Abstract
In India, clubroot caused by Plasmodiophora brassicae Woronin has been present on cabbage and cauliflower crops (Brassica oleracea L. var. capitata and botrytis) for nearly 80 years in the Eastern Himalayan Darjeeling Hills of West Bengal and South Indian Nilgiri Hills in Tamil Nadu. Since the early 1980s, P. brassicae has spread rapidly on the most popular cultivars of cultivated yellow sarson (Brassica rapa L. var. trilocularis (Roxb.) Kitam.) and Indian mustard (Brassica juncea L.) grown in the red and lateritic soils (Alfisols) and the Terai regions situated in the Himalayan foothills of the northern part of West Bengal State. Soils of both regions are acidic in nature (pH 5.3–6.7). Clubroot on tori sarson (B. rapa L. ssp. toria) is reported from the neighbouring Odisha State. The sporadic occurrence of the disease on cole vegetables is also reported from northeast Himalayan states such as Manipur Nagaland Sikkim and Mizoram. At present, clubroot disease is endemic only in West Bengal State. The present report summarizes the research conducted on clubroot disease in India, especially in the brassica oilseed and cole vegetables of West Bengal. Specifically, this review summarizes work to: (i) develop and assess new and existing clubroot management strategies; (ii) monitor clubroot occurrence and spread; (iii) identify and characterize sources of resistance; and (iv) evaluate variability of the pathogen in populations in different agro-climatic regions of West Bengal.
Résumé
La hernie, causée par Plasmodiophora brassicae Woronin, infecte les choux et les choux-fleurs (Brassica oleracea L. var. capitata et botrytis) depuis près de 80 ans dans les collines du Darjeeling de l’Est himalayen du Bengale-Occidental et les collines des Nilgiri du sud de l’Inde, au Tamil Nadu. Au début des années 1980, P. brassicae s’est rapidement propagé aux cultivars les plus populaires de sarson à graines jaunes (Brassica rapa L. var. trilocularis [Roxb.] Kitam.) et de moutarde indienne (Brassica juncea L.), cultivés dans les sols rouges et latéritiques (alfisols) et dans les régions du Terai des contreforts himalayens du nord du Bengale-Occidental. Les sols des deux régions sont acides (pH de 5.3 à 6.7). La hernie trouvée sur le sarson du groupe Tori (B. rapa L. ssp. toria) a été signalée dans l’État voisin de l’Odisha. L’incidence sporadique de la maladie sur les légumes de la famille du chou a également été signalée dans les États du Nord-Est himalayen comme le Monipur, le Nagaland, le Sikkim et le Mizoram. Actuellement, la hernie n’est endémique qu’au Bengale-Occidental. Cet article présente un aperçu des recherches menées sur la hernie en Inde, spécialement sur les oléagineuses du genre Brassica et les légumes de la famille du chou cultivés au Bengale-Occidental. Il propose une synthèse des travaux visant notamment à: (i) évaluer les stratégies, courantes et nouvelles, de gestion de la hernie et à en élaborer de nouvelles; (ii) surveiller l’incidence et la propagation de la hernie; (iii) répertorier et caractériser les sources de résistance; et (iv) évaluer la variabilité de l’agent pathogène dans des populations provenant de différentes régions agroclimatiques du Bengale-Occidental.
Introduction
Clubroot disease caused by the protist pathogen Plasmodiophora brassicae Woronin was reported for the first time in India from the Darjeeling Hills in the Eastern Himalayan region of West Bengal by Chattopadhyay and Sengupta (Citation1952), and also from the South Indian Nilgiri Hills in Tamil Nadu by McRae (Citation1928) and Sharangapani (Citation1930). Since the 1980s, this disease has spread rapidly on oilseed brassicas grown in the plains of West Bengal State, causing crop losses of 32.5% as estimated from harvesting data (Laha et al. Citation1985). The first report of an epidemic of clubroot on oilseed brassicas in India was in 1983–85 (Laha et al. Citation1985). Disease incidence ranged from 30% on Indian mustard (Brassica juncea L.) to 70% on yellow sarson (Brassica rapa L. var. trilocularis (Roxb.) Kitam) (Chattopadhyay & Bagchi Citation1989). Except for exotic germplasm of B. napus, B. nigra and B. carinata, all of the indigenous popular cultivars of brassica oilseeds and vegetables grown in the Darjeeling Hills and plains of north and south West Bengal state are highly susceptible (Chattopdhyay et al. Citation1989, Citation1991). Currently, clubroot is endemic to the Darjeeling Hills, causing substantial damage to oilseed and vegetable brassicas, especially to seed production in cauliflower (Brassica oleracea L. var. botrytis). Clubroot on tori sarson (Brassica rapa L. ssp. toria) is reported from Odisha State (Das et al. Citation1987). There is also a report of clubroot incidence in Nepal on cauliflower and other cultivated cole crops (Timila et al. Citation2008). With the rapid expansion of brassica cultivation, clubroot has spread rapidly in the Himalayan states, causing 35–40% crop loss.
Since the mid 1980s, the rapid expansion in the areas cultivated with brassica oilseed and vegetables in West Bengal State along with high planting densities and the indiscriminate use of nitrogen fertilizers (especially urea which contributes to soil acidity) has exacerbated the disease intensity. Clubroot disease is now endemic to the brassica-growing areas of West Bengal State. Plasmodiophora brassicae is very well acclimatized to the various agro-climatic regions of this state. Gradual irreversible wilting, yellowing of the leaves and root galling (club formation) are the common visual symptoms on brassica hosts that may result in total death of infected plants within a short time. A popular and widely cultivated yellow sarson cultivar, ‘Benoy (B-9)’, is highly susceptible to clubroot in the plains of South and North Bengal. There are wide differences in the host specialization and virulence patterns of the pathogen populations within and between clubroot-infested fields in the different agro-climatic regions of West Bengal State. The present paper briefly reviews the disease situation, pathogen variability, genetic resistance, abiotic stress factors, epidemiology and integrated management strategies for clubroot in India.
Clubroot occurrence and spread
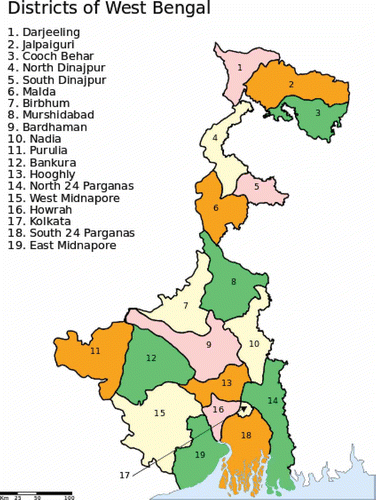
Clubroot disease was surveyed for two consecutive years during 1998–2000 (Bhattachary Citation2003) in an area covering the Darjeeling (altitude 2100 m), Kalimpong (1200 m) and Sonada (2550 m) sub-divisions in the Darjeeling Hills, situated in the Eastern Himalayan region and also in the foothills (Terai region) of North Bengal and plains of South Bengal. Kalimpong in the Darjeeling Hills was the hot spot for clubroot disease where all of the brassica plants were found to be susceptible to P. brassicae. Rayosak (Brassica juncea L. ssp. rugosa (Ronb), a local leafy vegetable, and cauliflower were found to be the most susceptible hosts at all locations surveyed. Among the oilseed brassicas in the Darjeeling Hills, Indian mustard (B. juncea) was severely diseased (rated 2–3 on a 0–3 scale). In the plains of North and South Bengal districts (soil pH 5.3–6.7) such as Coochbihar, Jalpaiguri, Birbhum, Bankura, West Midnapore and Purulia (), the B. rapa group of oilseeds was found to be the most susceptible host. The sporadic occurrence of clubroot on cole vegetables was also found in the foothills of Darjeeling, including the Coochbihar and Jalpaiguri districts of North Bengal. A more recent survey for clubroot in the Darjeeling Hills and plains of North and South Bengal (Baskey Citation2013) showed that clubroot disease is spreading at an alarming rate in the plains of North Bengal, expanding its host range from oilseed brassicas to vegetables. With the exception of the Nadia, North and South 24 Parganas and East Midnapore districts, clubroot was reported from all other districts at moderate to high disease severity (). Since 2003–2004, clubroot disease has started to appear on oilseed brassicas in the most fertile part of West Bengal, the Gangetic alluvial region (soil pH 6.7–7.3), covering the Hooghly Bardhaman and Howrah districts of South Bengal. It is possible that in this potato-growing region, farmers applied large amounts of nitrogenous fertilizers such as urea to boost production. The urea, through ammonification and nitrification processes, made the soils more acidic (pH 5.7–6.7). Symptoms of clubroot started appearing when farmers replaced potato with brassica oil seeds in their cropping sequences.
Fig. 1. (Colour online) Clubroot disease occurrence in West Bengal, India. The most severe symptoms have been observed in the Darjeeling Hills, Jalpaiguri and Coochbihar districts of North Bengal and Birbhum, Bankura, Purulia and West Midnapore on the red and lateritic soil regions of South Bengal. With the exception of Nadia, North 24 Parganas and the coastal areas of South 24 Parganas and East Midnapore, clubroot disease is prevalent in all districts of West Bengal. Recently, clubroot has also been found in the gangetic alluvial fertile and neutral soils of Howrah, Hooghly Bardhaman and Murshidabad districts.
Symptom development and crop loss assessment
Symptoms of gradual irreversible wilting of plants starts with a bluish tinge on the leaf surface followed by yellowing. These are characteristic and lead to the ultimate death of plants and the formation of clubs on the primary and secondary roots, except on radish where small clubs, which develop on tap roots, become black and ultimately rotten. In the case of late infections, losses attributable to clubroot disease in cauliflower seed production in the Darjeeling Hills may exceed 50% because crops are maintained in the field for longer periods. The above-ground clubroot symptoms on affected plants of yellow sarson in the red and lateritic region (Alfisol) include stunting and yellowing as the disease progresses; the roots, on examination, are swollen with the formation of clubs of various sizes. There is a reduction in the number of branches and pods as well as in the pod size in the inflorescences of diseased plants. In field plot experiments (Chattopdhyay Citation1991), losses of 47.7% and 54.0% were recorded in seed yield as a result of severe clubroot development in yellow sarson. Disease severity is dependent upon soil pH, land elevation, level of inoculum, and the susceptibility of host plants in the red and lateritic soils of West Bengal (Sen Citation2005).
Sources of inoculum and spread of clubroot disease
Soil is the primary source of P. brassicae inoculum wherever brassicas are grown. In the Darjeeling Hills, the long-distance spread of clubroot takes place through the transportation of infected seedlings from nurseries at Kalimpong which carry infested soil on the roots. The pathogen is also carried in cattle and other manures applied in the nurseries. It was also observed that clubroot incidence increased rapidly after irrigation or rain. Five years of rainfall data (1994–1998) from the Darjeeling Hills and plains of North Bengal showed that annual rainfall and especially late monsoon rainfall in autumn (September–October) increased soil moisture levels before winter brassica oilseed and vegetable cultivation, which stimulated early clubroot incidence and spread of the disease (Bhattacharya Citation2003).
Land elevation, cropping sequence and soil conditions on clubroot development
Crop losses of about 30–80% were observed in the case of the brassica leafy vegetable rayosak, which is cultivated under shade in moist and lowland conditions in the Darjeeling district (Bhattacharya Citation2003). Resting spore populations of 1 × 107/g soil were recorded at Kalimpong in highly infested soils, as estimated by bioassays. The cultivation of more than one brassica crop in a cropping sequence increases the risk of clubroot incidence. Inclusion of rice in a cropping sequence reduced disease incidence and severity in Kalimpong at Darjeeling Hills, possibly because the anaerobic conditions created in paddy rice fields reduced the viability of P. brassicae resting spores (Bhattacharya Citation2003). Physical, chemical and biological components of the soil environment affect the survival, growth and reproduction of P. brassicae (Dixon Citation2009a). In the case of rape and mustard cultivated under lowland moist conditions in the red and lateritic regions of West Bengal, clubroot development is favoured when the temperatures fluctuate between 18 °C and 25 °C, the soil is sandy, the moisture level is above 50% of field capacity and the pH is acidic (5.3–6.5) (Sen Citation2005).
Root hair infection test
A root hair infection test was conducted in the laboratory for two consecutive years on brassica hosts and also on non-brassica crop plants. Zoosporangia of P. brassicae were observed in the root hairs of all the tested brassica vegetables and oilseeds and some non-brassica plants. Among the non-brassicas, root hair infection was observed on lentil (Lens culinaris L.) and buckwheat (Fagopyrum esculentum Moench) but very few (0.02% and 0.07%, respectively) root hairs were found to be infected. Root hairs of black gram (Vigna mungo (L) Hopper) and cowpea (Vigna unguiculata (L.) Walp) were not infected. Statistically significant differences in root-hair infection rates were found among different B. juncea hosts and among B. oleracea hosts; the rate of root-hair infection was observed to be highest on B. juncea. No statistically significant difference in root-hair infection rates was found among B. rapa hosts (Bhattacharya Citation2003).
Clubroot reaction to brassica hosts and physiological specialization in P. brassicae
The results of three years of in situ tests under field conditions and in vivo tests under controlled conditions in a growth chamber (Bhattacharya Citation2003) showed that there was distinct physiological specialization in P. brassicae in different agro-climatic regions of India. All the popular and socially important cultivars of cauliflower, cabbage and oilseed brassicas belonging to the B. oleracea, B.rapa and B .juncea groups showed high disease severity as expressed as a Per cent Disease Index (PDI). The results from field screening and in vivo growth chamber studies in Kalimpong using field isolates of P. brassicae from rayosak showed that the B. juncea and B. oleracea groups of vegetables including Indian mustard developed high PDIs whereas the B. rapa group of oilseeds and vegetable brassicas are moderately susceptible hosts, including Chinese cabbage ‘Granaat’, which is recognized as universally susceptible. There was a statistically significant difference in the disease reactions between different brassica species, such as B. juncea, B. oleracea and B. rapa including tori sarson and yellow sarson. Das et al. (Citation1987) screened 18 tori sarson cultivars in Odisha state and found all but four to be highly susceptible. The results of screening of brassica hosts in the plains of South Bengal revealed that B. napus ‘Tower’ and ‘Midas’, B. carinata HC-1, HC-4 and HC-5, and line ACCBN-479 of the B. nigra group, were either resistant or highly tolerant to P. brassicae isolates from India (Chattopdhyay et al. Citation1991). ‘Midas’ was tolerant in the first year of the trial but in the following year, very small clubs formed on a few plants. All of the indigenous cultivars of B. juncea, yellow sarson and tori sarson were found to be moderately or highly susceptible to P. brassicae.
The variability in the virulence of P. brassicae populations was observed when a tori sarson cultivar (B-54), described as resistant in Odisha State (Das et al. Citation1987), was found to be highly susceptible in West Bengal State. Similarly, Benoy (B-9), a yellow sarson cultivar, is highly susceptible in the plains of South and North Bengal but the same genotype showed tolerant reactions in the Darjeeling Hills. These findings indicate the possible existence of different pathotypes of P. brassicae in the different agro-climatic zones of West Bengal.
Genetic resistance and development of clubroot-resistant lines
The development of clubroot-resistant brassica lines is difficult in India because of the narrow range of genetic diversity in the brassica hosts and differences in the virulence of P. brassicae populations in different agro-climatic regions of West Bengal (Bhattacharya Citation2003). Furthermore, no resistance sources have been found among the cultivated oilseed and vegetable brassicas, with all being found to be highly susceptible to clubroot disease in West Bengal (Chattopadhyay & Bagchi Citation1989; Chattopdhyay et al. Citation1991; Bhattacharya Citation2003). The cultivars belonging to B. napus, B. carinata and B. nigra have shown either a resistant or tolerant reaction to disease (Chattopdhyay et al. Citation1991). With the objective of screening of local brassica germplasm with local isolates of P. brassicae to produce resistant lines, an initiative was made to collect germplasm from the Darjeeling Hills. Among the local collections, two species (B. juncea ssp. rugosa (Roxby) Prain and Brassica rapa L. ssp. chinensis Olsson) usually grown as leafy vegetable crops in the region, showed variation with respect to different morphological characters and clubroot disease reaction. Interspecific and intraspecific crossing of these brassicas, however, resulted in poor seed setting. Moreover, disease inheritance studies on segregating generations could not be carried out due to infertility problems in the subsequent generations (Bhattacharya Citation2003).
Integrated management of clubroot disease of crucifers in West Bengal
The control of clubroot disease is difficult using conventional methods and practices. Although chemical control of the disease can be achieved in different parts of the world, there is not a single chemical fungicide available in the Indian market for clubroot disease management. Therefore, fungicidal control of clubroot in India has not been attempted. Very recently (2012) however, Perlka™ (calcium cyanamid), a calcium-based neutral fertilizer for clubroot disease management, has been registered in India for marketing. Previously, only a piecemeal approach has been taken with cultural practices and soil amendments. Prolonged survival of the pathogen, soil abiotic stress factors such as soil acidity, deficiencies in macro- and micronutrients, soil physical characters, differences in the pathogenicity of isolates, narrow ranges of genetic diversity in the brassicas and limited germplasm with resistance to the pathogen represent the major constraints for achieving satisfactory integrated management of the disease. Resistance to clubroot, although genetically controlled, may also be affected by environmental factors (Dixon Citation2009b).
Disease management with soil amendments
The treatment of soil with standard doses of fertilizers (N : P : K, 80 : 40 : 40 kg ha−1) along with lime (CaO, calcium oxide, quicklime) applied at 3 t ha−1 increased the soil pH from 5.7 to 7.2 and reduced clubroot disease incidence significantly. In plots of yellow sarson, there was a reduction in disease incidence of 19.6% in treated versus untreated controls. The effect on disease severity was also pronounced, with a reduction in the size and number of clubs on plants in treated plots (Chattopdhyay Citation1991).
The effects on clubroot disease incidence in Indian mustard of calcium borate, calcium carbonate and ammonium molybdate along with a standard dose of fertilizer (N : P : K, 80 : 40 : 40 kg ha−1) applied to highly infested plots (resting spore population of 2 × 107/g soil and pH 5.6 were studied (Sen Citation2005). The results of four consecutive years of trials (1996–2000) showed drastic reductions in the rate of club formation and root/shoot ratio with a combination of calcium, boron and molybdenum and also with only boron + molybdenum treatments. The optimization of yield of mustard and highest yield of 916 kg ha−1 were achieved with a combination of the standard dose of N, P, K fertilizers and calcium + boron + molybdenum. An effect of molybdenum in clubroot disease suppression in coarse sandy soil (pH 5.6) in acidic conditions has been demonstrated (Sen Citation2005).
Disease management with cultural practices
Yellow sarson and flaxseed (Linum usitatissimum L.) cultivated as an inter-crop in clubroot-infested plots reduced clubroot incidence and enhanced yield. Possibly, hydrogen cyanide present in root exudates of the flaxseed (Poulton Citation1990) acts as an inhibitor to resting spore germination and club formation on yellow sarson. Clubroot incidence was drastically reduced (30–50%) in this trial (Sen Citation1993).
Bait cropping with resistant, susceptible and non-brassicas
Reducing resting spore populations or initial levels of inoculum in infested soil by prior cultivation of bait crops, including both resistant and susceptible brassicas and non-brassicas, in order to stimulate resting spore germination before main cultivation of the brassica crop, is one of the effective means of integrated management of clubroot disease (Friberg et al. Citation2006; Bhattacharya & Dixon Citation2010). A field experiment was conducted in highly infested plots (resting spore concentration of 1 × 107/g soil) in Kalimpong in the Darjeeling Hills to determine the effectiveness of different species of brassica and non-brassica species as bait crops on disease severity and yield in cauliflower, cabbage, and local Indian leafy vegetables such as rayosak. The result of two years of field trials indicated that resistant hosts such as radish and toria sarson are the most effective bait crops when cultivated for 30–35 days before main crops like cabbage, cauliflower and rayosak are sown (Bhattacharya & Dixon Citation2010).
Disease management with plant products and biocontrol agents
Bhattacharya and Pramanik (Citation1998) studied the effect of plant products and biocontrol agents on two phases of the life cycle of P. brassicae. Indian neem tree (Azadirachta indica A. Juss.) oil emulsion in water (2% v/v) emulsified with 0.1% Tween 80 (Polyoxyethylene (20) sorbitan monooleate) when applied as a soil drench resulted in maximum inhibition (80%) of root hair infection of yellow sarson during the primary phase of colonization in a laboratory study. Only a very few small clubs were produced following this treatment. Field trials conducted with different formulations of the neem product (granular and suspension) revealed significant reductions in clubroot disease severity relative to the control (Bhattacharya & Pramanik Citation1998). There was little difference found between the effects of different doses and formulations of neem products when applied under field conditions. Inhibition of root hair infection by 10–15% following treatment with the antagonistic rhizobacteria Streptomyces graminifaciens G11 and Pseudomonas fluorescens H237 was observed in an in vitro test. These two antagonists also reduced club formation and disease severity values by 15–20% relative to the control in glasshouse experiments. The effects of antagonist rhizobacteria were less pronounced relative to neem products.
Conclusion and future prospects
Clubroot disease poses a substantial threat to brassica oilseed cultivation in the plains of North and South Bengal and to cole vegetables in the Darjeeling Hills of the Eastern Himalayan region. Short-duration toria sarson oilseeds (75–80 days), medium-duration yellow sarson (90 days), and long-duration Indian mustard (120–140 days) are the main oilseed brassica crops cultivated in West Bengal. Crop losses from clubroot disease may reach up to 100% and farmers have had to stop the cultivation of rape and mustard in heavily infested areas. The most popular and widely cultivated yellow sarson cultivar ‘Binoy’ (B-9) has been found to be highly susceptible. The genetic variability of existing brassica germplasm in India is very narrow. Moreover, all of the indigenous and commercial oilseed and vegetable brassicas are highly susceptible to P. brassicae. Exotic B. napus, B. carinata and B. nigra germplasm has already been shown to be resistant or tolerant to Indian isolates of P. brassicae (Chattopdhyay et al. Citation1991).
Possible sources of resistance may be found in primary secondary and tertiary gene pools of brassicas including both indigenous and exotic germplasm. Sources of race-specific to broad-spectrum resistance can be exploited from the diploid species B. rapa (AA), B. nigra (BB) and B. oleracea (CC), and the amphidiploids B. napus (AACC), B. juncea (AABB) and B. carinata (BBCC), and also through the interspecific and intergeneric hybridization of brassicas and genera belonging to Raphanus. Broadening the genetic basis of clubroot resistance by pyramiding different clubroot resistance genes into a single line will be an indispensable means to increasing the durability of resistance in existing indigenous popular varieties against a wide range of physiological races as well as to stop the outbreak of new races.
Brassica crops are cultivated at high altitudes in the Darjeeling Hills of the eastern Himalayan region and also in the Himalayan foothills and plains of West Bengal under different agro-climatic regions. Variability in the host and pathogen populations and in soil physical, chemical and biological characters is the main determinant of clubroot disease severity. There is a need to develop new methodologies for the detection and estimation of inoculum and survivability of P. brassicae that will help in devising better and more effective management of this pathogen. Research to develop molecular methods for the proper diagnosis and quantification of P. brassicae populations in the soil is needed. A Decision Support System can be developed, using soil physical, chemical and biological indicators, for the farmers of different agro-climatic zones of West Bengal, for efficient management of clubroot disease. Geographic information and positioning systems-based geopathological mapping of clubroot-infested areas, combined with information on soil physical, chemical and biotic stress factors, including the presence of antagonist microbes, will be useful in predicting disease severity in the various agro-climatic zones of West Bengal. A prediction model based on inoculum load, soil and environmental stress factors could be the basis for a decision support system to develop integrated management strategies for clubroot in West Bengal.
Acknowledgements
We gratefully acknowledge the financial support extended by the Indian Council of Agricultural Research (I.C.A.R.), New Delhi, in the form of an ad hoc project ‘Studies on race variation and characterization of Plasmodiophora brassicae causing Clubroot Disease of Crucifers in West Bengal’ (1998–2003).
References
- Baskey S. 2013. Screening of Brassica hosts against Plasmodiophora brassicae causing club root disease of crucifers to identify sources of resistance [dissertation]. Bidhan Chandra Krishi Viswavidyalaya; , Mohanpur, Nadia, West Bengal India.
- Bhattacharya I. 2003. Study of race variation and characterization of Plasmodiophora brassicae causing clubroot disease of crucifers in West Bengal (India) Mohanpur, Bidhan Chandra Krishi Viswavidyalaya, Final Report ICAR (Indian Council of Agricultural Research) Adhoc Project; . New Delhi, India.
- Bhattacharya I, Dixon GR. 2010. Management of clubroot disease (Plasmodiophora brassicae) of Brassicas using trap cropping techniques. In: Hansen M, editor. Proceedings of the 5th ISHS International Symposium on Brassicas and 16th Crucifer Genetics Workshop Acta. Hort.; , 867:157–164.
- Bhattacharya I, Pramanik M. 1998. Effect of different antagonist rhizobacteria and neem products on clubroot of crucifers. Indian Phytopathol. 51:87–90.
- Chattopadhyay AK, Bagchi BN. 1989. Occurrence of club root disease on rapeseed mustard in West Bengal. Indian J Mycol Res. 27:83–88.
- Chattopadhyay SB, Sengupta SK. 1952. Addition to the fungi of Bengal. Bull Soc Bengal (India). 16:2–6.
- Chattopdhyay AK. 1991. Studies on the control of clubroot disease of rapeseed mustard in West Bengal. Indian Phytopathol. 44:397–398.
- Chattopdhyay AK, Bagchi BN, Roychoudhury UK. 1991. Reactions of some cultures of rapeseed mustard against clubroot disease. Indian Phytopathol. 44:238–239.
- Das SN, Mishra SK, Swain PK. 1987. Reactions of some toria varieties to Plasmodiophora brassicae. Indian Phytopathol. 40:120.
- Dixon GR. 2009a. Plamodiophora brassicae in its environment. J. Plant Growth Regul. 28:212–228.
- Dixon GR. 2009b. The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. J Plant Growth Regul. 28:194–202.
- Friberg HJ, Lagerlöf J, Rämert B. 2006. Usefulness of nonhost plants in managing Plasmodiophora brassicae. Plant Pathol. 55:690–695.
- Laha JN, Naskar I, Sharma BD. 1985. A new record of clubroot disease of mustard. Curr Sci. 54:1247.
- McRae W. 1928. Report of the Imperial Mycologist Scientific Reports of the Agricultural Research Institute; , Pusa, 1927–1928 (pp. 56–70).
- Poulton JE. 1990. Cyanogenesis in plants. Plant Physiol. 94:401–405.
- Sen P. 1993. Control of clubroot disease of rape and mustard attained by intercropping. Indian Agric. 37:127–128.
- Sen P. 2005. Antagonistic effect of Ca, B and Mo on clubroot disease of rape and mustard. Indian Agric. 49:13–16.
- Sharangapani SG. 1930. Appendix Annual Reports of Economic Botanist to the Government of Bengal for the year 1929–30 Annual Report of Department of Agriculture; , Bengal (pp. 37–46).
- Timila RD, Correll JC, Duwadi VR. 2008. Severe and widespread clubroot epidemic in Nepal. Plant Dis. 92:312–317.
