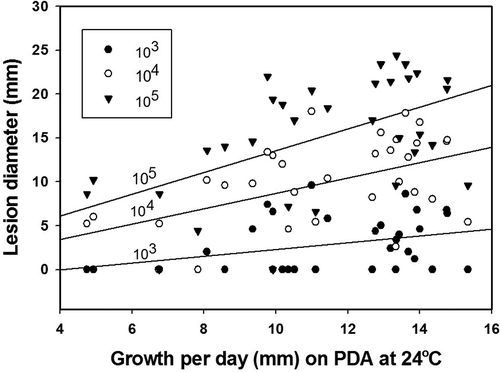
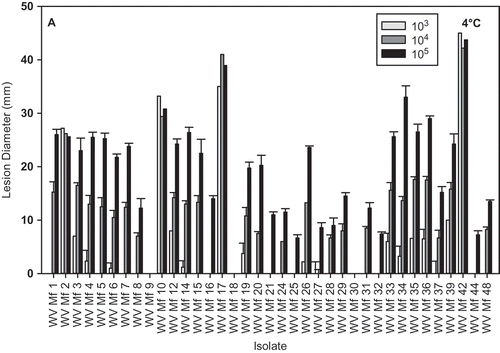
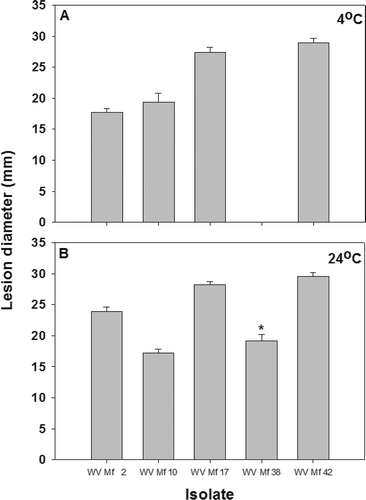
Isolate aggressiveness varied greatly at both 24 °C and 4 °C; however, the isolate distribution with regard to growth rate, in some cases, was similar for both temperatures (, ). All the isolates eventually, after prolonged incubation, caused fruit decay (data not shown). Inoculation of nectarines with mycelia resulted in the development of lesions that were comparable to lesions on fruit inoculated with the highest concentration of conidia, in four out of the five isolates tested ( A, B). No lesions developed on fruit inoculated with isolate WV-Mf 38 at either temperature during the duration of the experiment; however, small lesions developed after three days of additional incubation at 24 °C. The regression of the isolate growth per day on PDA against lesion diameter on nectarines was statistically significant for 24 °C () but not for 4 °C. The coefficient of determination increased as the concentration of the pathogen inoculum used for fruit inoculation increased, and was r2 = 0.1042 (P = 0.0376), r2 = 0.2484 (P = 0.0032), and r2 = 0.2585 (P = 0.0015) for fruit inoculated with 103, 104 and 105 conidia mL−1, respectively.
Fig. 5. Brown rot lesion development on wound-inoculated nectarine fruit with mycelium of different Monilinia fructicola isolates incubated at: A, 4 °C for 17 days; and B, 24 °C for 2 days. * Lesions developed on nectarines inoculated with isolate WV-Mf 38 only after additional incubation for three days.
Discussion
This is the first study to characterize Monilinia isolates from decayed stone fruits in West Virginia and to conclusively show that all isolates were M. fructicola. In amplifying the ITS regions, PCR primers were used that have been developed for species-specific probes to differentiate between M. fructicola, M. laxa and M. fructigena (Van Brouwershaven et al., Citation2010). Our results confirmed the specificity of the sequences used for developing these probes as they differentiated 38 M. fructicola isolates (obtained from decayed stone fruits) from the three control M. laxa isolates (Van Brouwershaven et al., 2010). Monilinia laxa was not found among the isolates obtained during this study. However, it was recently reported for the first time in the eastern United States (Villani & Cox, Citation2010).
The diversity in various characteristics observed among M. fructicola isolates from eastern West Virginia has practical implications. Growth characteristics on PDA, at low and ambient temperatures, in conjunction with aggressiveness on fruit, can be very useful in selecting the appropriate isolates for testing the efficacy of various disease management options, under cold storage and ambient conditions. The range of growth rates of the 38 M. fructicola isolates (4.8 to19.0 mm day−1 at 24 °C) was greater compared with 32 isolates from California, which ranged from 11.0 to 15.6 mm day−1 on PDA at 20 °C (Sonoda & Ogawa, Citation1982), and 14 isolates from various countries, which ranged from 9.4 to 16.6 mm day−1 at 22 °C (Van Leeuwen & van Kesteren, Citation1998). The growth range for isolates in this study was comparable to the 3.83–18.83 mm day−1 range for 35 isolates of M. laxa, and 3.5–23 mm day−1 for 10 isolates of M. fructigena at 18 °C from Spain (De Cal & Melgarejo, Citation1999). However, the average growth per day for M. fructicola was reported to be approximately twice as much as that of M. laxa or M. fructigena (Van Leeuwen & van Kesteren, Citation1998; Anonymous, Citation2009).
Isolate WV-Mf 34 which grew most vigorously at 4 °C and very well at 24 °C on PDA, was also one of the most aggressive isolates on nectarines at 4 °C and 24 °C. However, it does not sporulate well and this may present problems in the production of conidia for larger-scale tests. Isolate WV-Mf 42 was very aggressive on nectarines at both temperatures and sporulated moderately, and isolates WV-Mf 7, WV-Mf 17 and WV-Mf 35 sporulated profusely and were aggressive at both temperatures. Interestingly, isolate WV-Mf 4, which grew poorly on PDA at 4 °C, was among the most aggressive on nectarines at 4 °C. On the other hand, isolate WV-Mf 8, which grew relatively well at 4 °C and at 24 °C, was weakly aggressive on nectarines at both temperatures. It was observed that most of the profusely sporulating isolates were also very aggressive in causing fruit decay. However, some of the isolates that originally produced spores early in the development of the culture, produced fewer spores when cultured from conidial stocks on peach halves, and sporulation was greatly delayed. Nevertheless, when nectarines were inoculated with the mycelium from these isolates, they were very aggressive at both low and ambient temperatures. The progressively increasing significance of the regression of the growth rate on PDA at 24 °C against aggressiveness in causing decay on fruit as concentrations of the pathogen inoculum increased may simply reflect a greater ability of the pathogen to mobilize virulence factors at the higher inoculum levels and the optimum temperature for the pathogen growth to overcome resistance of the fruit, which did not occur at 4 °C.
The fenbuconazole sensitivity tests revealed EC50 values ranging from 0.003 to 0.129 μg mL−1. One isolate was completely resistant and it was confirmed to be M. fructicola by genetic analysis, but was very weakly pathogenic on nectarine fruit despite rapid growth rate at 24 °C on PDA. The EC50 value for the most susceptible isolate was identical to that reported for baseline isolates in New York (Wilcox & Burr, Citation1994), and was lower than for isolate DL25W (0.020 μg mL−1) and the average of 33 isolates previously unexposed to fenbuconazole (0.032 μg mL−1) from South Carolina (Dai et al., Citation2003; Schnabel & Dai, Citation2004), and those obtained for the reference isolates, ATCC16103 (0.020 μg mL−1) and ATCC46606 (0.016 μg mL−1), in this study. The reference values in our study are also similar to that obtained for unexposed isolate SCDL71.01 (0.019 μg mL−1) from South Carolina (Holb & Schnabel, Citation2007). Although the reference isolates used in this investigation were originally isolated in New York and Georgia (before fenbuconazole was registered), and should adequately represent baseline sensitivity, our findings are similar to work by Spiegel & Stammler (Citation2006), who found no significant difference among origin of isolates (country, host plant) in determining minimum inhibitory concentrations for Monilinia spp. to pyraclostrobin and boscalid. Studies by Schnabel et al. (Citation2004) and Zehr et al. (Citation1999) showed a range of sensitivity of M. fructicola isolates to propiconazole. The isolates that were exposed to propiconazole were less sensitive than the ones that were not exposed. Therefore, the varying levels of sensitivity observed for our isolates may have resulted from different levels of exposure to fenbuconazole in the field.
Vegetative compatibility grouping (VCG) can be helpful in describing the epidemiology of brown rot (Sonoda et al., Citation1982, Citation1990). Among the 38 isolates investigated, at least five morphotypes can be easily distinguished, which, in addition to the ‘typical’ isolates, included white isolates, those causing a rusty discolouration in the agar medium and on fruit tissue, and a few with a fluffy appearance of the mycelium. This diversity of morphotypes may suggest some level of genetic diversity; however, the low number of vegetative compatibility groups seems to indicate otherwise. However, this is not unexpected as the gene(s) controlling morphological variations may be distinct from those governing VCGs (Coppin et al., Citation1997). A high number of morphotypes may result from the heterokaryotic nature of the fungus. For example, Hoffmann (Citation1972, Citation1974) observed six different major morphotypes and a few subtypes (in two morphotypes) out of 132 isolates of M. fructigena tested, and six morphotypes among 26 isolates of M. laxa. In addition, conidial isolations carried out for several generations resulted in the appearance of new morphotypes, further implicating heterokaryon-based variation. Despite an extensive search for the sexual stage in the AFRS orchard in the spring of 2010, 2011 and 2012, we failed to uncover a single apothecium; and no apothecia have been detected in West Virginia from 1990 to the present (authors, unpublished observations). This was despite an environment conducive to apothecia formation and production of numerous sclerotized fruit on the ground. In 2010, the spring followed a winter with high snowfall, which provided ample moisture throughout the winter, and 2011 was also a wet year with a total rainfall of more than 250 mm by the time full bloom of peach and nectarine trees occurred. In West Virginia and neighbouring states, apothecia have been observed only once and that was in 1993 in eastern Maryland (Biggs, unpublished results).
Batra (Citation1991) indicated that not all stages of M. fructicola are present at every locality, and that conidia developed on overwintering mummies on trees could serve as the primary inoculum source in the spring. The low level of diversity in VCGs coupled with the lack of apothecial production in the field may indicate that the fungus is primarily undergoing asexual propagation in West Virginia. This is in stark contrast to the development of M. fructicola in California, where both ascospores and conidia serve as the primary sources of inoculum during flowering of stone fruit (Michailides et al., 2007). Thus, it is not surprising that many VCGs were found also among ascospore progenies in sexually reproducing populations of M. fructicola in the San Joaquin Valley of California (Free et al., Citation1996), and as many as 18 VCGs were found among 19 isolates from decayed fruits on a single tree in California (Sonoda et al., Citation1982). In contrast, we found only a few VCGs in eastern West Virginia as only two VCGs were found among six isolates, each isolated from a different tree in the AFRS orchard.
Results from this study can be used in the selection of the most appropriate isolates for evaluation of effectiveness of various treatments for controlling brown rot. For instance, we demonstrated the importance of using aggressive isolates in determining the true efficacy of biocontrol agents against brown rot in our preliminary work (http://www.ars.usda.gov/pandp/docs.htm?docid=17505).
Supplemental Material
Download Zip (1.3 MB)Acknowledgements
We thank Roger Lewis for technical assistance in isolation of fungi from fruit and initial observation of the cultures, Shane Yarrington for assisting with the single-spore isolations and fungicides tests, Jerry Matthews for assistance with VCG work, and Verneta Gaskins for extraction and preparation of DNA for sequencing.
References
- Altschul , S.F. , Gish , W. , Miller , W. , Myers , E.W. and Lipman , D.J. 1990 . Basic local alignment search tool . J. Mol. Biol. , 215 : 403 – 410 .
- Anonymous . 2009 . OEPP/EPPO . Monilinia fructicola. OEPP/EPPO Bulletin , 39 : 337 – 343 .
- Anonymous (2011). PPDB Fenbuconazole (Ref: RH 7592) Environmental Fate – Exotoxicology – Human Health – A to Z Index - Home. Available at http://sitem.herts.ac.uk/aeru/footprint/en/Reports/293.htm (http://sitem.herts.ac.uk/aeru/footprint/en/Reports/293.htm)
- Batra , L.R. 1991 . World Species of Monilinia (Fungi): their Ecology, Biosystematics and Control , 246 Berlin : Cramer . pages
- Dai , Q. , Sun , Z. and Schnabel , G. 2003 . Development of spontaneous hygromycin B resistance in Monilinia fructicola and its impact on growth rate, morphology, susceptibility to demethylation inhibitor fungicides, and sporulation . Phytopathology , 93 : 1354 – 1359 .
- De Cal , A. and Melgarejo , P. 1999 . Effects of long-wave UV light on Monilinia growth and identification of species . Plant Dis. , 83 : 62 – 65 .
- Coppin , E. , Debuchy , R. , Arnaise , S. and Pickard , M. 1997 . Mating types and sexual development in filamentous ascomycetes . Microbiol. Mol. Biol. Rev. , 61 : 411 – 428 .
- Emery , K.M. , Michailides , T.J. and Scherm , H. 2000 . Incidence of latent infection of immature peach fruit by Monilinia fructicola and relationship to brown rot in Georgia . Plant Dis. , 84 : 853 – 857 .
- Förster , H. and Adaskaveg , J.E. 2000 . Early brown rot infections in sweet cherry fruit are detected by Monilinia-specific DNA primers . Phytopathology , 90 : 171 – 178 .
- Förster , H , Kanetis , L. and Adaskaveg , J.E. 2004 . Spiral gradient dilution, a rapid method for determining growth responses and 50% effective concentration values in fungus–fungicide interactions . Phytopathology , 94 : 163 – 170 .
- Free , S.J. , Holz , B.A. and Michailides , T.J. 1996 . Mating behavior in field populations of Monilinia fructicola . Mycologia , 88 : 208 – 211 .
- Hoffman , G.M . 1972 . Heterokaryose bei Wildstaemmen von Monilinia fructigena . Phytopathol. Z. , 73 : 326 – 340 .
- Hoffman , G.M. 1974 . Zum Vorkommen von Heterokaryose bei Monilinia laxa. . Phytopathol. Z , 79 : 193 – 202 .
- Holb , I.J. and Schnabel , G. 2007 . Differential effect of triazoles on mycelia growth and disease measurements of Monilinia fructicola isolates with reduced sensitivity to DMI fungicides . Crop Prot. , 26 : 753 – 759 .
- Lane , C. R. 2002 . A synoptic key for differentiation of Monilinia fructicola, M. fructigena and M. laxa, based on examination of cultural characteristics . Bulletin OEPP/EPPO Bulletin , 32 : 489 – 493 .
- Lee , M-H. and Bostock , R.M. 2006 . Induction, regulation and role in pathogenesis of appressoria in Monilinia fructicola . Phytopathology , 96 : 1072 – 1080 .
- Luo , C.-X. , Cox , K.D. , Amiri , A. and Schnabel , G. 2008 . Occurrence and detection of the DMI resistance-associated genetic element ‘Mona’ in Monilinia fructicola . Plant Dis. , 92 : 1099 – 1103 .
- Michailides, T.J., Luo, Y., MA, Z., & Morgan, D.P. (2007). Brown rot of dried plum in California: new insights on an old disease. Available at http://www.apsnet.org/online/feature/prune/ (http://www.apsnet.org/online/feature/prune/)
- Northover , J. and Cerkauskas , R.F. 1994 . Detection and significance of symptomless latent infections of Monilinia fructicola in plums . Can. J. Plant Pathol. , 16 : 30 – 36 .
- Pusey , L.L. and Wilson , C.L. 1984 . Postharvest biological control of stone fruit brown rot by Bacillus subtilis . Plant Dis. , 68 : 753 – 756 .
- Pusey , P.L. , Hotchkiss , M.W. , Dulmage , H.T. , Baumgardner , R.A. , Zehr , E.I. , Reilly , C.C. and Wilson , C.L. 1988 . Pilot test for commercial production and application of Bacillus subtilis (B-3) for postharvest control of peach brown rot . Plant Dis. , 72 : 622 – 626 .
- Schnabel , G. and Dai , Q. 2004 . Heterologous expression of the P450 sterol 14a-demethylase gene from Monilinia fructicola reduces sensitivity to some but not all DMI fungicides . Pesticide Biochem. Physiol. , 78 : 31 – 38 .
- Schnabel , G. and Mercier , J. 2006 . Use of a Muscodor albus pad delivery system for the management of brown rot of peach in shipping cartons . Postharvest Biol. Technol. , 42 : 121 – 123 .
- Schnabel , G. , Bryson , P.K. , Bridges , W.C. and Brannen , P.M. 2004 . Reduced sensitivity in Monilinia fructicola to propiconazole in Georgia and implications for disease management . Plant Dis. , 88 : 1000 – 1004 .
- Smilanick , J.L. , Denis-Arrue , R. , Bosch , J.R. , Gonzales , A.R. , Henson , D. and Janisiewicz , W.J. 1993 . Control of postharvest brown rot of nectarines and peaches by Pseudomonas species . Crop. Prot. , 12 : 513 – 520 .
- Sonoda , R.M. and Ogawa , J.M. 1982 . Growth rate of Monilinia fructicola resistant and susceptible to benomyl on potato-dextrose agar and on peach fruit . Plant Dis. , 66 : 1155 – 1156 .
- Sonoda , R.M. , Ogawa , J.M. , Esser , T.E. and Manji , B.T. 1982 . Mycelial interaction zones among single ascospore isolates of Monilinia fructicola . Mycologia , 74 : 681 – 683 .
- Sonoda , R.M. , Ogawa , J.M. and Manji , B.T. 1990 . Population structure of Monilinia fructicola in Prunus persica var. nucipersica tree canopies . Florida Agric. Exp. St. J. Series No. , R-00769 : 893 – 895 .
- Spiegel , J. and Stammler , G. 2006 . Baseline sensitivity of Monilinia laxa and M. fructigena to pyraclostrobin and boscalid . J. Plant Dis. Protect. , 113 : 199 – 206 .
- Spotts , R.A. , Cervantes , L.A. and Facteau , T.J. 2002 . Integrated control of brown rot of sweet cherry fruit with a preharvest fungicide, a postharvest yeast, modified atmosphere packaging, and cold storage temperature . Postharvest Biol. Technol. , 24 : 251 – 257 .
- Van Brouwershaven , I.R. , Bruil , M.L. , Van Leeuwen , G.C.M. and Koxet , L.F.F. 2010 . A real-time (TaqMan) PCR assay to differentiate Monilinia fructicola from other brown rot fungi of fruit crops . Plant Pathol. , 59 : 548 – 555 .
- Van Leeuwen , G.C.M. and Van Kesteren , H.A. 1998 . Delineation of the three brown rot fungi of fruit crops (Monilinia spp.) on the basis of quantitative characteristics . Can. J. Bot. , 76 : 2042 – 2050 .
- Villani , S.M. and Cox , K.D. 2010 . Confirmation of European brown rot caused by Monilinia laxa on tart cherry, Prunus cerasus, in western New York . Plant Dis. , 94 : 783
- Wilcox , W.F. and Burr , J.A. 1994 . Base-line sensitivity of Monilinia fructicola to six DMI fungicides . Phytopathology , 84 : 1078
- Zehr , E.I. , Luszcz , L.A. , Olien , W.C. , Newall , W.C. and Toler , J.E. 1999 . Reduced sensitivity in Monilinia fructicola to propiconazole following prolonged exposure in peach orchards . Plant Dis. , 83 : 913 – 916 .