Abstract
Narrow-leaved lupin has the potential to become an important pulse crop for the Canadian Prairies because of its high protein content and its climatic adaptation. However, cultivation in the region is constrained by seedling blight and root rot caused by Fusarium avenaceum, an endemic fungal pathogen that reduces seedling establishment and crop yield. This pathogen attacks a broad range of crop and non-crop plants in the region. It varies in colony morphology, in the aggressiveness of individual isolates toward different crops, and in genetic makeup. However, genetically and phenotypically similar isolates can occur over a broad geographic range and diverse isolates can be found in the same field. Despite this diversity, the sexual stage has been observed only rarely in nature. The deleterious effects of the pathogen can be mitigated by optimizing seeding depth. Temperatures that are near optimum for plant growth also reduced the effect of the pathogen, but few studies showed a consistent relationship between seeding date and damage by the pathogen. Fungicidal seed treatments have been shown to reduce the incidence of seedling blight. Although there is some variation in resistance to the pathogen, no completely resistant cultivars have been found under western Canadian conditions. Development of management strategies to mitigate the effects of fusarium seedling blight and root rot on lupin crops will make cultivation of this crop a much more attractive option for western Canadian producers.
Résumé
Le lupin à feuilles étroites pourrait devenir une légumineuse importante à cultiver sur les Praires canadiennes à cause de sa forte teneur en protéines et de sa facilité d’acclimatation. Toutefois, dans cette région, sa culture est limitée par la fonte des semis et le pourridié causés par Fusarium avenaceum, un agent pathogène fongique endémique qui réduit le taux d’établissement des semis et le rendement de la culture. Cet agent pathogène s’attaque à une grande variété de plantes cultivées et sauvages de la région. Il varie sur le plan de la morphologie de la colonie, sur celui de l’agressivité des isolats individuels à l’égard de différentes cultures ainsi que sur le plan de la constitution génétique. Toutefois, il est possible de trouver des isolats semblables génétiquement et phénotypiquement sur une vaste aire de répartition géographique, et d’autres, différents, peuvent partager un même champ. Malgré cette diversité, la phase de reproduction sexuée n’a été que rarement observée dans la nature. Les effets nuisibles de l’agent pathogène peuvent être atténués en optimisant la profondeur des semis. Les températures optimales pour la croissance des plantes tendent également à inhiber l’action de l’agent pathogène, mais peu d’études montrent une relation constante entre la date des semis et les dommages causés par l’agent pathogène. On a montré que les traitements fongiques des semences ont engendré une réduction de l’incidence de la fonte des semis. Bien qu’il y existe une certaine variation en ce qui a trait à la résistance à l’agent pathogène, aucun cultivar entièrement résistant correspondant aux conditions prévalant sur les Prairies canadiennes n’a été trouvé. Le développement de stratégies de gestion visant à atténuer les effets de la fonte des semis et du pourridié causés par le Fusarium chez le lupin fera de cette culture une option beaucoup plus attrayante pour les producteurs de l’Ouest canadien.
Introduction
Narrow-leaved lupin (Lupinus angustifolius L.) is a legume crop species that is native to the Iberian Peninsula. The crop was introduced to northern Europe in the mid-19th century (Oldershaw Citation1920; Gladstones Citation1998) and cultivation spread worldwide after the First World War (Hondelmann Citation1984; Gladstones Citation1998). However, L. angustifolius did not become fully domesticated until the 1960s, when cultivars were developed with resistance to shattering and low alkaloid content in the seed (Cowling et al. Citation1998). At present, narrow-leaved lupin (, b) is grown for seed in many parts of the world, especially Western Australia and Europe, and may have potential as an alternative pulse crop for central Alberta, Canada. Lupin produces a high protein grain that is desirable as a livestock feed (Cox Citation1998). It can also serve as a green manure crop, providing a substantial benefit to subsequent crops, at least partly because of its ability to fix atmospheric nitrogen (Evans et al. Citation1989; Unkovitch et al. Citation1994; Vellasamy et al. Citation2000; Helgadóttir et al. Citation2004; Jensen et al. Citation2004). In addition, the deep roots of lupin plants loosen the subsoil, which can increase water availability for subsequent crops (Henderson Citation1989). Given these benefits and its adaptation to the regional climate, narrow-leaved lupin has the potential to become an important high-protein pulse crop for the Canadian Prairies. The Parkland regions (black soil zone) of Alberta, Saskatchewan and Manitoba have the most suitable climatic conditions for lupin production on the Canadian Prairies (Boles et al. Citation2003).
Fig. 1 (Colour online) Healthy and diseased lupin plants. (A) Healthy plants at flowering stage. (B) Mature podding stage. (C) Fusarium root rot causing yellowing and stunting symptoms on adult lupin plants. (D) Defoliation of a lupin plant as a result of fusarium root rot, as seen at the later growth stages. (E) Discolouration of the root and masses of spores on the root surface of infected plants.
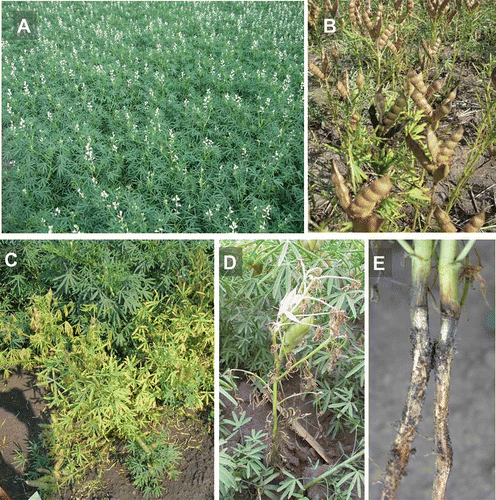
Notwithstanding the aforementioned advantages, however, there are a number of limitations to lupin production. Poor stand establishment has been reported in Alberta (Chang et al. Citation2005a, Citation2005b), where lupin seedlings are vulnerable to seedling blight and root rot (Wollenweber & Reinking Citation1935; Weimer Citation1944; Nowicki Citation1995; Chang et al. Citation2006; Holtz et al. Citation2011). The symptoms of root rot include pre-emergence damping-off and post-emergence collapse of seedlings, yellowing, stunting and wilting of the plants and girdling of the upper tap root (, d, e) (Bateman Citation1997; Chang et al. Citation2005b). Ultimately, the adult plants may also be stunted and wilted, and yield may be substantially reduced. While various soilborne fungi have been implicated in lupin seedling blight and root rot, studies from western Canada indicate that Fusarium avenaceum (Fr.) Sacc. (teleomorph: Gibberella avenacea Cook) is the predominant pathogen of lupin in this region (Chang et al. Citation2005b, Citation2006; Holtz et al. Citation2007). This fungal pathogen is a soil-dwelling, filamentous ascomycete endemic to the Prairie Provinces (Holtz Citation2008). It causes root rot and seedling blight on all of the major field crops grown on the Canadian prairies, including canola (Brassica napus L.), cereals and pulse crops. The pathogen can be disseminated between fields on infected seed (Nowicki Citation1995) and in infested soil and plant debris, by rain splash over short distances (Jenkinson & Parry Citation1994) and in air currents over long distances (Martin Citation1988). Moreover, F. avenaceum can persist in the soil for long periods in the absence of a susceptible host (Chang et al. Citation2011).
This review summarizes recent progress aimed at understanding the biology and impact of F. avenaceum on lupin on the Canadian prairies. The host range, genetic diversity, mating type composition and aggressiveness of the pathogen are discussed. In addition, possible management strategies for lupin root rot, including genetic resistance, fungicidal seed treatments and cultural practices such as seeding date, seeding depth and seeding rate selection are also assessed.
Host range
The host range of F. avenaceum is broad and includes more than 160 host genera (Booth Citation1971). Even individual isolates can exhibit broad host ranges, with the ability to infect plants from various families (Schneider Citation1958; Satyaprasad et al. Citation1997). In addition to infecting lupin, F. avenaceum also causes seedling blight and root rot of lentil (Lens culinaris L.) (Hwang et al. Citation1994, Citation2000) and clover (Melilotus officinalis L. Pall.) (Cormack Citation1937; Leslie & Summerell Citation2006), ear rot of corn (Zea mays L.) and head blight of cereals (Parry et al. Citation1995; Desjardins Citation2003; Yli-Mattila et al. Citation2004) and dry rot of potato (Satyaprasad et al. Citation1997) on the Canadian prairies. One study found that Canadian isolates of F. avenaceum caused root rot in alfalfa (Medicago sativa L.), bird’s-foot trefoil (Lotus corniculatus L.), canola (Brassica napus L.; B. rapa L.), sweet clover (Melilotus officinalis L.), faba bean (Vicia faba L.), flax (Linum usitatissimum L.) and in one cultivar of chickpea (Cicer arietinum L., kabuli type), but did not induce root rot in any cereal grain, bean, soybean (Glycine max L.) or field pea (Pisum sativum L.) (Holtz et al. Citation2007). In contrast, other studies have found that F. avenaceum caused severe root rot in cereal crops (Schneider Citation1958; Celetti et al. Citation1990), but generally did not cause severe root rot in canola (B. napus L.) or flax (L. usitatissimum) (Fernandez Citation2007).
Prevalence and pathogenicity of F. avenaceum
Fusarium avenaceum is often one of the main Fusarium species found in agricultural soils, but is also a common soil fungus in non-agricultural soils (Kommedahl et al. Citation1988; Hestbjerg et al. Citation1999). The fungus can survive saprophytically and also as a plant parasite. As a saprophyte, F. avenaceum survives on crop debris within the soil and on the soil surface (Hudson Citation1968; Hestbjerg et al. Citation1999), and may occur commonly alongside other important root pathogens (Summerell et al. Citation2003). In a survey of lupin fields conducted in Alberta in 2005 and 2006, F. avenaceum was recovered from 36% of the plants collected. It was the predominant microorganism identified at each location in both years and was also the only microorganism that was collected from every site each year (Holtz et al. Citation2011). When these isolates were inoculated onto lupin seedlings, 51% could consistently incite symptoms of root rot. This finding supported the conclusion of earlier studies that identified F. avenaceum as the predominant cause of seedling blight and root rot in lupin in Alberta (Chang et al. Citation2005b, Citation2006, Citation2008; Holtz et al. Citation2007). It was also consistent with studies that noted the widespread occurrence of F. avenaceum in the region (Hwang et al. Citation1994; Fernandez Citation2007). Various researchers in central and eastern Europe have found a similar predominance of F. avenaceum in lupin crops affected by seedling blight and root rot (Wollenweber & Reinking Citation1935; Schneider Citation1958; Debelyi et al. Citation1977).
In other parts of the world, F. avenaceum and F. oxysporum Schlecht. are generally the predominant species associated with root rot of lupin (Wollenweber & Reinking Citation1935; Debelyi et al. Citation1977; Kalis et al. Citation1990; Bateman Citation1997; Golubev & Kurlovich Citation2002). However, other Fusarium spp. have also been shown to cause root rot or seedling blight, including F. culmorum (W.G. Smith) Saccardo, F. poae (Peck) Wollenweber, F. equiseti (Corda) Saccardo, F. solani (Martius) Appel & Wollenweber emend. Snyder & Hansen, F. semitectum Berkeley & Ravenel, F. tricinctum (Corda) Saccardo and F. moliliforme sensu lato (Wollenweber & Reinking Citation1935; Weimer Citation1944; Nowicki Citation1995; Bateman Citation1997). During the time when the southeast USA was a major production area for lupin, the Fusarium spp. most commonly associated with root and hypocotyl rot were F. oxysporum, F. solani and F. moniliforme sensu lato (Weimer Citation1944). In Australia, which is the main region where L. angustifolius is produced, diseases caused by Fusarium spp. are not a major problem, despite the genus being associated with lupin (Sweetingham Citation1989).
In contrast, other Fusarium spp. are not important components of the root rot complex on lupin in Alberta. For example, Holtz et al. (Citation2011) found only two isolates of other Fusarium spp. (one isolate of F. acuminatum and one of F. oxysporum) that could consistently cause root rot on lupin. The absence of symptoms caused by F. oxysporum, F. redolens and F. solani contrasts with reports from northern Europe and the southern USA, where these species are highly aggressive against narrow-leaved lupin (Weimer Citation1944; Debelyi et al. Citation1977; DARCOF Citation2005). When F. oxysporum strains from Alberta were characterized, the authors concluded that they were non-pathogenic, endophytic strains, given their inability to cause root rot or vascular wilt (Holtz Citation2008; Holtz et al. Citation2011). Similarly, F. oxysporum and F. solani were only weakly pathogenic or non-pathogenic on lupin in Australia (Sweetingham Citation1989). Also, F. acuminatum has been associated with diseased lupin in Australia (Sweetingham Citation1989; Kalis et al. Citation1990), but it is not usually reported as a pathogen of this crop, even though it is pathogenic on other legume species (Hancock Citation1983; Hwang et al. Citation1994).
Isolates of F. avenaceum display a wide range of aggressiveness toward lupin, from non-pathogenic to highly virulent (Schneider Citation1958; DARCOF Citation2005; Holtz et al. Citation2011). The root rot reaction of the lupin cultivars ‘Arabella’ and ‘Rose’ to a collection of lupin isolates of F. avenaceum from Alberta ranged from highly susceptible to highly resistant. Both cultivars also had a similar pattern of rot root reaction in field trials in Alberta (Chang et al. Citation2011). A similar range of aggressiveness among isolates has been reported on other pulse crops (Hwang et al. Citation1994).
Intraspecific diversity
Fusarium avenaceum is genetically diverse and morphologically variable (Schneider Citation1958). Colonies of the fungus show a wide range of variation with respect to both colour and appearance on agar media (Booth Citation1971), and mutant degenerate forms are relatively common (Leslie & Summerell Citation2006). Microscopically, F. avenaceum is distinguished by the production of long, slender macroconidia; production of microconidia is rare and it does not produce chlamydospores.
European populations of F. avenaceum exhibit a high level of genotypic diversity (Yli-Mattila et al. Citation1996; De Nijs et al. Citation1997; Chelkowski et al. Citation1999; Golínska et al. Citation2002). For example, 67 haplotypes were identified among 68 F. avenaceum isolates (recovered mainly from L. albus) in the UK (Satyaprasad et al. Citation2000). In Australia, each isolate had a unique haplotype in a study using restriction fragment length polymorphism (RFLP) analysis (Benyon et al. Citation2000). In Alberta, each isolate of F. avenaceum from infected roots of lupin possessed a unique multi-locus genotype (Holtz et al. Citation2011), which indicates that a high level of genetic diversity exists in the pathogen population in this region.
Despite the extent of diversity observed in populations of F. avenaceum, hierarchical clustering has revealed no association with geographic origin or aggressiveness among isolates from Europe (Yli-Mattila et al. Citation1996; De Nijs et al. Citation1997; Chelkowski et al. Citation1999; Satyaprasad et al. Citation2000; Golińska et al. Citation2002). The lack of correlation between genetic and geographic distance is also maintained on a larger geographic scale. For example, F. avenaceum isolates from Germany, France and the UK did not cluster according to the country of origin (Turner et al. Citation1998). In a global collection dominated by American isolates of F. avenaceum from Lisianthus (Eustoma grandiflorum (Grise.)), there was no correlation between genetic relatedness, plant host or geographic origin (Nalim et al. Citation2009). Similarly, cluster analysis based on genetic markers from Canadian isolates did not demonstrate an association between the geographic location of the isolates and genetic relatedness (Holtz et al. Citation2011). Instead, the F. avenaceum isolates were grouped into two distinct clusters, with isolates from both clusters occurring together at many sites. Phylogenetic analysis also revealed high diversity and the presence of some European-like isolates within the Canadian pathogen population.
Like many other filamentous ascomycete fungi, Fusarium spp. have a single mating type (MAT) locus regulating sexual reproduction (Nelson Citation1996; Coppin et al. Citation1997; Kerényi et al. Citation2004). The two idiomorphs of the mating type locus are identified as MAT1-1 and MAT1-2. Unlike alleles, idiomorphs lack significant sequence similarity with each other, but occupy the same chromosomal position. Mating type may be determined in tests of cross-fertility with strains of known mating type (Leslie & Summerell Citation2006), or by PCR amplification of portions of each idiomorph with selective primers (Kerényi et al. Citation2004). The latter approach has been used to show that F. avenaceum is heterothallic and that each strain carries either the MAT-1 or MAT-2 idiomorph (Kerényi et al. Citation2004). The mating type composition of 42 isolates of F. avenaceum from lupin in Alberta was determined via PCR amplification of portions of each idiomorph (Holtz et al. Citation2011). Each mating type occurred at about equal frequency, MAT-1 (n = 25) and MAT-2 (n = 17), and isolates of each mating type were present in all but one of the locations examined.
The teleomorph of F. avenaceum has been reported only twice, once in Europe (Booth & Spooner Citation1984) and once in the USA (Cook Citation1967). This rare occurrence may indicate that F. avenaceum is a functionally asexual species. For Fusarium spp. and other fungi where sexual reproduction is rarely observed, several methods are available to detect sexual recombination within a population. Strictly clonal populations often have a predominance of identical genotypes and a low level of genetic diversity, because new genotypes arise mainly from mutation or immigration of new genotypes. These populations may also exhibit linkage disequilibrium, since the entire genome is effectively linked in clonal reproduction (Anderson & Kohn Citation1995). In randomly mating populations, however, recombinant genotypes generate genotypic diversity and meiotic recombination creates a more random association of alleles, which decreases linkage disequilibrium. In heterothallic species with random mating, the mating type ratio should approach 1:1 (Milgroom Citation1996; Peever et al. Citation2004). Many fungal populations reproduce both asexually and sexually, and have characteristics between the extremes of clonality and panmixis. From a disease management perspective, it is important to detect recombination, since a recombining pathogen population may be much more likely to overcome host resistance. Moreover, the production of sexual propagules may alter pathogen dispersal mechanisms and function as new sources of inoculum for initiating disease outbreaks (Taylor et al. Citation1999; McDonald & Linde Citation2002). The high genotypic diversity of isolates and the presence of both mating types across sites indicates that sexual reproduction may occur within populations of F. avenaceum in Alberta (Holtz et al. Citation2011), although additional work would be required to confirm this hypothesis.
To assess the influence of clustering based on genetic relatedness (described previously) and mating type of isolates from lupin in Alberta, variation in the aggressiveness of representative F. avenaceum isolates was assessed based on seedling emergence, shoot dry weight and root rot severity of the cultivars ‘Arabella’ and ‘Rose’ (Holtz et al. Citation2011). The incidence and severity of root rot were higher after inoculation with isolates from Cluster I than with isolates from Cluster II. In contrast, emergence tended to be lower when inoculated with isolates from Cluster II than Cluster I, although the difference was significant only for ‘Rose’. Inoculation with MAT-2 isolates resulted in higher root rot incidence and lower emergence and shoot weight than did inoculation with MAT-1 isolates. This indicated that MAT-2 isolates were slightly more aggressive than MAT-1.
Yield loss
An understanding of disease-yield relationships is needed to measure the efficacy and economic benefits of disease management strategies (Schoeny et al. Citation2001). Many studies have generated differences in disease levels using crop rotation (Slope & Etheridge Citation1971; Shipton Citation1975; Schoeny & Lucas Citation1999), combinations of sowing depth and artificial inoculation (Rothrock Citation1988; Hornby & Bateman Citation1990), seeding date (Bateman et al. Citation1990), seed treatment fungicides or cultivars that differed in level of resistance (Nkalubo et al. Citation2007), to quantify yield loss associated with a particular host–pathogen system. These studies demonstrate that root rot and seedling blight can have a substantial impact on seedling establishment and yield of many field crops. For example, fusarium wilt is a major disease of white lupin in Egypt, resulting in yield losses of up to 40% (Osman et al. Citation1986). Also, F. avenaceum caused 22–31% loss in stand establishment and a 19% seed yield loss in Canada (Chang et al. Citation2011), but yield loss differed among lupin genotypes (Chang et al. Citation2014). In other regions, F. avenaceum is seedborne at low levels in L. angustifolius (Nowicki Citation1995), but seed transmission is not an important mechanism of transmission on L. angustifolius in Alberta (Chang et al. Citation2005). The pathogen is already endemic in most agricultural fields in Alberta, so low levels of natural seed infection and seed-to-seedling infection would have a negligible impact on disease levels at most sites.
Management of fusarium root rot
Cultural practices
In Alabama, one study showed that crop rotations that included lupin once every 3 years had the lowest incidence of root and hypocotyl rots, but disease levels were higher under shorter rotations (Collins et al. Citation1996). However, the effect of rotation on pathogen populations was not consistent (Burch et al. Citation1997). Many of the crops that normally occur in rotation with lupin on the Canadian prairies, including canola, barley, lentil and field pea, are also highly susceptible to F. avenaceum (Gossen & Derksen Citation2003). As a result, crop rotation is unlikely to provide effective management of root rot on lupin in this region.
Cultural production practices, such as seeding date, depth and rate, have an impact on root rot of lupin, as has been reported for other pulse crops (Hwang et al. Citation2000; Chang et al. Citation2008). In central Alberta, there was no consistent effect of seeding date on the establishment of lupin across sites and years (Chang et al. Citation2011), but yield declined when seeding was delayed into mid-June (Lopetinsky et al. Citation2006). In Minnesota, sowing in mid-April resulted in the highest yield in white lupin (L. albus), and yield declined after this optimum date (Putnam Citation1993).
Lupin plants grew best under a 25 °C/15 °C temperature regime, suffering less damage from root rot than at higher or lower temperatures (Chang et al. Citation2011). Other studies report that root rot caused by F. avenaceum on white lupin is more severe at higher soil temperatures (Satyaprasad et al. Citation2000). Similarly, the severity of root rot on field pea caused by F. solani also increased as soil temperature increased from 10 °C to 30 °C (Tu Citation1994). Other studies have shown that a differential impact of temperature on the relative growth rates of host and pathogen can influence the severity of seedling blight and root rot (Leath Citation1947; Gardiner et al. Citation1987; Bhatti & Kraft Citation1992; Chang et al. Citation2004).
In a controlled environment study, seedling establishment declined as seeding depth increased from a 5- to 10-cm depth, and no seedlings survived in the 7- and 10-cm depth treatments in inoculated soil (Chang et al. Citation2011). Other studies have also demonstrated that deep seeding reduces seedling vigour in pulse crops (Johnston & Stevenson Citation2001), and that seedlings with low vigour are highly susceptible to seedling blight and root rot (Hwang et al. Citation2001, Citation2003). In Australia, deep seeding (7–10 cm) also reduced seedling emergence, delayed maturity and reduced yield of lupin (Corbett et al. Citation2001). Similar results were reported on chickpea (Chang et al. Citation2004) and lentil (Hwang et al. Citation2000). Under field conditions, however, seeding at 5–7 cm depth may be required in order to gain access to soil moisture and thereby ensure consistent and timely seed germination, despite any negative impact on subsequent seedling emergence and vigour (Wilson & Thurling Citation1996).
Seed treatments
Fungicidal seed treatments on lupin crops are reported to provide effective control of F. oxysporum (Mart.) Sacc. with benomyl-thiophanate-methyl/thiram (Fahim et al. Citation1983), as well as control of Pleiochaeta setosa (Kirchn.) Hughes and R. solani with iprodione (Evans et al. Citation1986; Sweetingham Citation1990), and control of Phomopsis leptostromiformis (Kühn) Bubak ex Lind with benomyl (Clarke & Kellock Citation1979; Salt Citation1980). In contrast, seed treatments did not provide effective control of seedling blight in other trials ( Kuznia et al. Citation1993; Sadowski Citation1994; Gowely et al. Citation1996). In Canada, several fungicide seed treatments were evaluated under field conditions for efficacy against F. avenaceum seedling blight. Apron Maxx RTA® (fludioxonil + metalaxyl), Crown® (carbathiin + thiabendazole) and Vitaflo® 280 (carbathiin + thiram) reduced seedling blight incidence and improved seedling establishment and seed yield relative to non-treated controls (Chang et al. Citation2011). Apron Maxx RTA® and Vitaflo® 280 are registered as seed treatments to manage seedling blight and root rot caused by Fusarium, Rhizoctonia and Pythium spp. on the major pulse crops grown in Canada, so these fungicides may also have the potential to control seedling blight of lupin.
Cultivar resistance
Sources of genetic resistance to root rot in lupin are limited (Kurlovich et al. Citation1995; Kuptsov et al. Citation2004). In Europe, eight genealogically distinct fusarium root rot-resistant genotypes were identified in L. angustifolius (‘Bordako’, ‘Apva’, ‘Tapa’, ‘Pch-1’, ‘Vps-1’, ‘Sideral-D’, ‘Wild-accB’ and GB-14), and four resistant genotypes were identified in L. albus (FUS-1, MA, ‘Nelly’ and ‘EgyptBestFus’) (Kutpsov et al. Citation2006). Six loci for resistance to F. avenaceum have been identified, and all of these resistance genes have been introgressed into resistant cultivars of lupin, including ‘Boddako’ and ‘Borweta’ in Belarus, ‘Kristal’ and ‘Snejet’ in Russia, ‘Arabella’ and ‘Boltensis’ in Germany, and ‘Rose’ and ‘Galant’ in Denmark (Kuptsov et al. Citation2004, Citation2006). Resistance to fusarium wilt in L. albus has been reported from Russia (Kurlovich et al. Citation1995), Ukraine (Raza et al. Citation2000) and Egypt (Raza et al. Citation1998).
The cultivars ‘Arabella’ and ‘Rose’ were not resistant to isolates of F. avenaceum collected in Alberta or in field trials (Chang et al. Citation2014). Indeed, only three of the 19 lupin cultivars and lines assessed were even moderately resistant to F. avenaceum in Alberta, but the rankings were not consistent from year to year. This indicates that the development of resistant cultivars for Canada may be more complicated than simply incorporating resistance identified previously from other regions.
Conclusion
Production of narrow-leaved lupin represents an opportunity to further diversify cropping rotations in western Canada. The crop has many benefits from a production perspective, but seedling blight and root rot represents a serious constraint to its adoption in the region. Additional research is needed on the biology and impact of F. avenaceum as a pathogen of lupin on the Canadian prairies. It is clear, however, that lupin production will require the development of a disease management programme that integrates crop rotation, shallow seeding of seed with high vigour, fungicidal seed treatments, and eventually, cultivars with strong genetic resistance.
Acknowledgements
We thank past and present sponsors of our research on lupin pathology, including the Alberta Crop Industry Development Fund and the Alberta Pulse Growers Commission, whose support made much of the work reviewed here possible.
References
- Anderson JB, Kohn LM. 1995. Clonality in soilborne, plant-pathogenic fungi. Annu Rev Phytopathol. 33:369–391.
- Bateman GL. 1997. Pathogenicity of fungi associated with winter loss and injury in white lupin. Plant Pathol. 46:157–167.
- Bateman GL, Hornby D, Gutteridge RJ. 1990. Effects of take-all on some aspects of grain quality of winter wheat. Ann Appl Biol. 25:339–348.
- Benyon FH, Burgess LW, Sharp PJ. 2000. Molecular genetic investigations and reclassification of Fusarium species in sections Fusarium and Roseum. Mycol Res. 104:1164–1174. doi:10.1017/S0953756200002914
- Bhatti MA, Kraft JM. 1992. Effects of inoculum density and temperature on root rot and wilt of chickpea. Plant Dis. 76:50–54. doi:10.1094/PD-76-0050
- Boles D, Olson MA, Lopetinsky KJ, Eyolfson A, Bamber A. 2003. Evaluation of pulse crop diversification potential in south central Alberta. Farming for the future on farm demonstration project (#2000CR17). Final Report. 14 pp.
- Booth C. 1971. The genus Fusarium. Kew, Surrey: Commonwealth Mycological Institute; 237 pp.
- Booth C, Spooner BM. 1984. Gibberella avenacea, teleomorph of Fusarium avenaceum, from stems of Pteridium aquilinum. Trans Br Mycol Soc. 82:178–180. doi:10.1016/S0007-1536(84)80231-4
- Burch KB, Bowen KL, Van Santen E, Reeves DW. 1997. Effects of tillage and crop rotation on seedling diseases and soil fungi associated with white lupine in Alabama. Phytopathology. 87:S13–14. (abstr.).
- Celetti MJ, Johnston HW, Kimpinski J, Platt HW, Martin RA. 1990. Incidence of soil-borne plant pathogens isolated from barley and winter wheat, and other crops in the rotation, on Prince Edward Island. Plant Pathol. 39:606–611. doi:10.1111/j.1365-3059.1990.tb02541.x
- Chang KF, Hwang SF, Ahmed HU, Strelkov SE, Gossen BD, Turnbull GD. 2014. Disease reaction to Fusarium avenaceum and yield losses in narrow-leafed lupin lines. Can J Plant Sci. (in press).
- Chang KF, Hwang SF, Gossen BD, Howard RJ, Lopetinsky K, Olson M. 2005a. First report of Rhizoctonia solani AG-4 and AG-2-2 on Lupinus angustifolius in Canada. Plant Dis. 89:685. doi:10.1094/PD-89-0685C
- Chang KF, Hwang SF, Gossen BD, Strelkov SE, Turnbull GD, Bing DJ. 2011. Effect of seeding practices, temperature and seed treatments on fusarium seedling blight of narrow-leaved lupin. Can J Plant Sci. 91:859–872. doi:10.4141/cjps2010-039
- Chang KF, Hwang SF, Gossen BD, Turnbull GD, Howard RJ, Blade SF. 2004. Effects of soil temperature, seeding depth and seeding date on rhizoctonia seedling blight and root rot of chickpea. Can J Plant Sci. 84:901–907. doi:10.4141/P03-024
- Chang KF, Hwang SF, Gossen BD, Turnbull GD, Wang H, Howard RJ. 2008. Effects of inoculum density, temperature, seeding depth, seeding date and fungicidal seed treatment on the impact of Rhizoctonia solani on lentil. Can J Plant Sci. 88:799–809.
- Chang KF, Hwang SF, Lopetinsky K, Olson M, Bowness R, Turnbull GD, Calpas J, Bing DJ, Howard RJ. 2006. Occurrence of lupine diseases in central Alberta in 2005. Can Plant Dis Surv. 86:107–108.
- Chang KF, Lopetinsky K, Olson M, Bowness R, Hwang SF, Turnbull GD, Strydhorst S, Clayton G, Harker N, Bing DJ, et al. 2005. Disease situation of lupines occurring in Alberta in 2003 and 2004. Can Plant Dis Surv. 85:87–88.
- Chang KF, Lopetinsky K, Olson M, Bowness R, Hwang SF, Turnbull GD, Strydhorst S, Clayton G, Harker N, Bing DJ, Lupwayi N, Cole D, Byer J. 2005b. Diseases of lupins in central and northern Alberta in 2003 and 2004. Can. Plant Dis. Survey. 85:87–88.
- Chelkowski J, Bateman GL, Mirocha C. 1999. Identification of toxigenic Fusarium species using PCR assays. J Phytopathol. 147:307–311. doi:10.1111/j.1439-0434.1999.tb03835.x
- Clarke RG, Kellock AW. 1979. Control of seed-borne Phomopsis Sp. on lupins.. Plant Pathol Soc Newsl. 8:34. doi:10.1071/APP9790034
- Collins DJ, Bruch KB, Reeves DW, Van Santen E. 1996. Effects of crop rotation and tillage on seedling diseases of white lupin. Phytopathology. 86:S59. (abstr.).
- Cook RJ. 1967. Gibberella avenacea sp. n., perfect stage of Fusarium roseum f. sp. cerealis ‘Avenaceum. Phytopathology. 57:372–376.
- Coppin E, DeBuchy R, Arnaise S, Picard M. 1997. Mating types and sexual development in filamentous ascomycetes. Microbiol Mol Biol Rev. 61:411–428.
- Corbett AJ, Mock IT, Matassa V. 2001. Effect of plant density and sowing date on narrow-leaf lupin production in the Victorian Mallee. In: Rowe et al., editors. Proceedings of the 10th Australian Agronomy Conference; 2001, Hobart (TAS). [cited 2013 Aug]. Available from: http://www.regional.org.au/au/asa/2001/p/13/corbett.htm#TopOfPage
- Cormack MW. 1937. Fusarium spp. as root parasites of alfalfa and sweet clover in Alberta. Can J Res C. 15c:493–510. doi:10.1139/cjr37c-037
- Cowling WA, Huyghe C, Swiecicki W. 1998. Lupin breeding. In: Gladstones JS, Atkins CA, Hamblin J. editors. Lupins as crop plants: biology, production and utilization. New York: CAB International; p. 93–129.
- Cox B. 1998. Marketing and trade. In: Gladstones JS, Atkins CA, Hamblin J. editors. Lupins as crop plants: biology, production and utilization. New York: CAB International; p. 437–454.
- [DARCOF] Danish Research Centre for Organic Food and Farming. 2005. 1.4 Grain legumes for organic farming. Improvement of disease resistance. FØJOII-44. Final Report.
- De Nijs M, Larsen JS, Gams W, Rombouts FM, Wernars K, Thrane U, Notermans S. 1997. Variations in random amplified polymorphic DNA patterns and secondary metabolite profiles within Fusarium species from cereals from various parts of the Netherlands. Food Microbiol. 14:449–457. doi:10.1006/fmic.1997.0111
- Debelyi GA, Zekunov AV, Minenko AK. 1977. Lupin mutants resistant to Fusarium diseases. Selectsiya i Semenovodstov. 6:63–64. [in Russian].
- Desjardins AE. 2003. Gibberella from A (venaceae) to Z (eae). Annu Rev Phytopathol. 41:177–198. doi:10.1146/annurev.phyto.41.011703.115501
- Evans J, O’Connor GE, Seymour AR, Carmichael A. 1986. Sensitivity of lupin (Lupinus angustifolius L.) root nodulation to iprodione (Rovral) fungicide. Australas Plant Pathol. 15:66–67. doi:10.1071/APP9860066
- Evans J, O’Connor GE, Turner GL, Coventry DR, Fettell N, Mahoney J, Armstrong EL, Walsgott DN. 1989. N2 fixation and its value to soil N increase in lupin, field pea and other legumes in south-eastern Australia. Aust J Agric Res. 40:791–805. doi:10.1071/AR9890791
- Fahim MM, Osman AR, Sahab AF, El-Kader M. 1983. Agricultural practices and fungicide treatments for the control of Fusarium wilt of lupin. Egypt J Phytopathol. 15:35–46.
- Fernandez MR. 2007. Fusarium populations in roots of oilseed and pulse crops grown in eastern Saskatchewan. Can J Plant Sci. 87:945–952. doi:10.4141/P06-145
- Gardiner DC, Horst RK, Nelson PE. 1987. Symptom enhancement of fusarium wilt of chrysanthemum by high temperatures. Plant Dis. 71:1106–1109. doi:10.1094/PD-71-1106
- Gladstones JS. 1998. Distribution, origin, taxonomy, history and importance. In: Gladstones JS, Atkins CA, Hamblin J. editors. Lupins as crop plants: biology, production and utilization. New York: CAB International; p. 1–40.
- Golínska B, Kwásna H, Narózna D, Kostecki M, Wakulínski W, Kròliczac J, Màdrzak CJ, Golínski P. 2002. Phenotypic and genetic diversity among the Fusarium avenaceum isolates. Phytopathol Pol. 22:47–60.
- Golubev AA, Kurlovich BS. 2002. Diseases and pests. In: Kurlovich BS, editor. Lupins: geography, classification, genetic resources and breeding. St. Petersburg, Russia: Intan; p. 287–312.
- Gossen BD, Derksen DA. 2003. Impact of tillage and crop rotation on ascochyta blight (Ascochyta lentis) of lentil. Can J Plant Sci. 83:411–415. doi:10.4141/P02-088
- Gowely AM, Abdel Rahman AG, Soliman GI. 1996. Studies on the behavior of some fungicides in controlling damping-off and root rot diseases in lupin plants. Bull Fac Agric Univ Cairo. 47:331–340.
- Hancock JG. 1983. Seedling diseases of alfalfa in California. Plant Dis. 67:1203–1208. doi:10.1094/PD-67-1203
- Helgadóttir A, Hermannsson J, Kristjánsdóttir TA. 2004. Annual lupins grown for green fodder in Iceland. In van Santen E, Hill GD, editors. Wild and cultivated lupins from the tropics to the poles. Proceedings of the 10th International Lupin Conference, 2002 19–24 June; Laugarvatn, Iceland; p. 101–104.
- Henderson C. 1989. Lupins as a biological plough: evidence for and effects on wheat growth and yield. Aust J Exp Agric. 29:99–102.
- Hestbjerg H, Elmholt S, Thrane U, Jensen UB. 1999. A resource-saving method for isolation of Fusarium and other fungi from individual soil particles. Mycol Res. 103:1545–1548.
- Holtz MD. 2008. Characterization of Fusarium pathogens of lupin in central Alberta, Canada [dissertation]. Edmonton (AB) :University of Alberta. 125 p.
- Holtz MD, Chang KF, Hwang SF, Gossen BD, Strelkov SE. 2011. Characterization of Fusarium avenaceum from lupin in central Alberta: genetic diversity, mating type and aggressiveness. Can J Plant Pathol. 33:61–76. doi:10.1080/07060661.2011.536651
- Holtz MD, Chang KF, Hwang SF, Strelkov SE. 2007. Evaluation of root pathogens of narrow-leaved lupine (Lupinus angustifolius) in Alberta. Can J Plant Pathol. 29:210. [abstr.].
- Hondelmann W. 1984. The lupin: ancient and modern crop plant. Theor Appl Genet. 68:1–9.
- Hornby D, Bateman GL. 1990. Artificial infestation of soil with Gaeumannomyces graminis var. tritici to study the relationship between take-all and wheat yields in field experiments. Soil Use Manag. 6:209–216. doi:10.1111/j.1475-2743.1990.tb00837.x
- Hudson HJ. 1968. The ecology of fungi on plant remains above the soil. New Phytol. 67:837–874. doi:10.1111/j.1469-8137.1968.tb06399.x
- Hwang SF, Gossen BD, Chang KF, Turnbull GD, Howard RJ. 2001. Effect of seed damage and metalaxyl seed treatment on pythium seedling blight and seed yield of field pea in Alberta, Canada. Can J Plant Sci. 81:509–517. doi:10.4141/P00-155
- Hwang SF, Gossen BD, Chang KF, Turnbull GD, Howard RJ, Blade SF. 2003. Etiology, impact and control of rhizoctonia seedling blight and root rot of chickpea on the Canadian prairies. Can J Plant Sci. 83:959–967. doi:10.4141/P02-165
- Hwang SF, Gossen BD, Turnbull GD, Chang KF, Howard RJ, Thomas AG. 2000. Effect of temperature, seeding date, fungicide seed treatment and inoculation with Fusarium avenaceum on seedling survival, root rot severity and yield of lentil. Can J Plant Sci. 80:899–907. doi:10.4141/P99-177
- Hwang SF, Howard RJ, Chang KF, Park B, Burnett PA. 1994. Etiology and severity of fusarium root rot of lentil in Alberta. Can J Plant Pathol. 16:295–303. doi:10.1080/07060669409500734
- Jenkinson P, Parry DW. 1994. Splash dispersal of conidia of Fusarium culmorum and Fusarium avenaceum. Mycol Res. 98:506–510. doi:10.1016/S0953-7562(09)80468-1
- Jensen CR, Joernsgaard B, Andersen MN, Christiansen JL, Mogensen VO, Friis P, Petersen CT. 2004. The effect of lupins as compared with peas and oats on the yield of the subsequent winter barley crop. Eur J Agron. 20:405–418. doi:10.1016/S1161-0301(03)00057-1
- Johnston AM, Stevenson FC. 2001. Field pea response to seeding depth and P fertilization. Can J Plant Sci. 81:573–575. doi:10.4141/P00-166
- Kalis RA, Stewart EL, Meronuck RA. 1990. Fungi isolated from white lupins in Minnesota. Phytopathology. 80:1043.
- Kerenyi Z, Moretti A, Waalwijk C, Olah B, Hornok L. 2004. Mating type sequences in asexually reproducing Fusarium species. Appl Environ Microbiol. 70:4419–4423. doi:10.1128/AEM.70.8.4419-4423.2004
- Kommedahl T, Abbas HK, Burnes PM, Mirocha CJ. 1988. Prevalence and toxigenicity of Fusarium species from soils of Norway near the Arctic Circle. Mycologia. 80:790–794. doi:10.2307/3807556
- Kuptsov N, Christiansen JL, Raza S, Jonsgaard B. 2004. Greenhouse screening for fusarium wilt resistance in lupin. In: van Santen E, Hill GD, editors. Wild and cultivated lupins from the tropics to the poles. Proceedings of the 10th International Lupin Conference, 2002 19–24 June; Laugarvatn, Iceland; p. 253–255.
- Kurlovich B, Korneichuk NS, Kiselev II. 1995. Breeding fusarium resistant lupin forms for agricultural environment of Russia based on ecologico-geographical approach. J Appl Genet. 36:241–249. [in Russian].
- Kutpsov V, Kolomiets EM, Kuptsov N, Joernsgaard B. 2006. Environmentally friendly methods to combat fungal disease of lupins. In Where old and new world lupins meet. In van Santen E, Hill GD, editors. Proceedings of the 11th International Lupin Conference, 2005 4–9 May; Guadalajara, Jalisco, Mexico; p. 135–138.
- Kuznia RA, Meronuck RA, Stewart EL. 1993. Effect of seed treatments on stand establishment, root necrosis, and yield of Lupinus albus. Plant Dis. 77:892–895.
- Leath LD. 1947. Growth rates of host and pathogen as factors determining the severity of pre-emergence damping-off. J Agric Res. 75:161–179.
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Ames, IA: Blackwell Publishing.
- Lopetinsky K, Strydhorst S, Olson MA, Kaufman J. 2006. Building the low tannin faba bean and narrow leafed lupin industries in Alberta: a compilation of research projects and people. Proc. Farm Tech 2006, Jan 25–27, 2006, Edmonton, AB, p.148.
- Martin RA. 1988. Use of a high through-put jet sampler for monitoring viable airborne propagules of Fusarium in wheat. Can. J. Plant Pathol. 10:359–360. doi:10.1080/07060668809501713
- McDonald BA, Linde C. 2002. The population genetics of plant pathogens and breeding strategies for durable resistance. Euphytica. 124:163–180. doi:10.1023/A:1015678432355
- Milgroom M. 1996. Recombination and the multilocus structure of fungal populations. Annu Rev Phytopathol. 34:457–477. doi:10.1146/annurev.phyto.34.1.457
- Nalim FA, Elmer WH, McGovern RJ, Geiser DM. 2009. Multilocus phylogenetic diversity of Fusarium avenaceum pathogenic on Lisianthus. Phytopathology. 99:462–468. doi:10.1094/PHYTO-99-4-0462
- Nelson MA. 1996. Mating systems in ascomycetes: a romp in the sac. Trends Genet. 12:91–131.
- Nkalubo S, Melis R, Laing MD, Opio F. 2007. Yield loss associated with anthracnose disease on Ugandan market-class dry bean cultivars. Afr Crop Sci Proc. 8:869–874.
- Nowicki B. 1995. Patogenic fungi associated with blue lupine seeds. Acta Agrobot. 48:59–64. [in Polish, English abstract.]. doi:10.5586/aa.1995.016
- Oldershaw AW. 1920. The value of lupins on poor light land. Agriculture (Lond.). 26:982–991.
- Osman AR, Fahim MM, Sahab AF, El-Kader M. 1986. Losses due to wilt of lupin. Egypt J Phytopathol. 15:27–34.
- Parry DW, Jenkinson P, McLeod L. 1995. Fusarium ear blight (scab) in small grain cereals? A review. Plant Pathol. 44:207–238. doi:10.1111/j.1365-3059.1995.tb02773.x
- Peever TL, Salimath SS, Su G, Kaiser WJ, Muehlbauer FJ. 2004. Historical and contemporary multilocus population structure of Ascochyta rabiei (teleomorph: Didymella rabiei) in the Pacific Northwest of the United States. Mol Ecol. 13:291–309. doi:10.1046/j.1365-294X.2003.02059.x
- Putnam DH. 1993. An interdisciplinary approach to the development of lupin as an alternative crop. In: Janick J, Simon JE, editors. New Crops. New York: Wiley and Sons Inc; p. 266–277.
- Raza S, Christiansen JL, Jørnsgård B, Ortiz R. 2000. Partial resistance to a fusarium root disease in Egyptian white lupin landraces. Euphytica. 112:233–237. doi:10.1023/A:1003904805737
- Raza S, Koriem A, Abou-Zeid N, Christiansen JL, Jørgensgärd B. 1998. Screening for resistance to wilt disease caused by Fusarium oxysporum in white lupin (Lupinus albus) in Egypt. Egypt J Appl Sci. 13:45–53.
- Rothrock CS. 1988. Relative susceptibility of small grains to take-all. Plant Dis. 72:883–886. doi:10.1094/PD-72-0883
- Sadowski S. 1994. Biological and chemical control of root rot of white lupin (Lupinus albus L.) cultivar Wat. Phytopathol Polonica. 19:43–47.
- Salt GA. 1980. Effect of fungicides on survival of lupins, Lupinus albus cv. Kievsky. Rothamsted Experiment Station. Report 1979, Part 1. Harpenden, England, p. 175.
- Satyaprasad K, Bateman GL, Read PJ. 1997. Variation in pathogenicity on potato tubers and sensitivity to thiabendazole of the dry rot fungus Fusarium avenaceum. Potato Res. 40:357–365.
- Satyaprasad K, Bateman GL, Ward E. 2000. Comparisons of isolates of Fusarium avenaceum from white lupin and other crops by pathogenicity tests, DNA analyses and vegetative compatibility tests. J Phytopathol. 148:211–219. doi:10.1046/j.1439-0434.2000.00494.x
- Schneider R. 1958. Untersuchungen über variation und pathogenität von Fusarium avenaceum (Fr.) Sacc. J Phytopathology. 32:129–148. [in German]. doi:10.1111/j.1439-0434.1958.tb01774.x
- Schoeny A, Jeuffroy MH, Lucas P. 2001. Influence of take-all epidemics on winter wheat yield formation and yield loss. Phytopathology. 91:694–701. doi:10.1094/PHYTO.2001.91.7.694
- Schoeny A, Lucas P. 1999. Modeling of take-all epidemics to evaluate the efficacy of a new seed-treatment fungicide on wheat. Phytopathology. 89:954–961. doi:10.1094/PHYTO.1999.89.10.954
- Shipton PJ. 1975. Yield trends during take-all decline in spring barley and wheat grown continuously. EPPO Bull. 5:363–374. doi:10.1111/j.1365-2338.1975.tb02486.x
- Slope DB, Etheridge J. 1971. Grain yield and incidence of take-all (Ophiobolus graminis Sacc.) in wheat grown in different crop sequences. Ann Appl Biol. 67:13–22. doi:10.1111/j.1744-7348.1971.tb02904.x
- Summerell BA, Salleh B, Leslie JF. 2003. A utilitarian approach to Fusarium identification. Plant Dis. 87:117–128. doi:10.1094/PDIS.2003.87.2.117
- Sweetingham MW. 1989. Fungi associated with root and hypocotyl diseases of seedling lupins in Western Australia. Aust J Agric Res. 40:781–789. doi:10.1071/AR9890781
- Sweetingham MW. 1990. Coping with brown spot and root rots of lupins. West Aust J Agric. 31:5–13.
- Taylor JW, Jacobson DJ, Fisher MC. 1999. The evolution of asexual fungi: reproduction, speciation and classification. Annu Rev Phytopathol. 37:197–246. doi:10.1146/annurev.phyto.37.1.197
- Tu JC. 1994. Effects of soil compaction, temperature and moisture on the development of the fusarium root rot complex of pea in southwestern Ontario. Phytoprotection. 75:125–131. doi:10.7202/706059ar
- Turner AS, Lees AK, Rezanoor HN, Nicholson P. 1998. Refinement of PCR-detection of Fusarium avenaceum and evidence from DNA marker studies for phenetic relatedness to Fusarium tricinctum. Plant Pathol. 47:278–288. doi:10.1046/j.1365-3059.1998.00250.x
- Unkovich MJ, Pate JS, Hamblin J. 1994. The nitrogen economy of broadacre lupin in southwest Australia. Aust J Agric Res. 45:149–164. doi:10.1071/AR9940149
- Vellasamy G, Hill GD, McKenzie BA. 2000. The advantage of lupins in crop rotations in New Zealand. In: van Santen E, Wink M, Weissmann S, Römer P, editors. Lupin, an ancient crop for the new millennium. Proceedings of the 9th International Lupin Conference, 1999 20–24 June, Klink/Müritz; p. 13–18.
- Weimer JL. 1944. Some root rots and a foot rot of lupines in the southeastern part of the United States. J Agric Res. 68:441–457.
- Wilson CE, Thurling N. 1996. Effect of sowing depth and water potential on seedling emergence of Lupinus species. Aust J Exp Agric. 36:463–471. doi:10.1071/EA9960463
- Wollenweber HW, Reinking OA. 1935. Die Fusarien, ihre Beschreibung, Schadwirkung und Bekämpfung. Verlag Paul Parey, Berlin. 355 pp.
- Yli-Mattila T, Paavanen S, Hannukkala A, Parikka P, Tahvonen R, Karjalainen R. 1996. Isozyme and RAPD-PCR analyses of Fusarium avenaceum strains from Finland. Plant Pathol. 45:126–134. doi:10.1046/j.1365-3059.1996.d01-105.x
- Yli-Mattila T, Paavanen-Huhtala S, Parikka P, Konstantinova P, Gagkaeva TY. 2004. Molecular and morphological diversity of Fusarium species in Finland and north-western Russia. Eur J Plant Pathol. 110:573–585. doi:10.1023/B:EJPP.0000032397.65710.69
