Abstract
Abstract: SYP-7017, a broad-spectrum fungicide, belongs to the chemical group of strobilurins. Baseline sensitivity of Sclerotinia sclerotiorum (Lib) de Bary to SYP-7017 was determined using 120 strains collected during 2010 and 2011 from rapeseed fields without a previous history of strobilurin usage in Jiangsu Province, China. The median effective concentration (EC50) values for SYP-7017 that inhibited mycelial growth ranged from 0.006 to 0.047 µg mL−1 (mean of 0.016 µg mL−1). No cross-resistance between SYP-7017 and carbendazim or iprodione was detected. On detached rapeseed leaves, SYP-7017 at 100 µg mL−1 provided over 90% control efficacy. Moreover, SYP-7017 exhibited good characteristics of absorption and translocation, and also exhibited excellent protective activity in pot experiments. In field trials, control efficacies of SYP-7017 at 75 g a.i. ha−1 and 187.5 g a.i. ha−1 were 80.2% and 91.58%, respectively, higher than other traditional fungicides. These results suggest that SYP-7017 has strong antifungal activity and a potential application in controlling S. sclerotiorum.
Résumé
Le SYP-7017, un fongicide à large spectre, appartient au groupe des strobilurines. La réceptivité de base de Sclerotinia sclerotiorum (Lib.) de Bary au SYP-7017 a été déterminée à l’aide de 120 souches collectées en 2010 et 2011 dans des champs de colza de la province du Jiangsu, en Chine, dans lesquels les strobilurines n’avaient jamais été utilisées. Les valeurs de la concentration létale médiane (CL50) du SYP-7017 qui inhibaient la croissance mycélienne variaient de 0.006 à 0.047 µg mL−1 (moyenne de 0,016 µg mL−1). Aucune résistance croisée entre le SYP-7017 et le carbendazime ou l’iprodione n’a été détectée. Sur des feuilles détachées de colza, le SYP-7017, utilisé à une concentration de 100 µg mL−1, a affiché un taux d’efficacité de plus de 90%. En outre, le SYP-7017 a présenté de bonnes caractéristiques d’absorption et de translocation, et ce, en plus d’assurer une excellente protection au cours d’expériences en pots. Au cours d’essais en champ, le taux d’efficacité du SYP-7017 à 75 g i. a. ha−1 et 187,5 g i. a. ha−1 était de 80.2% et de 91.58 %, respectivement, ce qui est plus élevé que ce qu’offrent les autres fongicides traditionnels. Ces résultats suggèrent que le SYP-7017 possède une puissante action antifongique et une capacité à maîtriser S. sclerotiorum.
Keywords:
Introduction
Sclerotinia sclerotiorum (Lib.) de Bary, a ubiquitous plant pathogenic fungus, can infect more than 400 species of cultivated plants worldwide, such as sunflower, carrot, lettuce, soybean, canola and oilseed rape (Brassica napus L.) (Wang et al. Citation2009). Leaves, stems and pods at different developmental phases can be infected (Kuang et al. Citation2011). In many countries, such as Canada, USA, Australia and China, the disease caused by S. sclerotiorum is destructive (Bardin & Huang Citation2001; Bolton et al. Citation2006; Kuang et al. Citation2011). For example, on the coast of Jiangsu province of China, Sclerotinia stem rot caused by S. sclerotiorum can reduce yield ranging from 10% to 80%, and oil quality declines as well.
With expanding agricultural production, the rapeseed industry in China occupies over 7.5 million hectares at present, producing an annual crop valued at nearly $5 billion. However, Sclerotinia stem rot caused by S. sclerotiorum has been a long-term threat to this industry. In practice, application of foliar fungicides is still the main method in most oilseed rape crops for controlling the disease. A benzimidazole fungicide, carbendazim (MBC), which has been used for control of Sclerotinia stem rot in China for more than 30 years, was demonstrated to be ineffective (Kuang et al. Citation2011). A point mutation in the β-tubulin gene (E198A) in resistant isolates of S. sclerotiorum was identified as the resistance mechanism of the fungus to benzimidazole fungicides in the field (Li et al. Citation2003). Therefore, alternative chemicals such as dicarboximide fungicides (e.g. dimethachlon, iprodione, procymidone and vinclozolin) have been used as a substitute to control diseases caused by S. sclerotiorum (Ma et al. Citation2009), Sclerotinia homoeocarpa F. T. Bennett and Sclerotinia minor Jagger (Smith et al. Citation1995; Jo et al. Citation2006). However, due to repeated applications of dicarboximide fungicides, resistant strains of S. sclerotiorum (Ma et al. Citation2009), Sclerotinia homoeocarpa (Jo et al. Citation2006), and Alternaria spp. (Ma & Michailides Citation2004) have been detected recently. Therefore, in order to achieve and optimize disease control and minimize the risk of resistance development, the preferred strategy is to find new fungicides with contrasting modes of action.
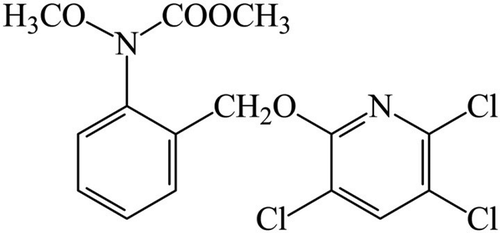
SYP-7017 (N-methoxy – N-[2 – [[(3, 5, 6 – trichloro-pyridine-2 – yl) oxy] methyl] phenyl] carbamate) () is a new fungicide belonging to the strobilurin group which was discovered and patented by the Shengyang Study Institute of Chemical Industry (Shenyang, China). This fungicide has been registered recently in China for the control of Puccinia recondite f. sp. tritici, Uncinula necator (Schw.) Burr., and Fabraea maculate (Lev.) Atk. (Gisi et al. Citation2002). However, its application for control of Sclerotinia stem rot has not been reported. Therefore, the present study was designed to: (i) establish the baseline sensitivity (sensitivity before exposure to the fungicide) to SYP-7017 in S. sclerotiorum populations from different oilseed rape fields in Jiangsu Province of China; (ii) determine cross-resistance patterns between SYP-7017 and other groups of fungicides; and (iii) test the efficacy of SYP-7017 for controlling Sclerotinia stem rot of rapeseed in pot and field experiments.
Materials and methods
Fungicides and medium
Technical grade carbendazim (MBC, 98%) and SYP-7017 (95%), provided by Shengyang Study Institute of Chemical Industry, were dissolved in 0.1 mol L−1 (0.1 N) hydrochloric acid (HCl) and methanol, respectively, to make a stock solution of 10 mg L−1. Iprodione (96.2%) was provided by Wenzhou Pesticide Factory (Zhejiang, China). It was dissolved in methanol (>99.5%) to 10 mg mL−1 for the stock solutions, which was stored at 4 oC in the dark. The stock solutions were added to autoclaved media when they cooled to approximately 50 °C. MBC (50% WP), iprodione (50%WP) and SYP-7017 (20% EC) were purchased in Nanjing.
Potato dextrose agar (PDA) was prepared with 200 g potato, 20 g agar and 20 g dextrose per litre of distilled water. Alkyl ester agar (AEA) medium was made from 5 g yeast extract, 6 g NaNO3, 1.5 g KH2PO4, 0.5 g KCl, 0.25 g MgSO4, 20 mL glycerin and 20 g agar in 1 L distilled water.
Collection of isolates
Isolates of S. sclerotiorum used in the study were collected from 15 oilseed rape fields in Jiangsu Province of China during 2010 and 2011. Mature sclerotia collected from infected stems of rapeseed plants were sterilized in 75% ethanol for 1 min and rinsed three times in sterilized water. Surface-sterilized sclerotia were then cut into two pieces and placed on PDA plates amended with 0.5 g L−1 streptomycin. After incubation of the plates at 25 °C for 3 days, a mycelial tip from each colony was transferred into a fresh PDA plate (Wang et al. Citation2009). Pure cultures were obtained by transfer of a single sclerotium and then maintained on PDA slants at 4 °C.
Baseline sensitivity to SYP-7017 in vitro
The effect of SYP-7017 on mycelial growth (Li & Si Citation2011) was determined in vitro by transferring mycelial plugs (5 mm in diameter) from the leading edge of an actively growing colony to a series of AEA plates containing 0.008, 0.016, 0.03, 0.063 or 0.125 μg mL−1 SYP-7017. Plates without SYP-7017 were used as a control. Additionally, in order to inhibit the alternative respiratory pathway, salicylhydroxamic acid (SHAM) (Sigma–Aldrich, Saint Louis, MO) at a concentration of 50 µg mL−1 was also added into the AEA plates amended with SYP-7017 including control (Duan et al. Citation2012). The plates were incubated at 25 °C for 2 days in a growth incubator. For each plate, the EC50 values were calculated by measuring the average colony diameter in two perpendicular directions. In the present study, the EC50 values were calculated by regressing percentage growth inhibition against the log of fungicide concentration (Kuang et al. Citation2011). Each concentration has three replicates and the test was repeated twice.
Analysis of cross-resistance
Five field isolates (chosen arbitrarily) and five iprodione-resistant isolates (induced and stored in our lab) were used to determine the cross-resistance according to previous studies (Wang et al. Citation2009; Kuang et al. Citation2011). Fresh mycelial plugs (5 mm in diameter) were transferred from the margins of the colonies onto PDA or AEA plates containing a series of concentrations of carbendazim, iprodione or SYP-7017. Each concentration has three replicates and the experiment was performed three times. After 2 days of incubation at 25 C in a growth incubator, two perpendicular diameters of each fungal colony were measured and averaged (the diameter of the plug was subtracted). EC50 values were determined as described above.
Efficacy and translocation of SYP-7017 on rapeseed leaves
Fully expanded oilseed rape leaves of rapeseed plants ‘Suyou 1’ (Jiangsu Fengqing Seed Science Company) were detached and soaked for 15 s in water as control, SYP-7017 (20% EC) at 25, 50, 100 μg mL−1, or iprodione (50% WP) at 100 μg mL−1. After naturally drying the excess water (in the air), each leaf was wounded, avoiding the midrib, with a sterilized needle and inoculated with a 5-mm mycelial plug taken from the edge of a 3-day-old colony of isolate JK19 which was arbitrarily selected. Inoculated leaves were maintained on moist filter paper in a growth chamber (25 C, 12 h photoperiod and 80% relative humidity). After 3 days, lesions were measured and the lengths of the long and short axes were averaged, and the control efficacy was calculated by [(lesion diameter in the water control − lesion diameter in the treatment)/(lesion diameter in the water control − 0.5)] × 100 (Kuang et al. Citation2011). There were six leaves for each treatment and the experiment was repeated three times.
A pot experiment was conducted to study the translocation of SYP-7017 in rapeseed leaves. The leaves of rapeseed plants ‘Suyou 1’ were daubed using a brush with water, or SYP-7017 at 300 µg mL−1 on the middle of the leaf midrib (about 1 cm2). One day later, three inverted mycelial plugs (5 mm in diameter) were placed on the tip, middle, and bottom of each wounded leaf on the same side of the leaf midrib (avoiding a major vein). The plants were placed in a growth chamber at the same conditions as described above. After 3 days, rapeseed leaves were detached from the plants and then photographed. The experiment was performed three times with six leaves per treatment.
Protective and curative activity of SYP-7017 in pot experiments
Protective and curative activity of SYP-7017 against Sclerotinia stem rot was tested according to a previous study (Duan et al. Citation2013). Mycelial plugs (5-mm diameter) were taken from the edge of a 3-day-old colony of the arbitrarily selected isolate JK19. For protective activity, leaves of rapeseed plants ‘Suyou 1’ of similar growth stage were wounded, avoiding the midrib, with a sterilized needle and sprayed with water, iprodione at 100 µg mL−1, or SYP-7017 at 25 µg mL−1, 50 µg mL−1 or 100 µg mL−1 1 day and 3 days before inoculation, respectively. For curative activity, wounded leaves were sprayed as above 1 day and 3 days after inoculation, respectively. The inoculated plants were kept at 23 °C with 85% humidity and 12-h photoperiod. Then the lesion diameter was measured and the control efficacy was calculated as described above. Three leaves per treatment were used and the experiment was conducted three times.
Control of Sclerotinia stem rot by SYP-7017 in field experiments
The same oilseed rape field naturally infested with S. sclerotiorum in Taizhou of Jiangsu Province was selected in 2011 and 2012 for field experiments. The field was divided into 24 plots measuring 5 × 6 m2 each. Six treatments with four replicates each were arranged in a randomized complete block design. The treatments were: (1) water control, 750 L ha−1; (2) carbendazim (50% WP), 375 g a.i. ha−1; (3) iprodione (50% WP), 375 g a.i. ha−1; (4) SYP-7017, 75 g a.i. ha−1; (5) SYP-7017, 187.5 g a.i. ha−1; and (6) SYP-7017, 375 g a.i. ha−1. Fungicides were applied twice as foliar sprays with one week between sprays with a Jacto Heavy-Duty HD400 sprayer (Agrolex, Singapore) when 90% of the major stems were flowering. No other fungicides were applied and the treatments were given in accordance with standard farm practices. Disease incidence was surveyed about 1 week before harvest, by examining 120 randomly selected plants in each plot for the symptoms of rapeseed stem rot. Disease severity was assessed according to previous studies (Wang et al. Citation2009; Kuang et al. Citation2011).
Statistical analysis
Data from repeated experiments were combined for analysis because variances between experiments were homogeneous. Statistical analysis was performed using SPSS 14.0 (SPSS Inc., Chicago, IL) according to previous studies (Kuang et al. Citation2011; Duan et al. Citation2013). The EC50 of the isolates was estimated by linear regression of the log of the colony diameter versus the fungicide concentration. The ANOVA procedure of SPSS and Fisher’s LSD (P < 0.05) were used to determine significant differences on the EC50 values.
Results and discussion
In this study, we established the baseline sensitivity of S. sclerotiorum to SYP-7017. The frequency distribution of the EC50 values of 120 isolates was a unimodal curve (), ranging from 0.006 to 0.047 µg mL−1 (mean 0.016 µg mL−1). All isolates were sensitive to SYP-7017 and there was no resistant subpopulation among the isolates used in the present study. As expected, there was no cross-resistance between SYP-7017 and other commonly used fungicides (), which could be explained by same or different mode of actions (Leroux et al. Citation1999).
Fig. 2 Frequency distribution of SYP-7017 EC50 values for 120 isolates of Sclerotinia sclerotiorum collected during 2010 and 2011.
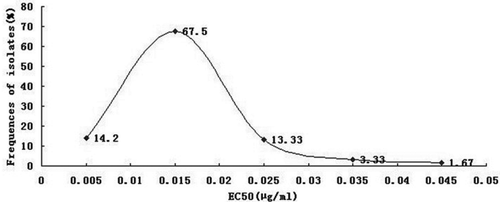
Table 1. Analysis of cross-resistance of isolates of S. sclerotiorum from rapeseed plants.
On detached rapeseed leaves, SYP-7017 exhibited excellent antifungal activity and had better control efficacy than iprodione (). When the fungicide was applied 1 day and 3 days before inoculations, the control efficacy of SYP-7017 at each treatment was higher compared with fungicide applied after inoculation, respectively, indicating that protective activity of SYP-7017 was better than curative activity (). Compared with the control (a), there was no disease lesion visible on the tip, middle and bottom when SYP-7017 was daubed on the bottom of the rapeseed leaves (d). When SYP-7017 was daubed on the middle of the rapeseed leaves, no disease lesion was visible on the tip and middle (c) and no disease lesion was visible on the tip when SYP-7017 was daubed on the tip of the rapeseed leaves (b). The data of translocation imply that transport direction of SYP-7017 in rapeseed leaves is from base to tip, manifesting that SYP-7017 exhibited good characteristics of absorption and translocation, which could be useful guidelines for field application.
Fig. 3 (Colour online) Translocation of SYP-7017 in rapeseed leaves. ∆ a was treated with water; b, c and d were daubed with SYP-7017 at 300 µg mL−1. → Means the area daubed with fungicide.
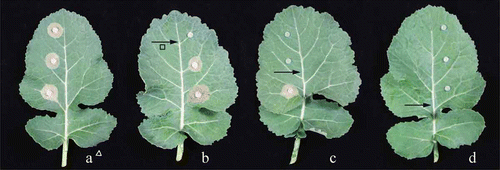
Table 2. Efficacy of SYP-7017 in controlling S. sclerotiorum isolate JK19 on detached rapeseed leaves.
Table 3. Protective and curative activity of SYP-7017 in pot experiments.
In the field experiment, because of dry weather which was unfavourable for disease development, the symptoms of rapeseed stem rot were not severe and the disease incidence for control plots was only 16.87%. The data showed that SYP-7017 exhibited better efficacy than iprodione; MBC recorded minimum disease control of 37.05% compared with other treatments (). The control efficacy of SYP-7017 (EC 20%) at 375 g a.i. ha−1 could be up to 100%, which was even better than that by boscalid or fludioxonil in previous studies (Wang et al. Citation2009; Kuang et al. Citation2011). Moreover, SYP-7017 has been found highly effective in controlling diseases caused by Botrytis cinerea Pers. and Magnaporthe oryzae (He-bert) Barr. (Li & Si Citation2011). Considering the excellent control efficacy of Sclerotinia stem rot and no cross-resistance with other fungicides, SYP-7017 could be used in integrated disease management programmes.
Table 4. The average control efficacy of rapeseed stem rot by SYP-7017 in the field in 2011 and 2012.
Previous studies reported that the resistance mechanism of strobilurin fungicides is mainly amino acid residue single-point substitution of the cytochrome b in fungi and the substitution in the field resistance was G143A, F129L or G137R, respectively (Ishii et al. Citation2001; Torriani et al. Citation2009). Although resistance to strobilurin fungicides has been reported in several fungi, such as Sphaerotheca fuliginea (Schlecht.:Fr.) Pollacci, Pyrenophora teres f. teres, and Plasmopara viticola (de Bary) Berl. & de Toni., no resistance has been detected in P. recondite f. sp. tritici, U. necator (Schw.) Burr. and S. sclerotiorum (Lib.) de Bary (Amand et al. Citation2003; Fraaije et al. Citation2003; Torriani et al. Citation2009). Moreover, our efforts to induce SYP-7017-resistant mutants of S. sclerotiorum were unsuccessful in this research, which indicated that this fungus might not develop resistance easily to SYP-7017.
Because limited gene sources hampered the breeding for resistance to Sclerotinia stem rot (Lu Citation2003), the disease is still a threat to oilseed rape production worldwide. The best method for controlling of benzimidazole and dicarboximide resistance depends on reducing selection pressure by limiting exposure to fungicides with the same or similar mechanism of action, and exploring new fungicides with different biochemical mechanisms of action. The current study demonstrates that SYP-7017 has strong antifungal activity on inhibiting mycelial growth of S. sclerotiorum both in vitro and in vivo, and has no cross-resistance with other fungicides. SYP-7017 has a potential application for managing Sclerotinia stem rot caused by the resistant population to benzimidazole and dicarboximide fungicides.
Acknowledgements
This study is sponsored by the Science and Technology Support Programs from Jiangsu Province and the Ministry of Science and Technology (No. 201103016 and 201303023).
References
- Amand O, Calay F, Coquillart L, Legat T, Bodson B, Moreau JM, Maraite H. 2003. First detection of resistance to QoI fungicides in Mycosphaerella graminicola on winter wheat in Belgium. Commun Agric Appl Biol Sci. 68:519–531.
- Bardin SD, Huang HC. 2001. Research on biology and control of Sclerotinia diseases in Canada1. Can J Plant Pathol. 23:88–98. doi:10.1080/07060660109506914
- Bolton MD., Thomma BPH J, Nelson, BD. 2006. Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol. 7:1–16. doi:10.1111/j.1364-3703.2005.00316.x
- Duan YB, Ge CY, Liu SM, Chen CJ, Zhou MG. 2013. Effect of phenylpyrrole fungicide fludioxonil on morphological and physiological characteristics of Sclerotinia sclerotiorum. Pestic Biochem Physiol. 106:61–67. doi:10.1016/j.pestbp.2013.04.004
- Duan YB, Liu SM, Ge CY, Feng XJ, Chen CJ, Zhou MG. 2012. In vitro inhibition of Sclerotinia sclerotiorum by mixtures of azoxystrobin, SHAM, and thiram. Pestic Biochem Physiol. 103:101–107. doi:10.1016/j.pestbp.2012.04.004
- Fraaije BA, Lucas JA, Clark WS, Burnett FJ. 2003. QoI resistance development in populations of cereal pathogens in the UK. In: Proceedings of the BCPC International Congress, 2003 10–12 November. The British Crop Protection Council, Alton, Hampshire, UK. Crop Science Technol. 2:689–694.
- Gisi U, Sierotzki H, Cook A, McCaffery A. 2002. Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides. Pest Manag Sci. 58:859–867. doi:10.1002/ps.565
- Ishii H, Fraaije BA, Sugiyama T, Noguchi K, Nishimura K, Takeda T, Amano T, Hollomon DW. 2001. Occurrence and molecular characterization of strobilurin resistance in cucumber powdery mildew and downy mildew. Phytopathology. 91:1166–1171. doi:10.1094/PHYTO.2001.91.12.1166
- Jo YK, Niver AL, Rimelspach JW, Boehm MJ. 2006. Fungicide sensitivity of Sclerotinia homoeocarpa from golf courses in Ohio. Plant Dis. 90:807–813. doi:10.1094/PD-90-0807
- Kuang J, Hou YP, Wang JX, Zhou MG. 2011. Sensitivity of Sclerotinia sclerotiorum to fludioxonil: in vitro determination of baseline sensitivity and resistance risk. Crop Prot. 30:876–882. doi:10.1016/j.cropro.2011.02.029
- Leroux P, Chapeland F, Desbrosses D, Gredt M. 1999. Patterns of cross-resistance to fungicides in Botryotinia fuckeliana (Botrytis cinerea) isolates from French vineyards. Crop Prot. 18:687–697. doi:10.1016/S0261-2194(99)00074-5
- Li HX, Lu YJ, Zhou MG, Wang XF. 2003. Mutation in β-tubulin of Sclerotinia sclerotiorum conferring resistance to carbendazim in rapeseed field isolate. Chin J Oil Crop Sci. 25:56–60.
- Li KK, Si NG. 2011. Application technology of the new fungicide: SYP-7017. Pesticides. 7:48–49.
- Lu G. 2003. Engineering Sclerotinia sclerotiorum resistance in oilseed crops. Afr J Biotechnol. 2:509–516.
- Ma HX, Feng XJ, Chen Y, Chen CJ, Zhou MG. 2009. Occurrence and characterization of dimethachlon insensitivity in Sclerotinia sclerotiorum in Jiangsu Province of China. Plant Dis. 93:36–42. doi:10.1094/PDIS-93-1-0036
- Ma Z, Michailides T.J.. 2004. Characterization of iprodione-resistant Alternaria isolates from pistachio in California. Pestic Biochem Physiol. 80:75–84. doi:10.1016/j.pestbp.2004.06.007
- Smith FD, Phipps PM, Stipes RJ, Brenneman TB. 1995. Significance of insensitivity of Sclerotinia minor to iprodione in control of Sclerotinia blight of peanut. Plant Dis. 79:517–523. doi:10.1094/PD-79-0517
- Torriani SFF, Brunner PC, McDonald BA, Sierotzki H. 2009. QoI resistance emerged independently at least 4 times in European populations of Mycosphaerella graminicola. Pest Manag Sci. 65:155–162. doi:10.1002/ps.1662
- Wang JX, Ma HX, Chen Y, Zhu X, Yu W, Tang Z, Chen C, Zhou M. 2009. Sensitivity of Sclerotinia sclerotiorum from oilseed crops to boscalid in Jiangsu Province of China. Crop Prot. 28:882–886. doi:10.1016/j.cropro.2009.06.012