Abstract
This study assessed the efficacy of phosphites, Trichothecium roseum (TR) and a combination of phosphites and TR compared with lime sulphur (LS) to control brown rot, caused by Monilinia fructicola, in an organic orchard with high inoculum pressure under subtropical conditions in Brazil. The treatments were applied from blossom to harvest on two peach cultivars (‘Granada’ and ‘Chimarrita’) during 2005 and 2006. Disease incidence was assessed from full bloom to post-harvest. Two other experiments were performed under post-harvest conditions using mature fruit harvested from organic and conventional orchards and inoculated with 106 conidia mL−1 of TR followed by M. fructicola. The incidence of blossom blight ranged from 25% to 64% on flowers collected from untreated controls, whereas all treatments reduced blight over both cultivars and years. For ‘Chimarrita’, disease incidence reached 8% in mature fruit on the tree and 43% on fruit post-harvest. In both years, TR treatment reduced fruit infection by 50% and 63% during the growing season and post-harvest, respectively, when compared with untreated trees. ‘Granada’ did not set enough fruit to be assessed. The TR applied as a post-harvest treatment to fruit also significantly reduced final disease incidence. Applications of TR combined with phosphites or lime-sulphur were not better than TR applied alone, and TR was equivalent to LS, indicating that both treatments have the potential to reduce brown rot in organic peach production.
Cette étude a évalué l’efficacité des phosphites, de Trichothecium roseum (TR) et d’une combinaison de phosphites et de TR comparativement au sulfure de calcium (SC) pour maîtriser la pourriture brune, causée par Monilinia fructicola, dans un verger organique brésilien subissant des pressions d’inoculum élevées dans des conditions subtropicales. Les traitements ont été appliqués de la floraison à la récolte sur deux cultivars de pêcher (‘Granada’ et ‘Chimarrita’) en 2005 et 2006. L’incidence de la maladie a été évaluée de la pleine floraison jusqu’après la récolte. Deux autres expériences ont été menées dans des conditions d’après récolte avec des fruits matures récoltés dans des vergers biologiques et traditionnels inoculés avec106 conidies/ml de TR, suivi de M. fructicola. L’incidence de la brûlure de la fleur a varié de 25 à 64 % sur les fleurs collectées sur les plants témoins non traités, tandis que tous les traitements ont réduit l’incidence de la maladie sur les deux cultivars, et ce, durant les deux années. Chez ‘Chimarrita’, l’incidence de la maladie a atteint 8% chez les fruits matures non récoltés et 43% chez les fruits récoltés. Durant les deux années, le traitement avec TR a réduit l’infection des fruits de 50% et de 63% durant la saison de croissance et après la récolte, respectivement, comparativement aux arbres non traités. ‘Granada’ n’a pas produit suffisamment de fruits pour qu’on l’évalue. Le TR appliqué sur les fruits après la récolte à titre de traitement a également réduit l’incidence finale de la maladie. Les applications de TR combinées à des phosphites ou à du sulfure de calcium n’ont pas été plus efficaces que les applications de TR seul, et les résultats obtenus avec TR équivalaient à ceux obtenus avec le SC, ce qui indique que les deux traitements offrent la possibilité d’atténuer la gravité de la pourriture brune dans un verger de pêchers en production biologique.
Introduction
Blossom blight and fruit brown rot, caused by Monilinia fructicola (Wint.) Honey, are very important diseases of peach (Prunus persica (L.) Batsch), with disease incidences of up to 90% in the peach production area of Brazil (May-De Mio et al. Citation2008b). This pathogen can infect flowers, twigs and fruit, and losses are often high in orchards with organic production systems. These orchards usually have high inoculum pressure (Keske et al. Citation2011; Negri et al. Citation2011). Presently, approximately 1095 ha of peaches in the USA are cultivated on organic certified farms, with a production of 21 372 tons (USDA Citation2012). In the European Union, 3.9% of the total agricultural area is used for organic production (Willer et al. Citation2008). While organic production in Brazil comprises less than 1% of the total output, the domestic market for organic products is the most developed in Latin America. Organic production increased from 250 000 ha in 2001 to 880 000 ha in 2008 worldwide (Willer et al. Citation2008). For a grower to receive organic certification, it is necessary to use natural products instead of synthetic ones to manage diseases.
The peach production area in Brazil is located in the southern and south-east regions of the country. There are no biological agents registered for use to control diseases on peach, and organic growers commonly use Bordeaux mixture during the winter and lime sulphur during the growing season, which are not considered synthetic fungicides. Also, environmental considerations have led to an increased interest in using biocontrol agents and other non-synthetic fungicides to control brown rot (Larena et al. Citation2005; Holb & Schnabel Citation2008; Moreira & May-De Mio Citation2009). In organic agricultural systems, it is recommended that sanitation strategies be used to reduce the primary inoculum of the pathogen and that natural products and biological control agents be used to control the pathogen (Ooijkaas et al. Citation1998; Tripathi & Dubey Citation2004). An increase in the use of alternative products and antagonistic microorganisms for biological control of brown rot of stone fruit has been reported (Schena et al. Citation2003; Larena et al. Citation2005).
Brazilian organic growers can use products such as lime sulphur, copper sulphate, colloidal sulphur, iodine, micronutrients, and resistance inducers such as potassium phosphites for brown rot control. These products are registered for use on peaches by the Agricultural Ministry of Brazil (MAPA Citation1999). Holb & Schnabel (Citation2008) reported the use of lime sulphur to control brown rot in detached fruit. Phosphites have also been used as another treatment option. The phosphites, which are micronutrient-based compounds, are mainly marketed as foliar fertilizers and either directly affect the pathogens (Fenn & Coffey Citation1985) or induce defence mechanisms in the plants (Jackson et al. Citation2000; Sônego & Garrido Citation2005). Potassium phosphite applied pre-harvest, for example, reduced the number of brown rot-infected peach fruit 3 and 5 days after harvest by 60% and 28%, respectively, compared with untreated fruits (Moreira & May-De Mio Citation2009).
Several biological control agents have been evaluated for M. fructicola control worldwide. The microorganisms Aureobasidium pullulans (de Bary) G. Arnaud, Epicoccum purpuracens (Link) and Gliocladium roseum Bainier were successfully used in the USA against M. fructicola of cherry (Wittig et al. Citation1997). The fungus Penicillium frequentans Westling was applied in a conidial suspension during the pre-harvest period for the control of Monilinia laxa (Aderh. and Ruhland) Honey in peaches in Spain (Pascual et al. Citation2000; Guijarro et al. Citation2007). Also, during the bloom and pre-harvest periods, Larena et al. (Citation2005) used Epicoccum nigrum Link in field experiments in Spain, Italy and France, and reduced brown rot to 54% as compared with 84% in untreated trees. In Italy, Schena et al. (Citation2003) applied an isolate of A. pullulans pre-harvest and reduced brown rot of cherry by 47%. In Brazil, Trichothecium roseum (isolate UFPR F4) has been tested since 1997 to control brown rot of peach in integrated production systems. This isolate has been extensively tested in several laboratory studies and in post-harvest disease control (Moreira et al. Citation2002) and in field evaluations (Moreira et al. Citation2008; Negri et al. Citation2011).
The most critical phases to control brown rot of peach are from bloom to the end of the pit hardening stage and from the end of embryo growth to the week preceding harvest (Byrde & Willetts Citation1977; Luo & Michailides Citation2003). In Brazil, although infection can occur during all fruit developmental stages (May-De Mio et al. Citation2008a), depending on the inoculum levels and weather conditions during the season, most symptoms develop during the harvest and post-harvest periods.
In organic systems in Brazil, the only study that has provided any preliminary information regarding control of blossom blight and fruit brown rot of peach was carried out by Negri et al. (Citation2011). In this study, lime sulphur, calcium and boron (CaB) and K phosphites, and the antagonist T. roseum applied during flowering and pre-harvest, reduced disease incidence; but no significant reduction in brown rot was observed during harvest. Furthermore, in organic systems in the USA, lime sulphur reduced disease incidence and the area of sporulating lesions when applied at post-harvest at a 1.5% concentration (Holb & Schnabel Citation2008). Lime sulphur is the standard product used in organic peach production to control blossom blight, infection of immature fruit, and fruit brown rot at harvest and post-harvest. However, brown rot control in organic peach orchards is unsatisfactory and new strategies must be studied. Usually the spray programmes tested on organic farms end 3 days before harvest (Negri et al. Citation2011) and fail to control the disease at harvest and post-harvest.
The aims of this study were to: (i) evaluate disease development caused by Monilinia fructicola under organic production systems in Brazil; (ii) determine the efficacy of calcium plus boron phosphites combined with K phosphites, Trichothecium roseum (TR) and a combination of phosphites and TR compared with lime sulphur (LS) for pre- and post-harvest disease development; and (iii) compare the efficiency of TR applied at post-harvest to reduce brown rot after inoculating fruit from conventional and organic systems.
Materials and methods
Field experiment
To evaluate the efficacy of various compounds, field experiments were carried out during the seasons of 2005 and 2006 in an organic peach orchard with cultivars ‘Granada’ and ‘Chimarrita’ (both medium to late season). The trees were 8 years old, trained in an open-vase shape with four scaffolds, and planted with a spacing of 6.5 m between rows and 5 m between trees in the row. The orchard was located at 27°11′07′′S latitude and 49°39′39′′W longitude, in Rio do Sul, Santa Catarina, Brazil. The experimental design used was completely randomized design (one tree per experimental unit, randomized for treatments) and five replications for the ‘Granada’ and six for the ‘Chimarrita’. The trees of each cultivar were separated in rows by untreated peach trees. All trees of each cultivar (‘Chimaritta’ or ‘Granada’) were in the same row. The same trees and design were used in 2005 and 2006 experiments.
The spray treatments, rates of compounds used, and timing (bloom to harvest) are listed in . The volume of suspension of each treatment applied was 20 L (blown with a fan-shape nozzle) using a backpack sprayer (Jacto®, Pompéia-SP, Brazil), with about 3 L per tree. Canopy height and shoot density in each tree were reduced during the winter pruning to obtain good spray coverage. To prevent spray drift from the experimental trees to neighbouring trees, a portable plastic curtain (4 × 4 m) was used to isolate each tree during the spraying process. Trees of ‘Granada’ only received the treatments during bloom that are described in , because fruit did not set due to adverse climatic conditions in both years of the experiment. In 2005, the sprays of ‘Chimarrita’ trees continued into the pre-harvest period and ended 1 week before harvest. In 2006, a spray was added one day before harvest. The treatment with K phosphite was used pre-harvest and the Ca phosphite was applied during bloom, based on the results of previous tests used in integrated production systems (Negri et al. Citation2011). For the treatment combining TR with phosphides, the phosphite spray was done early in the morning and the TR at the end of the afternoon. As described in an earlier study (Moreira & May De Mio Citation2007), phosphites do not have much influence on the growth of TR.
Table 1. Description of treatments, spray schedules and rates of treatments used to control blossom blight and brown rot in peaches of ‘Granada’ and ‘Chimarrita’ in Rio do Sul – SC, Brazil (2005 and 2006).
Rainfall, air humidity and temperature data were collected daily using a pluviometer and thermometer placed in the orchard in both years. The data are presented in and include a period of 10 days before and after flowering, immature fruit and mature fruit stages. The weather data and their relationship to disease incidence were interpreted based on the calculation of average data during the 10 days preceding the collection of flowers, green fruits and the fruit samples collected at harvest.
Table 2. Incidence (%) of blossom blight on flowers after incubation in the laboratory of cultivars ‘Granada’ and ‘Chimarrita’, collected before spraying (BS) and after spraying (AS) with the treatments, in 2005 and 2006, in Rio do Sul – SC, Brazil.
Table 3. Average incidence of latent infection of Monilinia fructicola on green fruit (after incubation in the laboratory) and brown rot incidence (%) at harvest and post-harvest on ‘Chimarrita’in 2005 and 2006 in Rio do Sul – SC, Brazil.
Preparation of T. roseum inoculum
An isolate (UFPR-F4) of T. roseum, stored at the Epidemiology and Disease Integrated Management Laboratory of Federal University of Paraná, was used in these studies. Production and preparation of spore inoculum of T. roseum were adapted from a previously described method (Negri et al. Citation2010). The fungus was grown on sterilized wheat seed, and a spore suspension of 106 conidia mL−1 was prepared by adding tap water to the wheat to wash the spores. Spore viability was determined by checking germination on three Petri dishes containing water agar (WA) (20 g of agar Himedia® L−1 of water). A 0.1 mL aliquot of each spore suspension was spread on the WA dishes, the dishes were incubated at 25 °C for 12 h in the dark and spore germination was determined by counting 100 spores at random in each Petri dish. A spore was considered germinated when the germ tube was 1.5 times the spore diameter. Spore suspensions with >80% germination were used in the experiments described.
Incidence of active infection causing blossom blight in flowers after artificial incubation
Two samples of 40 full-bloom flowers per experimental tree were collected to assess infection by M. fructicola, for both cultivars and seasons. To measure the effect of the treatment, the flowers were collected 24 h before and 24 h after spraying at full bloom (50% of opened flowers). The flowers were placed in a Gerbox®-type plastic box (11 × 11 × 3.5 cm), lined with sterilized filter paper and moistened with 10 mL of sterile water. The containers with the flowers were incubated at 25 °C in the dark for 3 days and at 4 °C for 3 more days (Luo et al. Citation2001). Disease was evaluated using a dissecting microscope by observing the signs of the pathogen (sporodochia) growing on the flower after the initial symptoms of blossom blight.
Incidence of latent infection on green fruit
The incidence of latent infection of M. fructicola in green fruit was carried out only with ‘Chimarrita’, because ‘Granada’ did not produce sufficient fruit for this evaluation. In 2005, samples of 90 fruits/treatment (15 fruit/sample tree) were collected on 13 October (fruit thinning stage), on 3 November (pit hardening stage) and on 10 December (10 days before harvest). The same sampling procedures were followed in 2006 with the samples collected on 8 September (fruit thinning stage) and 4 October (pit hardening stage). The difference in sampling dates in 2006 as compared with those of 2005 was due to an earlier (by almost one month) bloom of the trees in 2006. A total of 1350 and 900 fruit were evaluated in the first and second years of the experiment, respectively. The fruit were immersed in a solution of 70% ethanol, 2% sodium hypochlorite and 2% paraquat® for one minute, washed with sterile water (Northover & Cerkauskas Citation1994) and placed in sterilized PVC trays (40 × 30 cm). The trays were enclosed in a plastic bag containing wet paper towels and incubated at 25 °C for 7 days. After this time, the incidence of brown rot on fruits was recorded based on the signs of M. fructicola on the lesions.
Incidence of brown rot during the harvest and post-harvest periods
For ‘Chimarrita’, the incidence of brown rot at harvest was determined in the orchard by recording the percentage of fruits with characteristic symptoms of the disease and sporulation of the pathogen on two different dates each in 2005 and 2006. All fruit on the tree were evaluated: an average of 166 fruits per sample tree. For the post-harvest evaluations, two samples of 180 fruits per treatment (30 fruits per sample tree) for each harvest date were also collected. Fruits of one sample were immersed in a 0.5% solution of sodium hypochlorite for one minute and washed three times in sterile water. The other sample did not receive any disinfection. This was performed to verify whether the treatment with disinfection can reduce symptom expression. All the fruits were placed on tables covered with wet absorbing paper towels, left at room temperature (22 ± 1 °C) for 5 days, and then evaluated for disease.
Post-harvest experiment
To determine the efficacy of T. roseum to control brown rot applied during post-harvest, one experiment with two treatments, with and without applying T. roseum, was conducted in two different seasons using the cultivar ‘Chimarrita’. The fruits were collected from the conventional production orchard (CP) that had been treated with fungicides during the 2005 season (60 fruits sampled) and fruits were collected from the organic production orchard (OP) during the 2006 season (72 fruits sampled). The experimental design was completely randomized with two treatments and six replications using batches of 10 and 12 fruit in 2005 and 2006, respectively. The fruit were washed twice in water before starting the experiment. After drying excess water, the fruit were immersed in a suspension of 106 conidia mL−1 of T. roseum for one minute, 24 h prior to the inoculation with M. fructicola. Fruit were inoculated with 10 µL of a suspension with 104 conidia mL−1 of M. fructicola on three equidistant points around the top of the fruit (without wounding the fruit). For the control treatment, the fruit were inoculated but not immersed in the T. roseum suspension. The fruit were placed on a laboratory bench; they were assessed for brown rot every day for 5 days of incubation at 25 °C, 70% relative humidity and 12 h of light.
Statistical analysis
Disease incidence data for the field and post-harvest experiments were analysed using ANOVA and means were compared using Tukey’s multiple range test. The area under disease progress curve (AUDPC) was also calculated for post-harvest infection using 5 days assessment as described by trapezoidal integration (Berger Citation1988). A linear correlation was performed to analyse the relationship between the incidence of samples with visible brown rot symptoms (harvest and post-harvest) and the incidence of brown rot infection at each sampling date. To compare the T. roseum treatment with the untreated control, a t-test at P < 0.05 was performed. All analyses were performed by Statistica® Software 8.0 (Tulsa, OK, USA).
Results
Field experiment
A high incidence of flower infection, after incubation of samples in the laboratory, was observed in the untreated trees during bloom in both years of the experiment. The average disease incidence in 2005 and 2006 on flowers was 25% and 39% for ‘Granada’ and 15% and 64% for ‘Chimarrita’ (). The disease incidence was significantly reduced (P ≤ 0.05) after applying the treatments compared with the untreated trees (). Lime sulphur reduced disease incidence by 25–80%, phosphites combined with T. roseum, and T. roseum alone, were also effective in reducing blossom blight by 45–90% and 59–95%, respectively. The application of phosphites on ‘Granada’ in 2005 and on ‘Chimarrita’ in 2006 reduced the disease by 60% and 19%, respectively, compared with the untreated control. There were no significant differences in disease incidence between the two cultivars (Tukey test, P ≤ 0.05); however, disease incidence was higher in 2006 than in 2005 for both cultivars ().
In 2005, lime sulphur reduced the incidence of latent infections in green fruit by 50% (i.e. 4% disease incidence in lime sulphur vs. 8% in the untreated control), whereas the antagonist T. roseum and CaB and K phosphites reduced latent infections by 63% (i.e. 3 vs. 8%). However, in 2006 none of the treatments reduced the incidence of latent infection in green fruit ().
The incidence of brown rot infection at harvest in 2005 was 21% for the control, and none of the sprays reduced disease incidence (). However, in 2006, lime sulphur, phosphites together with T. roseum, and the antagonist T. roseum alone, significantly reduced (P < 0.05) disease incidence by 21%, 22% and 15%, respectively, when compared with the disease incidence of the untreated trees which was 33% (). The most effective treatment was T. roseum; nevertheless, the disease levels were still high.
No differences in disease levels after post-harvest storage were detected between disinfected and non-disinfected fruit (data are not shown). Incidence of post-harvest disease (the mean of disinfected and non-disinfected fruit) in the control was 43% and 45% in 2005 and 2006, respectively (). The antagonist T. roseum significantly reduced (P < 0.05) post-harvest disease to 16% and 22% in 2005 and in 2006, respectively (). Post-harvest disease incidence was significantly correlated with the latent infection incidence of fruit at different developmental stages in both years (r = 0.95, P value = 0.003 for 2005 and r = 0.92, P value = 0.009 for 2006).
The weather conditions were similar for both cultivars in 2005; however in 2006, low temperatures occurred during flower collection for both cultivars and a high amount of rain occurred before the harvest of ‘Chimarrita’ in 2006 ().
Table 4. Averages of temperature (T), relative humidity (RH) and rainfall, 10 days before the collection of flower, green fruit and mature fruit at harvest of ‘Granada’ and ‘Chimarrita’ in 2005 and 2006 in Rio do Sul – SC, Brazil.
Post-harvest experiment
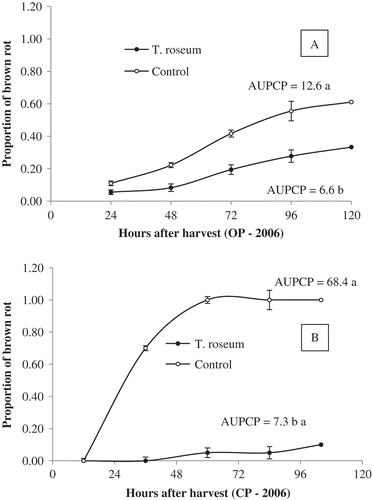
In both assays with fruit from a conventional commercial orchard or from an organic orchard, the TR treatment reduced disease incidence, expressed as the area under the disease progress curve (AUDPC). The AUDPC declined from 68.4 for the untreated control to 7.3 for the TR treatment in 2005 in conventional production in the absence of infection from the field (). In organic production during 2006, the AUDPC was reduced by approximately 50% (from 12.6 in the untreated control to 6.6 in the TR treatment) in the presence of infection from the field and absence of any fungicide treatments ().
Fig. 1 Progress of brown rot of peach ‘Chimarrita’ post-harvest after treatment with Trichothecium roseum (TR). (A) OP = Fruit were collected from an organic orchard (no conventional fungicides applied during the season). Inoculum for post-harvest brown rot infection was from field infections (fruit were not inoculated post-harvest). (B) CP = Fruits harvested from a conventional production orchard (with fungicides applied in the field). Harvested fruit were inoculated with 105 conidia mL−1 Monilinia fructicola 12 h after treatment with TR. In both cases, fruits were dipped in a 106 conidia mL−1 suspension of TR for 1 min. Proportion of brown rot of 0.8 is equal to 80% of incidence.
Discussion
The application of phosphites combined with T. roseum and also lime sulphur can be a choice for organic producers to control or minimize infection by M. fructicola on flowers. When the efficacy of T. roseum and of phosphites alone was separately compared, the antagonist was superior to the phosphites in both cultivars tested during the 2 years of the study with blossoms. The low efficacy of the phosphites during the flowering phase could be explained by the low concentration used, which would require a longer time for direct action on the pathogen and activation of the secondary metabolism of the plant to produce defence substances such as phytoalexins (Jackson et al. Citation2000) and other phenolic compounds (Nojosa et al. Citation2005). The efficacy of K phosphites applied during the pre-harvest season in controlling brown rot on peaches was also shown by Moreira et al. (Citation2008), but no disease control was observed when CaB phosphites were used at that stage.
Both cultivars had a high incidence of blossom blight expressed after the incubation period at the laboratory in both years of the study (), an indication of a high inoculum potential in the area and susceptibility of the cultivars. In the same orchard and cultivar, Keske et al. (Citation2011) detected 19.7% blossom blight on the trees during spring 2006. In the disease evaluation conducted before the spraying, no differences were detected between the treatments, suggesting a uniform inoculum distribution in the area in both 2005 and 2006 growing seasons. This disease occurrence was similar to previously determined levels; for example, Luo et al. (Citation2001) showed 40% disease incidence during the flowering of prunes after inoculation and high humidity. The high humidity and temperature conditions in 2005 and 2006, in the area where these studies were carried out, contributed to the high disease incidence levels (). May-De Mio et al. (Citation2008a) reported that full bloom and pre-harvest phases of peach are the most susceptible stages to M. fructicola infection in Brazil. Therefore, in conventional production, most of the fungicide sprays are applied during bloom and pre-harvest. However, in organic production, it is important to adopt strategies of disease control during bloom with the purpose of reducing flower blight and inoculum sources in cankers. Everhart et al. (Citation2011) reported that the cankers produced on twigs in a year contribute to the primary inoculum in the subsequent year(s).
The more favourable conditions and the earlier (22 days) blooming of the peach cultivars in 2006 compared with 2005 might have contributed to higher disease pressure and disease incidence in both cultivars in 2006. The incidence of latent infections during the period of fruit development varied from 3% to 10% () and correlated with post-harvest fruit rot (r = 0.86, P = 0.02), suggesting the considerable importance of latent infection during this phase under high inoculum pressure. Latent infection of green fruit of ‘Chimarrita’ in Brazil was previously demonstrated (May-De Mio et al. Citation2008a; Keske et al. Citation2011). This type of infection plays an important role primarily under conditions of high inoculum pressure and climatic conditions favourable for disease development. Mondino et al. (Citation1997) evaluated latent infection and verified a 49% incidence in peaches from orchards where disease was not controlled. Emery et al. (Citation2000) found up to 22% of latent infection in peaches from five different orchards. According to Gell et al. (Citation2008), there was a positive correlation between the incidence of latent infection and that of post-harvest brown rot.
The brown rot incidence in fruits at harvest and post-harvest () confirms the results reported by Ogawa et al. (Citation1995), Luo et al. (Citation2001) and Moreira & May-De Mio (Citation2009) who showed an increase of brown rot infection during the fruit maturation stage. The use of lime sulphur (LS) during the flowering and pre-harvest periods was an attempt to confirm this product as an alternative to reduce disease. The fungicidal effects of lime sulphur are well known in several other crops (Raseira & Quezada Citation2003) but have not been evaluated for peach in Brazil. A study with detached fruit showed the efficacy of lime sulphur at a 1.5% rate against brown rot providing a post-inoculation activity (Holb & Schnabel Citation2008). The same authors showed that LS could cause phytotoxicity to flowers. However, in southern Brazil, the recommendation for control of brown rot of peach is to apply LS during periods of dormancy (Raseira & Quezada Citation2003). Indeed, during dormancy, LS is used at a 10% rate, whereas in this study, LS was used at only a 0.3–0.4% rate when applied at flowering and pre-harvest stages, respectively, without any observable symptoms of phytotoxicity. The significant reduction of blossom blight and latent infection on green fruit resulting from LS application offers organic growers an alternative fungicide for organic production systems. However, further studies will be necessary to determine new application rates and timing for harvest and post-harvest brown rot control.
The effect of Trichothecium roseum (TR) in reducing blossom blight in both years and cultivars (‘Granada’ and ‘Chimarrita’) was also observed by Moreira et al. (Citation2008) in the late-season cultivar BR1 in a commercial orchard. The field tests using sprays with TR alone reduced brown rot by 83% in flowers and 95% in green fruit (Moreira et al. Citation2008). The disease reduction by using the antagonist TR in this study (, and ) agrees with results obtained using two other isolates of Trichothecium sp., which reduced brown rot infections by over 85% in inoculated peaches (Moreira & May-De Mio Citation2007). Furthermore, in 2006, low temperatures and frost at the beginning and end of the flowering period in the area where the study took place probably resulted in inconsistent response to the treatments applied during the pre-harvest period in comparison with 2005. In this year, no significant control was observed with one application of TR at harvest, but in 2006, TR significantly reduced brown rot when it was applied one day before harvest (). These results suggest that it is possible to reduce brown rot when the biological control treatment is applied during harvest. It is hypothesized that the pathogen growth is reduced in the presence of a population of antagonist spores.
The use of an antagonistic microbe at harvest could be an option to control brown rot in organic peach production. A reduction of the disease at harvest may contribute to a reduction of the inoculum in the post-harvest period, and it may also act against the primary inoculum of the pathogen in the fruit mummies left on the tree and those on the ground, and in cankers on branches. The same antagonist was also reported on peaches and nectarines grown in California (USA) (Hong & Michailides Citation1997) and used on beans to control white mould (Huang et al. Citation2000). Specifically, in soybeans, T. roseum prevented 90% of germination of uredospores of the rust fungus Phakopsora pachyrhizi Syd. and P. Syd. (Sangit & Jha Citation2002).
When the TR was applied at post-harvest to compare fruit from conventional and organic production systems, the greatest effect was observed when no field infections were present (Fig. ). Although the mechanism of antagonist action by the production of antibiotics and some hyperparasitic effects are already known (Moreira et al. Citation2002), further studies might elucidate the components involved in this process. No evidence of active colonization of fruit and plant tissues by T. roseum was obtained during the two years of this study or in a previous study using the same isolate in Brazil (Moreira et al. Citation2002, Citation2008). These data are in contrast to those reported by Hong & Michailides (Citation1997) who observed colonization of peach and nectarine fruit by T. roseum in commercial orchards in California. In addition, the antagonist developed and survived on fruit mummies on the tree and on the ground. The development and survival of T. roseum in the field are of major importance, as they demonstrate the ability of the antagonist to survive and persist during the peach dormant period and to act against the primary inoculum of M. fructicola. However, this should be validated in the field, by assessing the effect of TR spray on the mummies to reduce primary infections in the next season.
The present study corroborates and complements initial observations made by Negri et al. (Citation2011) that lime sulphur and T. roseum, alone or integrated with CaB and K phosphites, can decrease infection of peach flowers and fruit by M. fructicola in organically grown peach trees, providing an important option to organic peach growers. However, TR combined with phosphites or lime sulphur was no better than the TR applied alone. Future experiments with emphasis on treatments during harvest and post-harvest that can reduce the M. fructicola inoculum during these periods, and supplementary studies relating to doses of lime sulphur, survival of the antagonist in the field, and appropriate formulations are recommended.
Acknowledgements
We thank David P. Morgan for reviewing this manuscript. We also thank the Federal University of Parana, Federal Agro-Technical School of Rio do Sul and the Experimental Station of EPAGRI in Ituporanga, Santa Catarina, for making laboratories, equipment and other facilities available to us to carry out these experiments.
References
- Berger RD. 1988. The analysis of the effects of control measures on the development of epidemics. In: Kranz J, Rotem J. editors. Experimental techniques in plant disease epidemiology. Heidelberg: Springer-Verlang; p. 137–151.
- Byrde RJW, Willetts HJ. 1977. Infection. In: The brown rot of fruit: their biology and control. Oxford (UK): Pergamon Press; p. 87–110.
- Emery KM, Michailides TJ, Scherm H. 2000. Incidence of latent infection of immature peach fruit by Monilinia fructicola and relationship to brown rot in Georgia. Plant Dis. 84:853–857. doi:10.1046/j.1365-3059.2000.00422.x.
- Everhart SE, Askew A, Seymour L, Holb IJ, Scherm H. 2011. Characterization of three-dimensional spatial aggregation and association patterns of brown rot symptoms within intensively mapped sour cherry trees. Ann Bot. 108:1195–1202. doi:10.1093/aob/mcr029.
- Fenn ME, Coffey MD. 1985. Further evidence for the direct mode of action of fosetyl-Al and phosphorous acid. Phytopathology. 75:1064–1068. doi:10.1094/Phyto-75-1064.
- Gell I, De Cal A, Torres R, Usall J, Melgarejo P. 2008. Relationship between the incidence of latent infections caused by Monilinia spp. and the incidence of brown rot of peach fruit: factors affecting latent infection. Eur J Plant Pathol. 121:487–498. doi:10.1007/s10658-008-9268-3.
- Guijarro B, Melgarejo P, Torres R, Lamarca N, Usall J, De Cal A. 2007. Effects of different biological formulations of Penicillium frequentans on brown rot of peaches. Biol Control. 42:86–96. doi:10.1016/j.biocontrol.2007.03.014.
- Holb IJ, Schnabel G. 2008. The benefits of combining elemental sulfur with a DMI fungicide to control Monilinia fructicola isolates resistant to propiconazole. Pest Manag Sci. 64:156–164. doi:10.1002/ps.1492.
- Hong CX, Michailides TJ. 1997. Prune, plum, and nectarine as hosts of Trichothecium roseum in California orchards. Plant Dis. 81:112. doi:10.1094/PDIS.1997.81.1.112D.
- Huang HC, Bremer E, Hynes RK, Erickson RS. 2000. Foliar application of fungal biocontrol agents for the control of white mold of dry bean caused by Sclerotinia sclerotiorum. Biol Control. 18:270–276. doi:10.1006/bcon.2000.0829.
- Jackson TJ, Burgess T., Colquhoun I., Forte GE.. 2000. Action of the fungicide phosphite on Eucalyptus marginata inoculated with Phytophthora cinnamomi. Plant Pathol. 49:147–154. doi:10.1046/j.1365-3059.2000.00422.x.
- Keske C, Amorim L, May-De Mio LL. 2011. Peach brown rot incidence related to pathogen infection at different stages of fruit development in an organic peach production system. Crop Prot. 30:802–806. doi:10.1016/j.cropro.2011.03.005.
- Larena I, Torres R, De Cal A, Liñán M, Melgarejo P, Domenichini P, Bellini A, Mandrin JF, Lichou J, de Eribe XO, Usall J. 2005. Biological control of postharvest brown rot (Monilinia spp.) of peaches by field applications of Epicoccum nigrum. Biol Control. 32:305–310. doi:10.1016/j.biocontrol.2004.10.010.
- Luo Y, Michailides TJ. 2003. Threshold conditions that lead latent infection to prune fruit rot caused by Monilinia fructicola. Phytopathology. 93:102–111. doi:10.1094/PHYTO.2003.93.1.102.
- Luo Y, Morgan DP, Michailides TJ. 2001. Risk Analysis of Brown Rot Blossom Blight of Prune Caused by Monilinia fructicola. Phytopathology. 91:759–768. doi:10.1094/PHYTO.2001.91.8.759.
- MAPA - Ministério da Agricultura, Pecuária e Abastecimento. 1999. Instrução Normativa no 007:Brasília.
- May-De Mio LL, Amorim L, Fayad F. 2008a. Susceptibility of peaches (‘Chimarrita´) at different ages to Monilinia fructicola infection. Phytopathology. 98:S100.
- May-De Mio LL, Moreira LM, Monteiro LB, Justiniano JPR. 2008b. Infecção de Monilinia fructicola no período da floração e incidência de podridão parda em frutos de pessegueiro em dois sistemas de produção. Trop Plant Pathol. 33:173–180.
- Mondino P, Pérez E, Gepp V, Garcia S. 1997. Detección de infecciones quiescentes de Monilinia sp. sobre frutos verdes de duraznero. Fitopatol Bras. 22:287.
- Moreira LM, May-De Mio LL. 2007. Crescimento micelial de Monilinia fructicola e Trichothecium roseum em diferentes temperaturas e sensibilidade a fungicidas e fosfitos. Scientia Agraria. 8:337–341.
- Moreira LM, May-De Mio LL. 2009. Controle da podridão parda do pessegueiro com fungicidas e fosfitos avaliados em pré e pós-colheita. Ciênc Agrotec. 33:405–411. doi:10.1590/S1413-70542009000200007.
- Moreira LM, May-De Mio LL, Valdebenito-Sanhueza RM. 2008. Fungos antagonistas e efeito de produtos químicos no controle da podridão parda em pomar de pessegueiro. Summa Phytopathol. 34:272–276. doi:10.1590/S0100-54052008000300016.
- Moreira LM, May-De Mio LL, Valdebenito-Sanhueza RM, Maria LRZC., Possamai JC. 2002. Controle em pós-colheita de Monilinia fructicola em pêssegos. Fitopatol Bras. 27:395–398. doi:10.1590/S0100-41582002000400010.
- Negri G, Biasi LA, Wordell Filho JA., May-De Mio LL.. 2011. Manejo da queima das flores e da podridão-parda do pessegueiro cultivado em sistema orgânico. Rev Bras Frutic. [online]. 33:415–423. http://dx.doi.org/10.1590/S0100-29452011000500054
- Negri G, May-De Mio LL, Wordell Filho JA. 2010. Produção e armazenamento de Trichothecium roseum para uso como biopesticida no controle de podridão parda do pessegueiro. Scientia Agraria. 11:247–254.
- Nojosa GBA, Resende MLV, Resende AV. 2005. Uso de fosfitos e silicatos na indução de resistência. In: Cavalcanti LS, Di Piero, RM, Cia P, Pascholati SF, Resende, MLV, Romeiro, RS. Indução de resistência em plantas a patógenos e insetos. Piracicaba (SP): FEALQ; pp. 139–153.
- Northover J, Cerkauskas RF. 1994. Detection and significance of symptomless latent infections of Monilinia fructicola in plums. Can J Plant Pathol. 16:30–36. doi:10.1080/07060669409500785.
- Ogawa JM, Zehr EI, Bird GW, Ritchie DF, Uriu K, Uyemoto JK. 1995. Compendium of stone fruit diseases. St. Paul (MN): APS Press.
- Ooijkaas LP, Tramper J, Buitelaar RM. 1998. Biomass estimation of Coniothyrium minitans in solid-state fermentation. Enzyme Microb Technol. 22:480–486. doi:10.1016/S0141-0229(97)00246-9.
- Pascual S, Melgarejo P, Naresh M. 2000. Accumulation of compatible solutes in Penicillium frequentans grown at reduced water activity and biocontrol of Monilinia laxa. Biocontrol Sci Tech. 10:71–80. doi:10.1080/09583150029404.
- Raseira MCB, Quezada AC. Editors. 2003. Pêssego Produção. Embrapa Clima Temperado. Brasília: EMBRAPA. Available from: http://livraria.sct.embrapa.br/liv_resumos/pdf/00074330.pdf
- Sangit K, Jha DK. 2002. Trichothecium roseum: a potential agent for the biological control of soybean rust. Indian Phytopathol. 55:232–234.
- Schena L, Nigro F, Pentimone I, Ligorio A, Ippolito A. 2003. Control of postharvest rots of sweet cherries and table grapes with endophytic isolates of Aureobasidium pullulans. Postharvest Biol Tec. 30:209–220. doi:10.1016/S0925-5214(03)00111-X.
- Sônego OR, Garrido LR. 2005. Avaliação da eficácia de algumas marcas comerciais de fosfito de potássio e de fosfonato de potássio no controle do míldio da videira, circular Técnica no.60. Bento Gonçalves: EMBRAPA. Available from: http://www.cnpuv.embrapa.br/publica/circular/cir060.pdf
- Tripathi P, Dubey NK. 2004. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol Tech. 32:235–245. doi:10.1016/j.postharvbio.2003.11.005.
- [USDA] National Agricultural Statistics Service. 2012. 2011 Certified Organic Production Survey. 165 p. Available from: http://usda01.library.cornell.edu/usda/current/OrganicProduction/OrganicProduction-10-04-2012.pdf
- Wittig HPP, Johnson KB, Pscheidt JW. 1997. Effect of epiphytic fungi on brown rot blossom blight and latent infections in sweet cherry. Plant Dis. 81:383–387. doi:10.1094/PDIS.1997.81.4.383.
- Willer H, Yussefi-Menzler M, Sorensen N, editors. 2008. The World of Organic Agriculture. Statistics and Emerging Trends 2008. IFOAM: Bonn. Available from: http://www.organicworld.net/2008-corrigenda.asp (February 17, 2008).
