Abstract
Clubroot, caused by Plasmodiophora brassicae, has emerged as a serious disease threatening the cruciferous crop production industry in China. The distribution, occurrence, physiological races and transmission routes of P. brassicae are briefly described here, providing a basis for understanding the serious implications of clubroot disease in China. Strategies for managing clubroot in a Chinese context are also discussed. Approximately 3.2–4.0 million ha of cruciferous crops are infected by P. brassicae every year in China, causing yield losses of 20–30%. The identification of physiological races is of fundamental importance for breeding clubroot-resistant cultivars. Race 4 of the pathogen, as classified on the differentials of Williams, has been spreading all over China and is becoming increasingly damaging to cruciferous crops. Dissemination of P. brassicae is mainly via the movement of resting spores on seed or in infected plant material. Spore dispersal over short distances depends on the movement of infested soil, wind, water and livestock manure. Integrated control strategies include the development of resistant cultivars, soil liming, fungicide applications and use of biological control agents.
Résumé
La hernie, causée par Plasmodiophora brassicae, est apparue comme une grave maladie menaçant la culture des crucifères en Chine. La répartition, l’occurrence, les races physiologiques et les voies de transmissions de P. brassicae sont brièvement décrites dans cet article afin de fournir une base à la compréhension des graves conséquences de la hernie en Chine. Des stratégies visant à y gérer cette maladie sont également discutées. Approximativement, de 3.2 à 4.0 millions d’hectares de crucifères sont infectés annuellement en Chine, causant des pertes de rendement de 20 à 30%. L’identification des races physiologiques est essentielle à la sélection de cultivars résistants à la hernie. La race 4 de l’agent pathogène, selon les séries différentielles de Williams, s’est répandue partout en Chine et cause de plus en plus de dommages chez les crucifères. La dissémination de P. brassicae est principalement causée par le transport des spores de réserve trouvées sur les semences ou les plants infectés. Par ailleurs, sur de courtes distances, les spores sont dispersées par le transport de sol infesté, le vent, l’eau et le le fumier. Des stratégies de lutte intégrée incluent le développement de cultivars résistants, le chaulage des sols, l’application de fongicides et l’utilisation d’agent de lutte biologique.
Introduction
Clubroot in Brassica crops, caused by Plasmodiophora brassicae Woronin, is recognized as a serious soil-borne disease (Buczacki Citation1983) associated with appreciable yield losses (Wallenhammar Citation1996) and it is considered as one of the most economically important diseases of cruciferous crops. The disease is found throughout the world wherever vegetable brassicas and canola (Brassica napus L.) are grown (Linnasalmi & Tovianinen Citation1991; Engquist Citation1994; Wallenhammar Citation1996; Donald & Porter Citation2009) and is becoming important to emerging economies (Dixon Citation2009). Clubroot is known to occur in more than 60 countries and results in a 10–15% reduction in yields on a global scale (Dixon Citation2009).
In China, outbreaks of clubroot in districts growing cruciferous crops have been reported frequently in recent years (Yang et al. Citation1992; Liang et al. Citation2001; Wang et al. Citation2011c; Li et al. Citation2012a; Fan et al. Citation2013). China is a major producer of cruciferous crops, including 6 700 000 ha, 2 532 600 ha, 899 300 ha, 536 900 ha, 1 200 500 ha seeded to canola, Chinese cabbage (B. rapa L. ssp. pekinensis), pak choi (B. rapa L. ssp. chinensis), cabbage (B. oleracea L.) and turnip radish (Raphanus sativus L.), respectively, in 2012 (Chinese Agricultural Yearbook Citation2012). These crops have been infected by P. brassicae since the 1910s, when the disease was discovered in Taiwan and Fujian Provinces sequentially (Huang et al. Citation1955; Zhu Citation1956; Wang Citation1962). Since the 1990s, especially the late 1990s, the disease has appeared in the southwest and northeast of China, transmitted on seeds, in soil, infected plant material, irrigation water and animal manure (Li Citation1990; Tang et al. Citation1990; Chen et al. Citation1997; Li & Pan Citation1999). At present, clubroot is spreading around China, with the most severe disease outbreaks in the southwest, northeast and middle regions of the country. It is estimated that the clubroot pathogen infects 3.2–4.0 million ha of cruciferous crops annually (Wang et al. Citation2011a). The disease occurs in Chinese cabbage, cabbage, pak choi, turnip radish, tuber mustard, stem mustard and even medicinal crops such as Banlangen (Isatis tinctoria L.) (Wang et al. Citation2002; Xiao et al. Citation2002; Xiong et al. Citation2012). In recent years, it has spread into canola in Anhui, Sichuan, Hubei Provinces and also into Chongqing City (Ma et al. Citation2006; Huang et al. Citation2007; Wang et al. Citation2008, Citation2010). In some areas, P. brassicae infests vegetable fields very severely, resulting in the cultivation of cruciferous crops being discontinued.
Given the great economic value of cruciferous crops in China, the increased incidence of clubroot resulted in a large and coordinated research effort focused on improving clubroot management and knowledge. In China, there are now more than 20 public institutions involved in research into the control of clubroot of cruciferous crops. These institutions include the agricultural universities of Yunnan, Sichuan, Huazhong, Hunan, Hubei, Shănxi, China, Shenyang, Anhui and Tibet, comprehensive universities including Southwest, Zhejiang, and Science and Technology of East China, and the agricultural academies of Yunnan, Jiangxi, China (CAAS), Beijing, Shandong, Henan and Liaoning. These research programmes are aimed at: identifying physiological races, screening for resistant germplasm, breeding resistant Chinese cabbage, European cabbage and canola, and developing integrated management strategies, which include the use of chemical, physical, agricultural and biological methodologies (He & Wu Citation2013).
A brief history and the present state of clubroot research in China, including the occurrence, distribution, host range, disease cycle, routes of spread, physiological races and control methods, are summarized in this review.
Occurrence in China
Before China’s Liberation in 1949, little information about clubroot in China was available. There were, however, documented discoveries of clubroot being found in Taiwan in 1912 and 1936, as well as Fujian Province () in 1947 (Wang Citation1962). In 1953, clubroot was recognized as one of the important quarantine targets in the first National Plant Quarantine Conference (Wang Citation1962).
The frequency of reports on clubroot increased substantially in the 1950s–1960s as agricultural activity increased in order to feed the burgeoning populations serving the Socialist Industrialization. Clubroot disease was observed in 1950 in Nanchang City, Jiangxi Province, and five years later, the disease had spread to almost all areas of Jiangxi Province, with the general incidence above 70% (Huang et al. Citation1955). Clubroot prevailed in Zhejiang Province, including Zhuji, Huangyan, Yongjia and Jinhua counties, where the incidence ranged between 55% and 70% (Zhu Citation1956). Clubroot on turnip was first recorded in Jiamusi City in Heilongjiang Province in 1956, and 3 years later, it had spread to most cruciferous vegetable fields, with an incidence of about 10–42% (Wang Citation1962). Clubroot disease was also a serious problem on cruciferous vegetables in Altai, Fuhai and Burqin counties in Xinjiang Province (Wang Citation1962). Additionally, severe and widespread outbreaks of clubroot were also reported in many southern provinces, such as Guangdong, Guangxi, Jiangsu, Hunan, Sichuan and Yunnan. By 1962, clubroot had spread through 13 Chinese provinces (Wang Citation1962). Since then, records of clubroot gradually decreased (Yang et al. Citation1992; Li et al. Citation1993) until the 1990s, especially the end of the 1990s, when disease outbreaks were reported in the southwest and northeast regions of China (Li Citation1990; Tang et al. Citation1990; Chen et al. Citation1997; Li & Pan Citation1999). By 2010, clubroot was recognized as a major constraint reducing the production of cruciferous vegetables throughout the country. Yunnan Province was the most seriously affected region. For example, the infected areas in Eshan County of Yunnan Province increased from 1056 ha to 2388 ha from 2005 to 2012, respectively, more than doubling in the past 8 years (Fan et al. Citation2013).
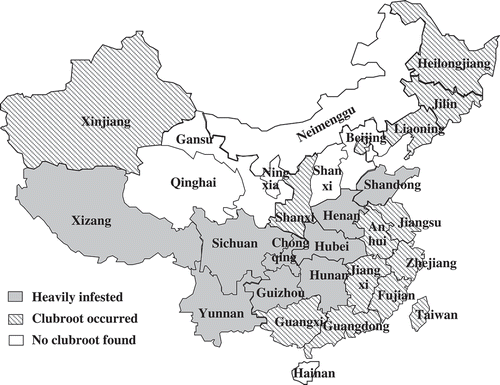
At present, clubroot is distributed throughout China, although it is more severe in the southwest, northeast and central regions than in other areas. The disease affects 3.2–4.0 million ha of cruciferous crops annually, accounting for more than a third of the area in which cruciferous crops are grown. The average rate of yield loss was reported to be 20–30% (Wang et al. Citation2011a). Clubroot infection is much more severe in Yunnan, Sichuan, Guizhou, Tibet, Chongqing, Hunan, Hubei, Henan, Shandong, Liaoning, Jilin, Heilongjiang and Shănxi Provinces of China. These regions are mostly mild, moist, and warm mountainous areas with acidic soils. The damage is not as severe in Guangxi, Guangdong, Jiangxi, Fujian, Anhui, Zhejiang, Jiangsu, Beijing, Shanghai, Xinjiang and Taiwan provinces. To date, no instances of clubroot have been reported in Gansu, Qinghai, Neimenggu, Ningxia and Shānxi Provinces. These regions with low rainfall commonly have alkaline soils. The current distribution of clubroot in China is illustrated in .
As the climate differences are significant between the south and the north, clubroot occurs at different times in different areas of China. The disease mainly occurs during July to August in the Northwest, while it develops during April to June and September to October in the Southwest. The time when clubroot occurs in the middle and lower streams of the Yellow River is April to June and October to November. It affects the middle and lower reaches of the Yangtze River around July to August and October to November. And the occurrence times for the Southern coast and the Northeast are January to March and October to December, respectively ().
Table 1. Geographic regions and season of clubroot occurrence in China.
Host range
All members of the Brassicaceae family are possible hosts for P. brassicae (Dixon Citation2009). Cultivated crops appear to be especially susceptible. Commonly cultivated brassica hosts in China include Brassica rapa (syn. campestris), B. rapa ssp. pekinensis (var. dissoluta, var. infarcta, var. laxa and var. cephalata), B. rapa ssp. chinensis (var. communis, var. rosularis, var. utilis, var. purpurea and var. Tai-tsai) (Shen et al. Citation2009; Zhang et al. Citation2013a; Zhao et al. Citation2013), B. oleracea (var. capitata, var. acephala, var. gemmifera, var. botrytis, var. italic, var. caulorapa, var. bullata and var. rubra) (Xiao & Guo Citation2002; Si et al. Citation2003; Wu et al. Citation2013), B. juncea L. (var. megarrhiza, var. crassicaulis, var. tumida, var. gemmifera, var. multisecta, var. leucanthus, var. longepetiolata, var. linearifolia, var. strumata, var. latipa, var. involuta, var. multiceps, var. rugosa, var. foliosa, var. capitata and var. utilis) (Xiao et al. Citation2002; Liu et al. Citation2012), B. napus (Ma et al. Citation2006; Huang et al. Citation2007; Wang et al. Citation2008, Citation2010), and R. sativus (var. longipinnatus and var. radiculus) (Zhang & Zhao Citation2012). Cruciferous medicinal crops such as I. tinctoria (Xiong et al. Citation2012), cruciferous ornamentals such as Matthiola incana (L.) R. Br. (Liu Citation2012), and cruciferous potherbs such as Armoracia rusticana (Lam.) P. Gaertner et Schreb., Nasturtium officinale R. Br., Capsella bursa-pastoris (Linn.) Medic. (Li Citation2012), and Eutrema wasabi (Siebold) Maxim. (He et al. Citation2003) are also infected by P. brassicae in China.
Physiological races
Physiological races are known to occur in P. brassicae (Honig Citation1931), with strains possessing differential abilities to infect a particular host species, lines or cultivars. Information on races of the pathogen attacking cruciferous crops and on the distribution of the races in the field is very important in breeding for resistant cultivars and in establishing cropping systems for the control of this disease.
Studies on the physiological specialization of P. brassicae were initiated many decades ago (Honig Citation1931) and various differential hosts have been proposed for the assessment of virulence in the pathogen (Ayers Citation1957; Williams Citation1966; Buczacki et al. Citation1975; Somé et al. Citation1996). In studies of pathogen populations from China, the differential set of Williams (Citation1966), consisting of two cabbage and two rutabaga cultivars ‘Jersey Queen’, ‘Badger Shipper’, ‘Laurentian’ and ‘Wilhelmsburger’, has been used most commonly (Shen et al. Citation2009; Ding et al. Citation2013; Ji et al. Citation2013; Peng et al. Citation2013; Wang et al. Citation2013b; Zhao et al. Citation2013). This research indicates that race (pathotype) 4 predominates in China.
In 2009, a total of 15 populations were characterized, of which 11 populations obtained from Liaoning, Shandong, Yunnan, Sichuan and Jilin Provinces were identified as race 4, while two other populations from Liaoning Province were identified as races 2 and 11, and another two populations from Sichuan Province were identified as races 7 and 10 (Shen et al. Citation2009). More recently, race 4 was also characterized from 11 populations collected from Heilongjiang, Jilin and Liaoning Provinces (Zhao et al. Citation2013). In populations of P. brassicae from canola, race 4 was found to be predominant in 16 locations, including Hubei, Anhui, Sichuan and Yunnan Provinces (Ji et al. Citation2013). Similarly, race 4 was determined to be the predominant race in 16 populations from Yunnan, Hubei, Shănxi, Sichuan, Shanghai and Liaoning Provinces (Ding et al. Citation2013). The findings were further confirmed in another recent study, in which four isolates collected from fields of Chinese cabbage, European cabbage and radish in Hubei Province also were classified as race 4 (Wang et al. Citation2013b). Race 4 appears to be predominant on cruciferous vegetables and is spreading throughout China, causing increasing damage.
Other races of P. brassicae also have been detected. In populations of P. brassicae from Hunan Province, races 1, 4, 9 and 13 were identified in 12 populations collected from clubroot-infected crops of Chinese cabbage, tuber mustard (B. juncea var. tumida Tsen & Lee), purple flowering stalk (B. rapa ssp. chinensis var. purpurea Hort.) and canola (Peng et al. Citation2013). Races 7 and 2 were detected, respectively, in populations from Sichuan and Yunnan Provinces (Ding et al. Citation2013). Therefore, although race 4 represents 78.5% of the populations characterized from China thus far, races 1 (2.5%), 2 (3.8%), 7 (2.5%), 9 (2.5%), 10 (1.3%), 11 (1.3%) and 13 (7.6%) have also been identified ().
Table 2. Distribution of Plasmodiophora brassicae races in China.
Disease cycle and primary inoculum
The disease cycle of P. brassicae consists of primary and secondary phases occurring in the root hairs and cortical cells of the host, respectively (Buczacki Citation1983). Primary inoculum is composed of resting spores dispersed from rotted host tissue into the surrounding soil (Kageyama & Asano Citation2009). These resting spores can remain viable in the soil for up to 20 years, with the potential to infect any nearby host (Wallenhammar Citation1996). Clubroot development is initiated by the germination of resting spores and subsequent encystment of primary zoospores in the root hairs. This primary infection, or root hair infection stage, is followed by the formation of primary plasmodia. The primary plasmodia cleave into zoosporangia, each containing 4–16 secondary zoospores (Ingram & Tommerup Citation1972). The cortical infection is induced by secondary zoospores released from zoosporangia in root hairs. Secondary zoospores penetrate the cortical tissues of the main roots. Inside invaded root cells, the pathogen develops into secondary plasmodia which are associated with cellular hypertrophy, followed by gall formation in the tissues. The secondary plasmodia will eventually cleave into a new generation of millions of resting spores within the root gall (Ikegayami et al. Citation1982). As the root tissues disintegrate, the resting spores are released back into the soil becoming the primary inoculum for subsequent seasons.
Pathogen transmission
Seed
Seed contaminated with resting spores of P. brassicae may represent a mode for the long-distance transmission of clubroot. Eriksson (Citation1930) described an account of clubroot on turnips (B. rapa var. rapa) in Sweden that was reportedly caused by seed-borne inoculum, while Gibbs (Citation1931) also attributed several cases of clubroot to seed-borne infestations. Using quantitative PCR to measure DNA of P. brassicae, Rennie et al. (Citation2011) conclusively showed that seed-borne dissemination of this pathogen is possible. Wang (Citation1981) reported that the occurrence of clubroot on cabbage (B. oleracea) in Henan Province, China, resulted from infested seeds obtained from a province where clubroot is prevalent. Similarly, it was suggested that seeds of white mustard (Sinapis alba L.) imported from Canada might have been the source of two cases of clubroot in New South Wales, Australia (Hind-Lanoiselet & Parker Citation2005).
Infected plant material
Long-distance transmission of P. brassicae may also occur via the movement of resting spores in infected plant material. Dissemination of clubroot is often associated with the spread of infested soil from a clubroot-infested field to an uninfested field on root vegetables.
The planting time of cruciferous vegetables varies in different regions of China. Domestic transportation of infected plants is the main route of long-distance transmission of P. brassicae (Li et al. Citation2010b). Out of season vegetables infested with clubroot are dispatched among different provinces, which creates opportunities for the spread of P. brassicae. Transplanting of cole vegetables from clubroot-infested fields onto non-infested fields could introduce the pathogen to new areas or augment existing inoculum levels in the soil.
Soil
As a soil-borne pathogen, the main way that P. brassicae spreads is via the movement of infested soil on farm and other machinery (Howard et al. Citation2010). Soil becomes infested when P. brassicae resting spores are released from decomposing host root tissue. These resting spores can also be dispersed to uninfected plants through the movement of infested soil and water (Dixon Citation2009; Kageyama & Asano Citation2009), or perhaps even by wind-borne dust (Rennie et al. Citation2012). Wind disperses spores that are picked up together with light, dry, dusty soil particles over even greater distances. Earthworms and possibly moles, root nematodes, and other insects may be vectors for P. brassicae in the soil (Dixon Citation2009).
Clubroot resting spores exhibit extreme longevity, which contributes to the severity of this disease. The half-life of resting spores has been estimated to be 3.6 to 4.4 years, while they can survive in the soil for nearly 20 years (Wallenhammar Citation1996; Dixon Citation2009). Using real-time PCR for direct detection and quantification of P. brassicae in soil samples, a population density of 104– 106 resting spores g−1 soil was found in naturally infested soil samples (Wallenhammar et al. Citation2011). Based on the correlation between the population density of resting spores and gall formation, the minimum population density for gall formation was calculated as 3.5 resting spores g−1 soil (Naiki et al. Citation1978). There are, however, several reports of single resting spores found to induce gall formation (Scott Citation1985; Narisawa et al. Citation1996). Considering that primary infection results in millions of resting spores, the population density of P. brassicae increases substantially after continuous cropping with susceptible plants.
Rain and irrigation water
Rain and flood water disseminate P. brassicae over substantial distances, especially on sloping land. Irrigation water contaminated with resting spores of P. brassicae could potentially infest large areas of fields. Plasmodiophora brassicae has been detected in irrigation pond sediments (Datnoff et al. Citation1984), dams and bores (Faggian et al. Citation1999). In a recent controlled study, resting spores of P. brassicae remained viable in water for 34 months and repeated irrigation with water containing as few as 10 spores mL−1 resulted in root galling (Donald Citation2005). In the same study, resting spores settled in undisturbed columns of water at a rate of 25 cm day−1. It was concluded that in a farm dam, most of the resting spore population could be expected to be concentrated in the sediment at the bottom of the dam. The risk of spreading disease through irrigation using contaminated farm dam water could be reduced by locating the irrigation intake pipe in the stillest part of the dam, mounted on a float to collect water from near the surface (Donald Citation2009). Water should not be applied to cruciferous vegetables if it has been drawn from ponds or creeks contaminated with P. brassicae spores resulting from runoff from infested fields.
In southern China, most areas of Yunnan, Hubei, Hunan, Sichuan and other provinces are mountainous regions. In these areas, cruciferous crops are planted in sloping or terraced fields, and fields at lower elevations can be easily infested by rain or irrigation water contaminated with P. brassicae resting spores coming from infested fields located at higher elevations.
Animal fodder and manure
Clubroot can also be spread on animal fodder or manure from livestock fed with P. brassicae-infested fodder (Gibbs Citation1931). Spores are spread in manure and on farm animals themselves, being capable of withstanding the highly acidic gut environment (Li Citation2013). It is thought that the movement of the pathogen from Europe resulted from transporting diseased fodder turnips or more likely swedes used to feed the livestock taken by early European settlers to America, Australasia and other similar centres of settlement (Dixon Citation2009).
One of the earliest suggestions that clubroot was an agricultural problem comes from the fourth century AD, when the Roman Pallidus described the development of spongy roots on rape, turnip and radishes grown in soil fertilized with manure in Italy (Watson & Baker Citation1969). This observation is believed to be the first record of what might have been clubroot transmission by livestock manure (Howard et al. Citation2010). In 1964, an outbreak of clubroot in previously uninfested fields was linked to the spread of manure from animals fed on diseased roots (Creelman Citation1965).
More recently, in 2007–2012, clubroot spread rapidly in HuoShaoPing, Hubei Province, China. More than 70% of the fields were infested within 5 years, with 60% yield loss (Li Citation2008). The survey found that livestock were raised free-range, and were feeding on P. brassicae-infected material. Local farmers often fertilized the fields with livestock manure directly without composting. Resting spores of P. brassicae can survive through the digestive tracts of cattle, pigs and chickens, and consequently can also be spread through the movement of infested animals and their manure (Li Citation2013).
Disease control
Resistant cultivars
Breeding of resistant cultivars is one of the most effective approaches for minimizing crop loss caused by P. brassicae infection (Diederichsen et al. Citation2009). Genetic resistance to clubroot can vary from broad-spectrum resistance, effective against several races or pathotypes of P. brassicae, to highly specific resistance effective only against one particular strain of the pathogen (Somé et al. Citation1996; Diederichsen et al. Citation2006). Thus, the durability of resistance is influenced by the composition of pathotypes within the region intended for deployment of that resistance source (Strelkov et al. Citation2011). As noted above, analysis of P. brassicae populations from China has revealed some pathogenic diversity in the virulence characteristics of the parasite, with at least eight different pathotypes (1, 2, 4, 7, 9, 10, 11 and 13) identified using the Williams (Citation1966) differential set (Shen et al. Citation2009; Ding et al. Citation2013; Ji et al. Citation2013; Peng et al. Citation2013; Wang et al. Citation2013b; Zhao et al. Citation2013).
Breeding for clubroot resistance (CR) in China today focuses on Chinese cabbage, European cabbage and canola. There are more than 10 private companies and 20 public institutions breeding resistant cultivars of these crops. A concerted effort to produce clubroot resistant hybrids has resulted in the recent release of several cultivars into the Chinese market (‘CR589’, Degao ‘CR117’, ‘Kanggen 51’, ‘Kanggen 25’ and ‘Kanggen 39’, ‘CR Huimin’, ‘CR Weimin’ and ‘CR Aimin’, ‘Kangda No. 3’, ‘Shennong 106’ of Chinese cabbage, ‘Xiyuan 6’ of European cabbage, and ‘A 35’, ‘Huayou 7’, ‘Yunyoushuang 1’ of canola) (Li et al. Citation2006; Piao et al. Citation2010; Geng et al. Citation2012; Zhang et al. Citation2012, Citation2013b). While the availability of these cultivars represents important progress in the management of clubroot in China, the cropping of resistant crops will have to be carefully organized to prevent erosion of that resistance. The virulence composition of P. brassicae has been shown to change quickly in response to the cropping of resistant cultivars, and previous experience in other countries has shown that genetic resistance can be eroded (Manzanares-Dauleux et al. Citation2000; Diederichsen et al. Citation2006; Hirai Citation2006; Yang et al. Citation2011; LeBoldus et al. Citation2012). In Canada, for instance, a break of 3 years is recommended between resistant canola cultivars grown on clubroot-infested fields, but resistance stewardship is complicated by a lack of knowledge on the sources of resistance used in commercial hybrids (Strelkov et al. Citation2011).
Liming
Liming is one of the components used in the integrated control of clubroot disease (Larson & Walker Citation1934; Haenseler Citation1939; Campbell et al. Citation1985; Murakami et al. Citation2002). Liming has two effects: the addition of calcium ions and the increase of soil alkalinity (Webster & Dixon Citation1991).
Clubroot development usually occurs in acidic soil conditions (Karling Citation1968; Myers & Campbell Citation1985; Rastas et al. Citation2012). Thus, maintaining a pH of 7.4 or higher with the application of various soil amendments, especially forms of lime, has been used as a strategy for reducing clubroot incidence in high-value horticultural crops. Hsieh (Citation1992a) analysed the physical and chemical properties of soil in relation to the clubroot disease index (severity). It was found that suppressive potential increased with rises in soil pH and soil exchangeable calcium content. No clubroot was found when the soil pH rose above 7.4 or the soil exchangeable calcium increased to 1210 ppm (Hsieh Citation1992a). In another paper, Hsieh (Citation1992b) suggested that calcium was the most active component in controlling clubroot, and a pH increase in soil might be its indirect effect. Calcium concentration may influence root hair infection as well as spore germination. Increasing calcium concentration around roots reduced the development of zoosporangia in root hairs and slowed the release of the secondary zoospores from zoosporangia (Webster & Dixon Citation1991). These effects can be associated with successful control of clubroot by liming of the soil (Donald & Porter Citation2009).
The impact of liming on clubroot severity and yield was assessed in field trials on cruciferous crops over several growing seasons in China. Application of lime in Lichuan City, Hubei Province, for 3 years at 150–200 kg per 667 m2 (2.25–3.375 t/ha) increased the soil pH by one unit, and the incidence of clubroot decreased 13.3% and the yield increased 14.3% (Wang et al. Citation2013a). Some researchers concluded, however, that using liming to reduce the clubroot was not necessarily practical or economically feasible. This is because several tons of lime per hectare were required to increase the pH of an acidic soil to a level at which clubroot severity was affected (Wang et al. Citation2011a). Moreover, long-term use of lime has potential detrimental effects on crop nutrition (Bornman et al. Citation1998). Dai et al. (Citation2004) suggested that adding a 1% lime solution to cruciferous crop roots at the 2, 4 and 6-leaf stages significantly reduced the disease incidence by at least 54%. This technology was of low cost, used lower dosage and had little negative influence on soil properties.
Calcium cyanamide
Calcium cyanamide is another amendment that has long been used to alleviate clubroot in the vegetable brassicas (Walker & Larson Citation1935; Klasse Citation1996). Calcium cyanamide breaks down to yield cyanamide and then urea, ammonia and nitrate. Calcium cyanamide and its break-down products increase soil pH, and serve as a source of nitrogen for the crop. Soil moisture encourages the dimerisation of cyanamide to dicyandiamide (Dixon Citation2009). Dicyandiamide is recognized as a nitrification inhibitor, which slows down soil nitrification and reduces the leaching of nitrates into groundwater (Vilsmeier & Amberger Citation1978). Ammonium produced by calcium cyanamide degradation persists for a long period of time (Kaushal et al. Citation2006).
ZhengfeidanTM, a granulated slow-release fertilizer consisting of calcium cyanamide (≥50% calcium oxide, ≥19% nitrogen and ≤0.3% calcium carbide), is commonly used in most parts of China. It has been shown to be associated with the suppression of clubroot of cruciferous crops (Zhu et al. Citation1995; Zhu & Fang Citation1998). The application of Zhengfeidan™ and Perlka™, commercial granular forms of calcium cyanamide, has been shown to be associated with reduced clubroot severity on stem mustard (Gao et al. Citation2004). The recommended rate for Zhengfeidan™ is 1000 kg ha−1 (Gao et al. Citation2004). The effects of Zhengfeidan™ and six fungicides, including dazomet, fluazinam, cyazofamid, PCNB (pentachloronitrobenzene), miconazole nitrate and metalaxyl-M + mancozeb, were evaluated for controlling clubroot of Chinese cabbage in Changyang City, Hubei Province, and the most effective clubroot management results were obtained with a combination of calcium cyanamide and dazomet (Zhao et al. Citation2008). Subsequent studies (Wang et al. Citation2011a) also suggested that application of calcium cyanamide enhanced control of P. brassicae.
Fungicide application
The application of fungicides is also an effective way to control clubroot on cruciferous vegetables and canola. Consistent control of clubroot has been reported for only a small number of active ingredients. Among the most widely evaluated group of chemicals for the control of clubroot are the benzimidazoles and their precursors, including benomyl, thiophanate, thiophanate methyl (Buczacki Citation1973; Doyle & Clancy Citation1986; Li et al. Citation2010a, Citation2012b), and the alkylene bisdithiocarbamates, including maneb (manganese ethylene bisdithiocarbamate), mancozeb (manganese ethylene bisdithiocarbamate complex with dithiocarbamate esters zinc), and zineb (zinc ethylene bisdithiocarbamate) (Li et al. Citation2010a). A number of other chemicals, most notably pentachloronitrobenzene (PCNB), trichlamide, flusulfamide, fluazinam and cyazofamid, are also effective against clubroot (Zhao et al. Citation2008; Donald & Porter Citation2009; Shang et al. Citation2009; Zhang et al. Citation2013c).
In a comprehensive field evaluation of various fungicides, Li et al. (Citation2010a) identified five effective products: fluazinam, mancozeb, thiophanate methyl, cyazofamid and PCNB. Zhang et al. (Citation2013c) evaluated the efficacy of clubroot control with fluazinam and thiophanate-methyl on Chinese cabbage in pot experiments and field trials, and fluazinam treatment resulted in better control. Combinations of fluazinam and cyazofamid were effective for the control of clubroot and are widely used in China (Zhao et al. Citation2008). Their control efficiency was better than for other chemical fungicides. Soaking seeds with 10% cyazofamid SC and treating seedbed soils and field soils with 50% fluazinam SC before seeding and transplantation improved the control efficiency to 85.7% and increased the yield by 42.3% (Shang et al. Citation2009). These results suggest that fluazinam and cyazofamid may be useful tools to reduce the impact of clubroot in China, but additional work is needed in order to optimize application rates and strategies.
Strict registration procedures have limited the use of many of the chemical products. In China, only one fungicidal active (fluazinam) is currently registered for control of clubroot of vegetable brassicas.
Biological control
Biological control was also identified as a possible component of an integrated disease management strategy for clubroot (Cheah et al. Citation2000). Several studies have illustrated the potential for reducing clubroot with soilborne fungi (Arie et al. Citation1999; Narisawa et al. Citation2000; Yang Citation2010; Sun et al. Citation2013), bacteria (Cheah et al. Citation2000; Xiong et al. Citation2009) and actinomycetes (Wang et al. Citation2011c). Some of these organisms produce anti-microbial metabolites active against P. brassicae (Arie et al. Citation1998; Yang Citation2010), while others induce resistance to the disease (Peng et al. Citation2011).
Reports of clubroot control in the field using these biological organisms are limited and there are no commercial bio-control products available in China. After a series of screenings, two actinomycete isolates for the bio-control of P. brassicae (A316 and A10) were identified, with glasshouse control efficiencies of 53% and 55%, respectively. Field control efficiencies of 46% and 48% were also observed and mutant strains showed field control efficiencies of 60% and 59% (Wang et al. Citation2011a, Citation2011b). In a series of continuous reports, Xiong et al. (Citation2009) identified a biocontrol strain XF-1 of Bacillus subtilis (Ehrenberg) Cohn, which could control clubroot of cruciferous crops effectively. The antagonistic substance, isolated from the culture of B. subtilis XF-1, could inhibit 50% and over 90% of the resting spore activity of P. brassicae for 2 and 9 days, respectively (Jia et al. Citation2011). The expression of a chitosanase gene of B. subtilis XF-1 in Escherichia coli and antagonistic activity against P. brassicae was studied (Zhao et al. Citation2011). The greenhouse and field experiments indicated that B. subtilis XF-1 could colonize the rhizosphere, root surface and root of Chinese cabbage successfully (Liu et al. Citation2012). After application of B. subtilis XF-1 in greenhouse and field experiments in Yunnan Province for 3 years, there was an 85–94% lower clubroot incidence, and the yield was increased by 150–200%. The strains are now in the process of being commercialized.
Microbial control of clubroot is attractive because certain soil organisms can colonize the host roots and rhizosphere, thereby providing durable protection. In addition to research with commercially available biofungicides, work is also underway to identify indigenous soil microorganisms that could serve as effective bio-control agents for clubroot of cruciferous vegetables and canola.
Acknowledgements
This work was supported by the Modern Agro-industry Technology Research System in China (CARS-25), the National Basic Research Program of China (973 Program, 2010CB126101) and was funded by the Key Laboratory of Horticultural Crops Genetic Improvement, Ministry of Agriculture in China. The authors would like to thank Professor G. R. Dixon and Professor S. E. Strelkov for their valuable comments and suggestions on the manuscript.
References
- Arie T, Kobayashi Y, Okada G, Kono Y, Yamaguchi I. 1998. Control of soilborne clubroot disease of cruciferous plants by epoxydon from Phoma glomerata. Plant Pathol. 47:743–748.
- Arie T, Kobayashi Y, Kono Y, Gen O, Yamaguchi I. 1999. Control of clubroot of crucifers by Phoma glomerata and its product epoxydon. Pestic Sci. 55:602–604.
- Ayers GW. 1957. Races of Plasmodiophora brassicae. Can J Bot. 35:923–932.
- Bornman JJ, Bornman L, Barnard RO. 1998. The effect of calcium carbonate and calcium hydroxide on plant growth during overliming. Soil Sci. 163:498–507.
- Buczacki ST. 1973. Glasshouse evaluation of some systemic fungicides for control of clubroot of brassicae. Ann Appl Biol. 74:85–90.
- Buczacki ST. 1983. Plasmodiophora. An inter-relationship between biological and practical problems. In: Buczacki ST, editor. Zoosporic plant pathogens: a modern perspective. London, UK: Academic Press Inc. p. 161–191.
- Buczacki ST, Toxopeus H, Mattusch P, Johnston TD, Dixon GR, Hobolth LA. 1975. Study of physiologic specialization in Plasmodiophora brassicae: proposals for attempted rationalization through an international approach. Trans Br Mycol Soc. 65:295–303.
- Campbell RN, Greathead AS, Myers DF, de Boer GJ. 1985. Factors related to control of clubroot of crucifers in the Salinas Valley of California. Phytopathology. 75:665–670.
- Cheah LH, Veerakone S, Kent G. 2000. Biological control of clubroot on cauliflower with Trichoderma and Streptomyces spp. N Z Plant Prot. 53:18–21.
- Chen SF, Liu XB, Hu BS. 1997. Occurrence and management of clubroot disease on Chinese cabbage in Aletai, Xingjiang. Plant Prot Tech & Ext. 17:21–22 (in Chinese).
- Creelman DW. 1965. Diseases of vegetable crops. Can Plant Dis Surv. 45:52–63.
- Dai YH, Yang L, Li SD, Liu JR, Zhang GX, Zhao YM. 2004. Technology of control Chinese cabbage root by adjusting soil pH. Chin Plant Prot. 8:20–22 (in Chinese).
- Datnoff LE, Lacy GH, Fox JA. 1984. Occurrence and populations of Plasmodiophora brassicae in sediments of irrigation water sources. Plant Dis. 68:200–203.
- Diederichsen E, Beckmann J, Schondelmeier J, Dreyer F. 2006. Genetics of clubroot resistance in Brassica napus ‘Mendel. Acta Hortic. 706:307–311.
- Diederichsen E, Frauen M, Linders EGA, Hatakeyama K, Hirai M. 2009. Status and perspectives of clubroot resistance breeding in crucifer crops. J Plant Growth Regul. 28:265–281.
- Ding YH, Jian YC, Yu YJ, Wang WH, Geng LH, Kang JG. 2013. Identification of pathotype of Plasmodiophora brassicae on crucifer vegetables in eight provinces of China. Chin Veg. 16:85–88 (in Chinese).
- Dixon GR. 2009. The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. J Plant Growth Regul. 28:194–202.
- Donald EC. 2005. The influence of abiotic factors and host plant physiology on the survival and pathology of Plasmodiophora brassicae of vegetable brassicas [dissertation]. Melbourne: University of Melbourne.
- Donald EC, Porter IJ. 2009. Integrated control of clubroot. J Plant Growth Regul. 28:289–303.
- Doyle OPE, Clancy KJ. 1986. Integrated chemical applications for the control of clubroot in brassicas. Proc British Crop Prot Conf – Pests Dis. 8:923–930.
- Editorial Committee of China Agriculture Yearbook. 2012. China Agriculture Yearbook. Beijing, China: China Agriculture Press.
- Engquist LG. 1994. Distribution of clubroot in Sweden and the effect of infection on oil content of oilseed rape. Sveriges Utsa ¨desfo ¨ renings Tidskrift. 104:82–86.
- Eriksson J. 1930. Fungous Diseases of Plants. 2nd ed. London: Bailliere Taindal & Co.
- Faggian R, Donald C, Porter IJ, Lawrie AC. 1999. Epidemiology of recent clubroot outbreaks using PCR to trace sources of inoculum. In: Proceedings of the Australasian Plant Pathology Society 12th Biennial Conference, Canberra.
- Fan GP, Yang QH, Ning JC, Qian CP, Li AW, Li ZS. 2013. Occurrence and management of clubroot on rape in Eshan city during 2005–2012. Chin Plant Protc. 8:46–48 (in Chinese).
- Gao MQ, Zhang SF, Xue P, Zhang JH, Wang XW. 2004. Evaluation of Zhengfeidan for control of clubroot of stem mustard. J Changjiang Veg. 9:47–48 (in Chinese).
- Geng XC, Shen XQ, Guo YF, Ma SF, Hong YT. 2012. Breeding of anti-clubroot AB lines type I of orange head Chinese cabbage. Acta Agric Boreali-occidentalis Sin. 21:155–161.
- Gibbs JG. 1931. Club-root in cruciferous crops. N Z J Agric. 42:1–17.
- Haenseler CM. 1939. The effect of various soil amendments on the development of club root (Plasmodiophora brassicae) of crucifers. Phytopathology. 29:9.
- He YQ, Wu YX. 2013. Research status of clubroot disease of cruciferous crops in China. International clubroot workshop, Edmonton, Alberta, Canada. June 19–21.
- He WX, Ning H, Yuan YC, Zhang FL. 2003. Plant quarantine problems and the countermeasures in wasabie plantation industry in Sichun. J Sichuan Agric Univ. 21:182–184 (in Chinese).
- Hind-Lanoiselet T, Parker P. 2005. Clubroot of canola and mustard. Primefact. 115:1–2.
- Hirai M. 2006. Genetic analysis of clubroot resistance in Brassica crops. Breed Sci. 56:223–229.
- Honig F. 1931. Der Kohlkopferreger (Plasmodiophora brassicae Wor.) Eine Monographie. Gartenbauwissenschaft. 15:116–225.
- Howard RJ, Strelkov SE, Harding MW. 2010. Clubroot of cruciferous crops-new perspectives on an old disease. Can J Plant Pathol. 32:43–57.
- Hsieh WH. 1992a. Ecology of Plasmodiophora brassicae. Bull Fungal Dis. 1:145–156.
- Hsieh WH. 1992b. Control of clubroot of crucifers. Bull Plant Dis Pests. 1:293–299.
- Huang QW, Ouyan L, Wang YJ. 1955. Occurrence and prevention of clubroot disease on cruciferous crops in Jiangxi. Bull Plant Prot. 8:1–4 (in Chinese).
- Huang Y, Ma SQ, Li XQ, Wang J, Hu XL. 2007. Morphology of Plasmodiophora brassicae and biological characteristic of the pathogenic resting spores in rapeseed. Sci Agric Sin. 40:1388–1394 (in Chinese).
- Ikegayami H, Naiki T, Ito T, Imuro Y. 1982. Ultrastructural growth process of Plasmodiophora brassicae in infected cells of Chinese cabbage and turnip (Studies on the clubroot of cruciferous plants IV). Res Bull Fac Agric Gifu Univ. 46:9–19.
- Ingram DS, Tommerup IC. 1972. The life history of Plasmodiophora brassicae Woron. Proc. Roy Soc. Lond Ser. B. 180:103–112.
- Ji HW, Ren L, Chen KR, Xu L, Liu F, Sun CC, Li J, Liu SY, Fang XP. 2013. Identification of physiological races of club root and resistance of rape cultivars to Plasmodiophora brassicae. Chin J Oil Crop Sci. 35:301–206 (in Chinese).
- Jia Y, Sun RK, Wu YX, Mao ZC, Sun HX, He YQ. 2011. Inhibition effect of Bacillus subtilis XF-1 extract on 11 fungal pathogens. J Anhui Agric Univ. 38:753–756 (in Chinese).
- Kageyama K, Asano T. 2009. Life cycle of Plasmodiophora brassicae. J Plant Growth Regul. 28:203–211.
- Karling JS. 1968. The plasmodiophorales. New York, NY: Hafner Publishing Co.
- Kaushal T, Onda M, Ito S, Yamazaki A, Fujikake H, Ohtake N, Sueyoshi K, Takahashi Y, Ohyama T. 2006. Effect of deep placement of slow-release fertilizer (lime nitrogen) applied at different rates on growth, N2 fixation and yield of soya bean. J Agron Crop Sci. 192:417–426.
- Klasse HJ. 1996. Calcium cyanamide-an effective tool to control clubroot-a review. Acta Hortic. 407:403–409.
- Larson RH, Walker JC. 1934. Soil treatment in relation to clubroot of cabbage. J Agric Res. 48:749–759.
- LeBoldus JM, Manolii VP, Turkington TK, Strelkov SE. 2012. Adaptation to Brassica host genotypes by a single-spore isolate and population of Plasmodiophora brassicae (clubroot). Plant Dis. 96:833–838.
- Li MY. 1990. Occurrence and prevention of clubroot disease of cruciferous crops. Beijing Agric Sci. 4:11–13 (in Chinese).
- Li BJ. 2008. Cruciferous crop root disease in Changyang, Hubei Province. Chin Veg. 7:55 (in Chinese).
- Li M. 2012. Characterization of the host range of Plasmodiophora brassicae and the role of glucosinolate glucoside in root infection by this pathogen [dissertation]. Wuhan: Huazhong Agricultural University (in Chinese).
- Li JP. 2013. Studies on the detection technology of Plasmodiophora brassicae and animal manure as a transmission route of the pathogen [dissertation]. Beijing: Chinese Academy of Agricultural Sciences (in Chinese).
- Li HM, Pan YH. 1999. Occurrence rules and control technology of clubroot disease on cruciferous crops. J Changjiang Veg. 9:15–16 (in Chinese).
- Li JK, Bai M, Xia XY, Zhaxi CR, Ou Z, Wei J. 1993. Research on the soil causes of clubroot on cruciferous vegetables in Yadong City, Tibet. J Tibet Univ. 8:70–72.
- Li WF, He JM, Li SK. 2006. Studied progress on the root swells sickness of mustard family plant. Southwest Chin J Agric Sci. 19:560–563 (in Chinese).
- Li Y, Xie XW, Shi YX, Xiang WS, Li BJ. 2010a. Studies of controlling clubroot of Chinese cabbage. Chin J Pestic Sci. 12:93–96 (in Chinese).
- Li JP, Zhu YQ, Li BJ. 2010b. Transmission of clubroot of cruciferous vegetables. Chin Veg. 5:21–22 (in Chinese).
- Li Y, Bai YJ, Miao ZY, Zhao Y, Zhao KH. 2012b. Effects of different germicides on the germination of Chinese cabbage. Northern Hortic. 22:142–144 (in Chinese).
- Li JP, Chai AL, Sun RF, Li BJ. 2012a. Recent research development on clubroot of cruciferae vegetables. Chin Veg. 8:1–4 (in Chinese).
- Liang Y, Zhang AF, Wang WX, Tang TS. 2001. Review on clubroot disease of crucifers. J Anhui Agric Sci. 29:746–749 (in Chinese).
- Linnasalmi A, Tovianinen A. 1991. Occurrence of clubroot and Plasmodiophora brassicae Wor. races in Finland. J Agr Sci Fin. 63:415–434.
- Liu WW. 2012. Control of clubroot on Matthiola incana. Yunnan Agric. 11:20 (in Chinese).
- Liu Y, Yin YP, Wang ZK, Luo YL. 2012. Expression of nitrilases in Brassica juncea var. tumida Tsen in root galls caused by Plasmodiophora brassicae. J Int Agric. 11:100–108 (in Chinese).
- Ma SQ, Huang Y, Wang ZY, Zhao YQ, Zeng FC. 2006. Modality and biological characteristic of Plasmodiophora brassicae in rape. J Sichuan Agric Univ. 24:161–165 (in Chinese).
- Manzanares-Dauleux MJ, Delourme R, Baron F, Thomas G. 2000. Mapping of one major gene and of QTL involved in resistance to clubroot in Brassica napus. Theor Appl Genet. 101:885–891.
- Murakami H, Tsushima S, Kuroyanagi Y, Shishido Y. 2002. Reduction of resting spore density of Plasmodiophora brassicae and clubroot disease severity by liming. Soil Sci Plant Nutr. 48:685–691.
- Myers DF, Campbell RN. 1985. Lime and the control of clubroot of crucifers: effects of pH, calcium, magnesium and their interactions. Phytopathology. 75:670–673.
- Naiki T, Kageyama K, Ikegami H. 1978. The relation of spore density of Plasmodiophora brassicae Wor. to the root hair infection and club formation in Chinese cabbage (Studies on the clubroot of cruciferous plant II). Ann Phytopathol Soc Jpn. 44:432–439.
- Narisawa K, Kageyama K, Hashiba T. 1996. Efficient root infection with single resting spores of Plasmodiophora brassicae. Mycol Res. 100:855–858.
- Narisawa K, Ohki KT, Hashiba T. 2000. Suppression of clubroot and Verticillium yellows in Chinese cabbage in the field by the root endophytic fungus, Heteroconium chaetospira. Plant Pathol. 49:141–146.
- Peng G, McGregor L, Lahlali R, Gossena BD, Hwang SF, Adhikari KK, Strelkov SE, McDonald MR. 2011. Potential biological control of clubroot on canola and crucifer vegetable crops. Plant Pathol. 60:566–574.
- Peng SS, Ren ZH, Huang XL, Liu MJ, Sun LF, Liu EM. 2013. Physiological race identification on clubroot of cruciferous crops caused by Plasmodiophora brassicae in Hunan. J Changjiang Veg. 6:46–49 (in Chinese).
- Piao ZY, Wu D, Wang M, Zhang T. 2010. Marker-assisted selection of near isogenic lines for clubroot resistant gene in Chinese cabbage. Acta Hortic Sin. 8:1264–1272.
- Rastas M, Latvala S, Hannukkala A. 2012. Occurrence of Plasmodiophora brassicae in Finnish turnip rape and oilseed rape fields. Agric Food Sci. 21:141–158.
- Rennie DC, Manolii VP, Cao T, Hwang SF, Howard RJ, Strelkov SE. 2011. Direct evidence of surface infestation of seeds and tubers by Plasmodiophora brassicae and quantification of spore loads. Plant Pathol. 60:811–819.
- Rennie DC, Turkington TK, Leboldus JM, Howard RJ, Kutcher HR, Strelkov SE. 2012. Dust monitoring for clubroot [Plasmodiophora brassicae] in Alberta. Can J Plant Pathol. 35:99–132.
- Scott ES. 1985. Production and characterization of single-spore isolates of Plasmodiophora brassicae. Plant Pathol. 34:287–292.
- Shang H, Yang PW, Dong LY, Liu SF, Yu HG, Li JR. 2009. Chemical control strategy against Chinese cabbage clubroot. Plant Prot. 6:157–159 (in Chinese).
- Shen XQ, Nie K, Wu Q, Zhang YG, Meng XH. 2009. Initial research report on differentiation identification of Chinese cabbage clubroot main physiological races. Chin Veg. 8:59–62 (in Chinese).
- Si J, Li CQ, Xiao CG, Ren XS, Wang XJ. 2003. Study on the inoculation methods of Plasmodiophora brassicae. J Southwest Agric Univ. 25:216–219 (in Chinese).
- Somé A, Manzanares MJ, Laurens F, Baron F, Thomas G, Rouxel F. 1996. Variation for virulence on Brassica napus L. amongst Plasmodiophora brassicae collections from France derived from single-spore isolates. Plant Pathol. 45:432–439.
- Strelkov SE, Hwang SF, Howard RJ, Hartman M, Turkington TK. 2011. Progress towards the sustainable management of clubroot [Plasmodiophora brassicae] of canola on the Canadian prairies. Prairie Soils Crops. 4:114–121.
- Sun LF, Ren ZH, Peng SS, Huang XL, Dong J, Liu EM. 2013. Screening of antagonistic microorganisms against Plasmodiophora brassicae and evaluation of their control effect. Hunan Agric Sci. 1:84–87 (in Chinese).
- Tang WH, Li H, Sun J. 1990. Ecological conditions and management of clubroot disease on Chinese cabbage in Beijing. Beijing Agric Sci. 6:6–9 (in Chinese).
- Vilsmeier K, Amberger A. 1978. Model experiments concerning the breakdown of powdered and granulated calcium cyanamide fertilisers. J Agron Crop Sci. 147:68–77.
- Walker JC, Larson RH. 1935. Calcium cyanamide in relation to control of clubroot of cabbage. J Agric Res. 51:183–189.
- Wallenhammar AC. 1996. Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation levels. Plant Pathol. 45:710–719.
- Wallenhammar AC, Almquist C, Soderstrom M, Jonssona A. 2011. In-field distribution of Plasmodiophora brassicae measured using quantitative real-time PCR. Plant Pathol. 61:16–28.
- Wang HF. 1962. Discussion of clubroot disease on cruciferous vegetables in China - from the disease found in Jiamusi City. J Northeast Agric Univ. 2:47–48 (in Chinese).
- Wang SZ. 1981. Changes of main crop diseases in Henan province during resent thirty years. J Henan Agric Univ. 4:127–133 (in Chinese).
- Wang X, Peng HJ, Gao MQ, Han HB, Zhang JH, Xiao CG. 2002. Preliminary identification of the pathogen of root tumour of stem mustard and factors influencing the incidence of the disease. Southwest Chin J Agric Sci. 15:75–78 (in Chinese).
- Wang J, Huang Y, Hu XL, Niu YZ, Li XL, Liang Y. 2008. Study on symptom, yield loss of clubroot and modality of Plasmodiophora brassicae in rape. Chin J Oil Crop Sci. 30:112–115 (in Chinese).
- Wang S, Zheng SJ, Shen L, Liao HM, Luo LM. 2010. Evaluation of control effect on rape clubroot of wormcast. Southwest Chin J Agric Sci. 23:1910–1931 (in Chinese).
- Wang J, Huang Y, Li XL, Li HZ. 2011a. Research progress in clubroot of crucifers. Plant Prot. 37:153–158 (in Chinese).
- Wang J, Huang Y, Yao J, Lin S, Li XL, Qin Y. 2011b. Identification and control effects of two antagonistic actinomycetes against clubroot. Sci Agric Sin. 44:2692–2700 (in Chinese).
- Wang J, Huang Y, Zhang Y. 2011c. Control of rapeseed clubroot by screened antagonistic microorganisms against Plasmodiophora brassicae. Chin J Oil Crop Sci. 33:169–174 (in Chinese).
- Wang MQ, Xiang YY, Zhou FZ. 2013a. Application effect of lime in aquatic vegetable bases. J Changjiang Veg. 4:63–65 (in Chinese).
- Wang WH, Yu YJ, Ding YH, Zhang FL, Yu SC, Zhang DS, et al. 2013b. Physiological race identification of clubroot (Plasmodiophora braassicae Wor.) from Changyang county, Hubei province and resistant resource screening for Chinese cabbage breeding. Chin Veg. 12:55–60 (in Chinese).
- Watson AG, Baker KF. 1969. Possible gene centers for resistance in the genus Brassica to Plasmodiophora brassicae. Econ Bot. 23:245–252.
- Webster MA, Dixon GR. 1991. Calcium, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Mycol Res. 95:64–73.
- Williams PH. 1966. A system for the determination of races of Plasmodiophora brassicae that infect cabbage and rutabaga. Phytopathology. 56:624–626.
- Wu DJ, Chen GK, Yang XQ, Yao YH, Ma Q, Xiao CG. 2013. Application of four kinds of cabbage clubroot disease classification standards evaluation. Southwest Chin J Agric Sci. 26:591–594 (in Chinese).
- Xiao CG, Guo XH. 2002. Biological characteristic of Plasmodiophora brassicae. Mycosystema. 21:597–603 (in Chinese).
- Xiao CG, Guo X.-H., Han MB, Peng HJ. 2002. Identification of the pathogen for clubroot on diseased stem mustard in Fuling, Chongqing and its major characteristics. J Southwest Agric Univ. 24:539–541 (in Chinese).
- Xiong GR, Zhao GF, Fan CM, He YQ. 2009. Identification and fungistatic effect of a biocontrol strain, XF-1. J Yunnan Agric Univ. 24:190–194 (in Chinese).
- Xiong GR, Zheng JF, Wu YX, He YQ. 2012. A new disease, clubroot of Isatis tinctoria L. J Agric Catastro. 2:3–4 (in Chinese).
- Yang LF. 2010. Preparation of compost and biocontrol of oilseed rape sclerotinia stem rot and clubroot with Trichoderma atroviride [dissertation]. Ya’an: Sichuan Agrictrial University (in Chinese).
- Yang YH, Zhang XF, Zou LJ. 1992. Studies of the occurrence of Chinese cabbage clubroot in Changsha. J Hunan Agric Univ. 18:854–858 (in Chinese).
- Yang YZ, Zhang XL, Yang WG, Tian WW, Wang Q. 2011. Studies on the resistance of rape variety to Plasmodiophora brassicae and screening. Chin Agric Sci Bull. 27:278–282 (in Chinese).
- Zhang ZM, Zhao YH. 2012. Identification of new symptoms of radish clubroot. Chin Plant Prot. 12:44–45 (in Chinese).
- Zhang T, Wu D, Zhao Z, Wang Z, Piao ZY. 2012. Development of near isogenic lines for clubroot resistance in Chinese cabbage and their assessment. Mol Plant Breed. 10:722–730 (in Chinese).
- Zhang XZ, Xie MJ, Kim BS, Song L. 2013c. Control efficacy of fluazinam to Chinese cabbage clubroot caused by Plasmodiophora brassicae. J Northeast Agric Univ. 1:77–80 (in Chinese).
- Zhang H, Xu H, Kuang YY, Song B, Chen LZ, Yuan XH. 2013a. Molecular marker of clubroot resistance genes in Brassica campestris ssp. chinensis. Jiangsu J Agric Sci. 29:633–636 (in Chinese).
- Zhang JW, Zhang JW, Sun MY. 2013b. Breeding of CR589, a new Chinese cabbage cultivar resistant to clubroot. J Changjiang Veg. 10:16–17 (in Chinese).
- Zhao YH, Xiang ZH, Zhang ZM. 2008. Evaluation of soil treatment agent for control of clubroot of brassicae. Hubei Plant Prot. 1:24–25 (in Chinese).
- Zhao J, Xiong GR, Wang ZY, Mao ZC, Wu YX, He YQ. 2011. Expression of chitosanase gene of Bacillus subtilis XF-1 in Escherichia coli and the chitosanase antagonism against the fungal pathogens. Chin J Biol Cont. 27:504–509 (in Chinese).
- Zhao Y, Bai YJ, Miao ZY, Li Y, Zhao KH. 2013. Identification of physiological races of Plasmodiophora brassicae causing clubroot in Chinese cabbage from Northeast China. J Hunan Agric Univ. 39:176–178 (in Chinese).
- Zhu GN. 1956. Clubroot on cruciferous vegetables. Bull Agric Sci. 11:677–678 (in Chinese).
- Zhu BY, Fang ZZ. 1998. Efficacy of calcium cyanamide for control of clubroot of Chinese cabbage. Shanghai Veg. 1:34–35 (in Chinese).
- Zhu BY, Ye ZX, Ouyang H, Xiong JS, Fang ZZ. 1995. Evaluation of calcium cyanamide for control of clubroot of Chinese cabbage. J Zhejiang Agric Sci. 6:300–301 (in Chinese).