Abstract
This study was carried out to investigate the potential role of banana weevils as vectors of Xanthomonas campestris pv. musacearum (Xcm), causal agent of banana wilt. Weevils captured from Xcm-infected plants were tested for presence of Xcm, and further raised on Xcm-infected corms for later use as vectors to transmit the pathogen to healthy tissue-cultured plantlets. Analysis of weevils captured from diseased fields revealed more weevils contained Xcm originating from ‘Mbwazirume’ compared with ‘Kayinja’ cultivars. Colonies of Xcm were recovered from the weevil external body surface, internal organs (mouth parts and abdomen) and faecal matter. There was significantly higher Xcm presence and cfu mL−1 on the external weevil body surface than within the internal organs. Bacterial populations declined progressively from the external body surface, internal mouth parts, internal abdominal parts and the faecal matter. Following placement of weevils previously fed on Xcm-exuding corms in close proximity to healthy potted plants, infection occurred, with characteristic disease symptoms observed on all cultivars evaluated except ‘Kayinja’ which remained symptomless. Isolation from both symptomatic and asymptomatic plants revealed erratic Xcm incidence and cfu g−1 that did not correlate to the number of weevils released in all cultivars, except for ‘Kayinja’. This study showed that Xcm can survive on and within the banana weevil and potentially spread the pathogen to neighbouring plants.
Résumé
Cette étude a été menée dans le but d’évaluer le possible rôle que jouent les charançons du bananier en tant que vecteurs de Xanthomonas campestris pv. musacearum (Xcm), l’agent causal du flétrissement du bananier. Les charançons capturés sur des plants infectés par Xcm ont été testés pour l’y déceler, puis élevés sur des cormes infectés afin de les utiliser comme vecteurs dans le but de transmettre l’agent pathogène à des plantules saines issues de la culture tissulaire. L’analyse des charançons capturés dans des champs infectés a révélé qu’un plus grand nombre d’insectes contenaient le Xcm provenant du cultivar ‘Mbwazirume’ que du cultivar ‘Kayinja’. Des colonies de Xcm ont été récupérées de la surface du corps, des organes internes (pièces buccales et abdomen) et des fèces des charançons. La surface de la carapace des charançons affichait un taux significativement plus élevé de Xcm et de cfu ml−1 que leurs organes internes. Les populations bactériennes décroissaient graduellement en passant de la surface du corps aux pièces buccales internes, aux organes internes de l’abdomen puis aux fèces. Après avoir placé les charançons s’étant préalablement nourris de cormes exsudant le Xcm sur des plants en pot sains, l’infection est survenue, tous les cultivars évalués affichant les symptômes caractéristiques de la maladie, sauf ‘Kayinja’ qui est demeuré intouché. L’isolement des plants symptomatiques et asymptomatiques a révélé une incidence irrégulière de Xcm ainsi que des cfu g−1 qui n’étaient pas corrélés au nombre de charançons relâchés sur tous les cultivars, sauf pour ‘Kayinja’. Cette étude a montré que Xcm peut survivre sur et dans les charançons du bananier et transmettre l’agent pathogène aux plants croissant à proximité.
Introduction
Bananas (including plantains) are cultivated annually on 10.5 million hectares in the tropical and subtropical regions of the world, with annual world production estimated at 27 million tonnes (FAO Citation2013). It is mainly consumed as a dessert banana, but in some parts of Africa, particularly in eastern and southern Africa, banana is also used to make beer or is steamed and eaten. Steamed banana is usually made from the East African highland bananas (genotype AAA-EA) and plantain (genotype ABB) which are grouped under the cooking type of bananas. The East African highland banana comprises about 70% of the bananas produced in the East African region and is a staple food for several of the East African countries, especially Uganda (Sharrock & Frison Citation1999).
Despite its importance in the diet, banana production has declined in Uganda to record low yields of around 5–30 t ha−1 y−1 compared with a potential yield of 70 t ha−1 y−1(Van Asten et al. Citation2004). The major biotic factors responsible for this decline are pests and diseases, including Xanthomonas wilt (BXW) caused by Xanthomonas campestris pv. musacearum) (Xcm) (Tushemereirwe et al. Citation2006) and the banana weevil (Cosmopolites sordidus Germar) (Gold et al. Citation2001). These two pests have been studied extensively in Uganda and found to cause significant losses to the banana production industry.
Symptoms due to BXW include progressive yellowing and wilting of leaves, shrivelling and rotting of the male buds, premature ripening and internal discolouration of fruits, and pockets of pale yellow bacterial ooze produced 5–15 min after diseased stems are cut (Tushemereirwe et al. Citation2004). On the other hand, banana weevils cause damage by larvae feeding within the corm and pseudostem, thus causing significant yield reduction, reduced suckering and shortened plantation life (Gold & Messiaen Citation2000; Gold et al. Citation2002; Messiaen Citation2002). It is known that adult weevils are free-living but are often found at the base of banana mats or associated with crop residues. They are nocturnally active, relatively sedentary and rarely fly. The weevils move freely within banana stands and can migrate into neighbouring fields, although few will disperse more than 50 m in 3 months (Gold et al. Citation1999). Adult weevils may survive up to 4 years while feeding on rotting banana tissue (Budenberg et al. Citation1993) and sometimes may feed on young suckers (Castrillon Citation2000), although the damage is negligible. Adult female weevils deposit their eggs inside the plant tissue at the base of the pseudostem or an exposed corm.
Despite the fact that weevils do feed on and oviposit in the banana plant and are able to move within and between farms, their potential as vectors for Xcm or any other pathogen is unknown. Potentially, the feeding, reproduction and movement of banana weevils within and across farms from adjacent areas could potentially enhance the transmission of Xcm within and between mats and fields. This study was aimed at establishing the potential of adult weevils as vectors for Xcm by investigating: (i) weevil banana cultivar preference; (ii) their potential to carry Xcm in their gut or on their body surface; and (iii) their potential to transmit BXW from infected corms to healthy banana plants.
Materials and methods
This study was conducted on site and in the laboratory located at Kifu National Forest Reserve in Mukono district and the National Agricultural Research Laboratories Institute (NARL), Kawanda, respectively. Four banana cultivars – ‘Kayinja’ (genotype ABB), ‘Mpologoma’ (East African highland banana (EA), genotype AAA), ‘Nakitembe’ (genotype AAA-EA) and ‘Musakala’ (genotype AAA-EA) – obtained from Agro-Genetic Technologies in Buloba, Mityana district, Central Uganda were planted in pots in the Kifu forest reserve for the on-site trials. A fresh Xcm isolate obtained from a symptomatic plant in Mukono, central Uganda was used for this study. All Xcm-like colonies were identified by PCR using Xcm 38 primers (Adikini et al. Citation2011).
Weevil trap, capture and maintenance
Adult male and female banana weevils were captured from ‘Kayinja’ and ‘Mbwazirume’ banana plantations showing BXW symptomatic plants using split pseudostem traps (25–30 cm) from recently harvested pseudostems (30–70 cm above the collar) obtained from a disease-free zone. Traps were placed next to banana mats in fields with high weevil infestation and weevils were recovered using sterile forceps and placed into sterile 5 mL vials at 3-day intervals for a period of 31 days. Subsamples of these weevils were kept at 4°C in the laboratory at NARL for later isolation of Xcm. A total of 1500 weevils were maintained for a period of 4 months on fresh ‘Mbwazirume’ (a weevil-susceptible cultivar) banana corms (i.e. corms were changed weekly) with visible Xcm ooze inside a 20 L perforated closed bucket for later inoculation studies.
DNA extraction and PCR
Genomic DNA was extracted from all Xcm colonies isolated from the captured weevils, weevil body parts and treatment plants using TES buffer (Mahuku Citation2004). A loopful of each Xcm colony was suspended in 500 µL of 1 M NaCl in Eppendorf tubes and vigorously vortexed. The supernatant was discarded and the process repeated twice, to reduce and separate the cells and finally washed twice in sterile distilled water to reduce the salt concentration. The bacterial pellets were suspended in 500 µL of TES extraction buffer (0.2 M Tris-HCl (pH 8), 10 mM EDTA (pH 8), 0.5 M NaCl, 1% SDS containing 50 µg mL−1 proteinase K, and vortexed for 30 s to thoroughly mix. Tubes were incubated in a water bath at 65°C for 15 min, after which 250 µL of 7.5 M ammonium acetate was added and tubes gently inverted thrice to mix. Tubes were incubated on ice for 10 min and later centrifuged at 15 000 rpm for 15 min and the supernatant transferred to a fresh tube. An equal volume (500 µL) of ice-cold isopropanol was added and tubes incubated in a refrigerator at −20°C overnight. Tubes were centrifuged at 15 000 rpm and 4°C for 10 min to pellet the DNA and the supernatant discarded. The DNA pellet was washed with 800 µL of cold 70% ethanol and tubes inverted on clean paper towels for 30 min to air-dry the DNA pellet.
DNA integrity (concentration and purity) of each sample was determined using the NanoDrop® 2000C spectrophotometer (Thermo Fisher Scientific Inc., Pittsburgh, PA) and adjusted to 50 ng µL−1, for PCR using Xcm 38 PCR primers (Adikini et al. Citation2011). Amplification was carried out in a 20 μL reaction with a final concentration of 0.3 µM of each of the primers, Xcm 38 F and Xcm 38 R, 1.5 mM MgCl2, 0.2 µM of each dNTPs (Promega, Madison, WI), 1× PCR green buffer, 1 unit of HotStarTaq Plus DNA polymerase (Qiagen, Canada) and 2 µL of template. The PCR amplification was performed in the Ependorf Mastercycler (Ependorf AG, Hamburg, Germany) and consisted of initial denaturation at 95°C for 5 min; 35 cycles consisting of 94°C for 20 s, annealing at 60°C for 20 s, extension at 72°C for 1 min and a final extension at 72°C for 10 min. The amplified PCR products were separated by gel electrophoresis using 1.5% agarose in 1× TAE. The gel was stained with ethidium bromide (0.5 µg mL−1) and visualized with a UV transilluminator.
Isolation and detection of Xcm from weevils directly trapped from diseased banana fields
A total of 68 weevil samples per banana cultivar (i.e. ‘Kayinja’ or ‘Mbwazirume’), each comprised of a vial of 3 weevils maintained at 4°C, were used for this study. Three mL of PBS-T, pH 7.4 (Na2CO3, 1.19 g; KHPO4, 0.2 g; KCl, 0.2 g; NaCl, 8.0 g; Tween-20 0.5mL) were added to each vial and vortexed for one min to dislodge any Xcm bacteria from the weevil body surface. One mL of this surface rinse was serially diluted twice. For each dilution, 20 µL of the suspension was spread onto three Petri dishes containing a semi-selective growth medium of yeast peptone glucose agar (YPGA – yeast extract 5 g, peptone 5 g, glucose 10 g, and agar 15 g, 5-fluorouracil and cephalexin in 1000 mL of water) (Mwangi et al. Citation2007) and incubated at 28°C for 72 h. Each sample was monitored for growth of Xcm characteristic colonies and a score of ‘1' assigned for Xcm presence and '0' for colony absence.
To determine if the same weevils had Xcm in their internal organs, weevils were surface sterilized by washing thrice with 3 mL of 15% sodium hypochlorite, followed by a single wash with 70% ethanol, and finally rinsed thrice with 3 mL of sterile distilled water by vortexing for 30 s. Twenty µL of the final rinse of each weevil sample was plated to confirm that no Xcm from the external body surface was present. The weevils were ground using a sterile mortar and pestle and 3 mL of sterile distilled water added. Xcm was isolated from the diluent as described for the external body parts above.
All data were subjected to analysis of variance using GenStat statistical software (Edition 12) and where significance was noted, LSD (Least Significance Difference) at 5% was employed to separate the means.
Xcm isolation from faecal matter, gut and external body parts of weevils maintained on diseased corms
Xcm isolation from the body surface
A total of 50 weevils maintained on the Xcm oozing corm samples were placed in separate vials. Three mL of PBS-T were added to each vial and vortexed for one min to dislodge any Xcm from the weevil body surface. One mL of this surface rinse was serially diluted twice. For each dilution, 20 µL was plated and incubated as described above. The plates were monitored for growth of Xcm colonies and number of colonies counted. The mean cfu mL−1 for each weevil sample was computed as follows:
Xcm isolation from the internal gut section
Fifty weevils were surface-sterilized as described above and rinsed thrice with 3 mL of sterile distilled water by vortexing for 30 s. 20 µL of the final rinse of each weevil sample was plated to confirm that no Xcm from the external body surface was present. A stereomicroscope was used to dissect each weevil into head, thorax and abdomen regions. Internal viscera were removed from the body segments using sterile forceps and scalpels under the stereomicroscope. The organs were ground using a mortar and pestle in 3 mL of PBS-T buffer and 1 mL of the macerate serially diluted, plated on semi-selective YPGA and incubated as above. Xcm presence and colony counts per gut section/body part were determined. The cfu mL−1 of each sample and body part were computed.
Xcm isolation from the faecal matter
Fifty weevils maintained on Xcm oozing corms were surface-sterilized as described above, rinsed thrice with 50 mL of sterile distilled water and blotted dry with sterile paper towels in the laminar flow hood. 20 µL of the final rinse of each weevil sample was plated to confirm that no Xcm from the external body surface was present. Five weevils were sealed in a clean Petri dish with perforated parafilm to allow air circulation and kept in the dark at room temperature overnight to collect the faecal matter. The weevils were removed and Petri dishes washed with 3 mL of PBS-T by shaking the covered Petri dishes for 5 min at 100 rpm on a mechanical shaker. One mL of the resulting suspension was plated and data captured as previously described in the section(s) above.
Xcm transmission by weevils fed on Xcm oozing corms
This experiment was conducted to determine the potential of weevils maintained on Xcm oozing corms to transmit Xcm to healthy potted plantlets. Four different banana cultivars: ‘Kayinja’ (Musa ABB), ‘Mpologoma’ (Musa EA-AAA), ‘Nakitembe’ (EA-AAA) and ‘Musakala’ (EA-AAA) were used in this study. A total of 30 plants per cultivar were each planted in 20 L buckets containing sterilized soil and arranged in a completely randomized design. Plants were grown for 3 months prior to treatment application with continued weeding and watering as required. No fertilizers, pesticides or herbicides were applied to the plants. Treatments were applied in orders of 10 weevils (T10), 5 weevils (T5) and 1 weevil (T1) per pot by placing the weevils maintained on Xcm oozing corms close to the plant. Controls included plantlets that received no weevils as a negative control and plantlets inoculated in the corm by injecting with 1 mL of freshly prepared Xcm suspended in sterile distilled water and adjusted to 0.1 O.D600 (corresponding to 1.0×108 cfu mL−1) as a positive control. Plants were maintained in a confined area outdoors and observed for disease symptoms over a period of 60 days post-inoculation. Corm, pseudostem and leaf samples from symptomatic and asymptomatic plants were aseptically sampled at the end of the experiment and the presence of Xcm confirmed on agar medium (Mwangi et al. Citation2007) and using PCR (Adikini et al. Citation2011).
Pseudostem samples were taken from 10 cm above the collar region; leaf samples included the leaf petiole and lamina; while the entire corm was sampled. About 3 g of each sample was macerated with sterile mortar and pestle in 5 mL of sterile distilled water. One mL of the resulting macerate was diluted two times. For each dilution, 20 µL was plated onto three Petri plates containing semi-selective YPGA medium and incubated at 28°C for 72 h. Characteristic Xcm colonies were counted and the mean colony forming units (cfu) g−1 of plant tissue for the triplicate plates was computed as indicated below:
Results
Detection of Xcm in weevils captured from Xcm infected plants
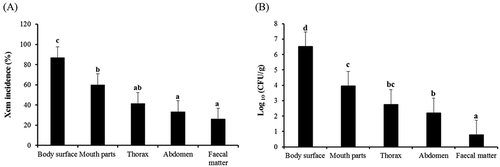
Xanthomonas campestris pv. musacearum was detected on both the external and internal body parts of the weevils captured from banana fields with plants showing characteristic banana Xanthomonas wilt symptoms (). Significant differences (P < 0.001) in Xcm incidence in the banana weevils were observed between the two cultivar types, and the external and internal weevil surfaces/organs. For example, more weevils with Xcm were observed from ‘Mbwazirume’ compared with ‘Kayinja’ fields (). Similarly, there was a higher Xcm incidence on the weevil body surfaces compared with the internal body surfaces/organs (). The Xcm-like colonies from both cultivar types and body parts were confirmed to be positive for Xcm with PCR ().
Table 1. Proportion of weevils that had Xcm on the external and internal body surfaces captured from ‘Mbwazirume’ and ‘Kayinja’ fields with banana Xanthomonas wilt symptomatic plants.
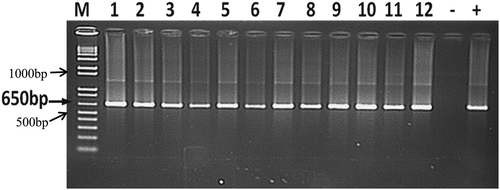
Fig. 1 Agarose gel photo of PCR amplification of Xcm isolates from different studies. Lane 1 to 5: denotes DNA fragments of Xcm isolates from weevils captured from ‘Kayinja’ and ‘Mbwazirume’ fields; Lane 6 to 9: DNA fragments of Xcm isolates from ‘Kayinja’, ‘Musakala’, ‘Nakitembe’ and ‘Mpologoma’, respectively, inoculated with weevils previously fed on Xcm oozing corms; and Lane 10 to 12: DNA fragments of Xcm isolates from weevil head (including mouth parts), thorax and abdomen. M is a 1kb ladder.
Occurrence of Xcm on the weevil body surface, internal body parts and faecal matter
Xanthomonas campestris pv. musacearum was isolated from the external body surface, internal parts (i.e. the mouth, thorax and abdomen) and the faecal matter of the weevils that had been maintained on Xcm oozing corms (, b). These colonies were confirmed by PCR using Xcm specific primers (). Significant differences (P < 0.001) were observed in Xcm incidence and colony forming units (cfu mL−1) between the external and internal body parts and faecal matter of the weevils (, b). Significantly more weevils had Xcm on their external body surfaces. The mouth parts ranked second in Xcm incidence and cfu mL−1; incidence and cfu mL−1 declined along the gut from the mouth parts to the abdomen, with the least observed in the faecal matter. No significant differences (P < 0.05) were observed between the number of weevils with Xcm in the abdomen and faecal matter (, b).
Transmission of Xcm to healthy plants by weevils
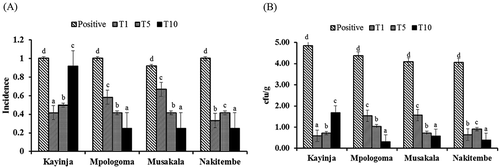
Weevils previously fed on Xcm oozing corms were able to infect healthy 3 month old potted banana plantlets. The first symptomatic plants were recorded 36 days post-inoculation on positive control plants. The plants that did not receive any weevils remained healthy for the duration of the experiment and no Xcm was isolated from them (). ‘Mpologoma’, ‘Musakala’ and ‘Nakitembe’ plants subjected to the different weevil treatments showed symptoms such as death of tissues around the point of inoculation, wilting of leaves and death of the entire plant; while ‘Kayinja’ remained asymptomatic for the duration of the experiment (). Plants that received treatment T10 showed symptoms earlier than those that received T5 and T1. PCR analysis of isolates of Xcm from representative plants from each cultivar confirmed presence of Xcm, even in ‘Kayinja’ plants that were asymptomatic ().
Table 2. Presence and absence of banana Xanthomonas wilt characteristic symptoms on plants treated with 1 weevil (T1), 5 weevils (T5) and 10 weevils (T10) 60 days post-inoculation. Positive controls were inoculated with known Xcm isolate while the negative controls did not receive any treatment.
Significant differences (P < 0.001) were also observed in Xcm incidence and cfu g−1 in the different treatments as well as interaction between treatment and banana cultivar. Incidence was inconsistent and did not correlate to the number of weevils released to the plants, except for ‘Kayinja’ (). Similar trends were observed in cfu g−1 for each cultivar (). The highest number of cfu g−1 were observed in ‘Kayinja’, ‘Mpologoma’ and ‘Musakala’ for treatment levels T10, T5 and T1, respectively (). The least colony forming units were observed in ‘Mpologoma’ (T10) and ‘Nakitembe’ (T10). Trends in cfu g−1 were similar in ‘Mpologoma’ and ‘Musakala’ with the highest cfu g−1 in plants treated with T1 and the least in plants treated with T10 (). In ‘Kayinja’, plants treated with T10 had the highest cfu g−1 while those treated with T1 had the least; however, for ‘Nakitembe’, plants treated with T5 had the highest number of cfu g−1 while those treated with T10 had the least number of cfu g−1 ().
Discussion
This study determined the potential of banana weevils to act as vectors for Xcm. Presently, Xcm has been confirmed to be transmitted by insects such as bees under field conditions (Tinzaara et al. Citation2006). Meki et al. (Citation2010) confirmed the indirect role of nematodes in soilborne Xcm transmission through nematode-inflicted root damage in banana. In this study, Xcm was recovered from within the internal weevil organs as well as the outer surface of weevils captured from fields with diseased plants. Similarly, Xcm was recovered from the outer surface, internal organs and faecal matter of weevils that were fed on Xcm oozing corms in the laboratory. Windels et al. (Citation1976) reported contamination of corn seed by picnic beetles (Glischrochilus quadrisignatus) (McCoy & Brindley Citation1961; Luckmann Citation1963) carrying Fusarium oxysporum (Windels & Windels Citation1974) internally and externally. The presence of Xcm in the different body parts of the banana weevil and its subsequent occurrence in faecal matter raises questions on banana Xanthomonas wilt spread and management. For example, if Xcm can persist through the digestive processes in the insect gut, weevil foraging on infected plant material could potentially transmit Xcm to healthy banana plants while foraging or ovipositing eggs. Weevil eggs are usually laid in the crown of the rhizome and pseudostem base (Abera et al. Citation1999). Females oviposit their eggs in small crevices chewed in the plant tissue (Beccari Citation1967), thus creating an opportunity to infect the plant with Xcm.
Under the current Xanthomonas wilt management practices, farmers cut and chop into pieces diseased plants that often release excessive Xcm ooze. Weevils are attracted to volatiles emanating from cut banana pseudostems, rhizomes and suckers (Gold & Messiaen Citation2000; Gold et al. Citation2002) and will thus burrow under these Xcm oozing corm or pseudostem pieces. When these pseudostems desiccate, or during oviposition and feeding, these weevils could move and spread bacteria to infect healthy plants within the field. In pot trials to prove this, weevils previously fed on Xcm oozing corms successfully infected healthy banana plantlets, irrespective of the number of weevils. These results suggest that the control of banana weevils is equally important to the management of banana Xanthomonas wilt.
Banana weevils are able to move between farms (Gold & Bagabe Citation1997; Gold et al. Citation2002). Flight among adult banana weevils is rare and adults mainly move by crawling at night with estimated distances of 10 m over several months (Gold et al. Citation1999), with only a small proportion moving beyond 25 m in 6 months (Gold & Messiaen Citation2000). For example, Gold et al. (Citation1999) found only less than 3% of marked weevils in a plot other than that of their release after 3 years in a mark–recapture experiment. However, similar experiments reported 60% of marked weevils to have moved more than 10 m over a 2 week period (Gold et al. Citation2001). Weevil movement is influenced by both host plant volatiles and their aggregation pheromone (Tinzaara et al. Citation2003), although the distance over which they can sense the volatiles and orient towards the host plant in the field is unknown (Gold et al. Citation1999). These studies suggest that the role of the banana weevil as a vector of Xcm could be influenced by various factors that could include but are not limited to: the predominant cultivar and management practices and agro-ecological conditions. From our results, we observed higher Xcm incidence and cfu mL−1 on weevils captured from ‘Mbwazirume’ fields compared with those from ‘Kayinja’ fields. This could be attributed to the high attractiveness of ‘Mbwazirume’ to the banana weevil. Cultivar preferences associated with corm hardiness and chemical characteristics (antibiosis) are responsible for weevil preferences to banana cultivars (Gold et al. Citation1993). For example, Kiggundu (Citation2000) showed higher weevil damage in ‘Mbwazirume’ compared with ‘Kayinja’. However, fewer cfu g−1 were recovered from ‘Mbwazirume’ and the other highland bananas compared with ‘Kayinja’ in the pot trials. This inconsistency may be due to differential colonization of Xcm on weevils. Also, it can be attributed to the fact that samples used for Xcm isolations in the highland cultivars had full-blown symptoms and had started to senesce whereas ‘Kayinja’ samples were fresh though symptomless. It has been observed that recovery of Xcm from plants with full-blown symptoms and at the point of senescence is inhibited by the high population of saprophytic microbes (Were Evans, personal communication). This study suggests that weevils can play a role as vectors of Xcm and hence the need to incorporate weevil management in the integrated disease management of banana Xanthomonas wilt.
Acknowledgements
Special thanks are owed to the International Institute of Tropical Agriculture (IITA) and Association for Strengthening Agricultural Research in East and Central Africa (ASARECA) for funding all activities of this study. Many thanks to Francis Ssebulime and Ronald Senabulya for their valuable contribution to this work.
Additional information
Funding
References
- Abera AMK, Gold CS, Kyamanywa S. 1999. Timing and distribution of attack by the banana weevil (Coleoptera: Curculionidae) in East African highland banana (Musa spp.). Fla Entomol. 82:631–641.
- Adikini S, Tripathi L, Beed F, Tusiime G, Magembe EM, Kim DJ. 2011. Development of a specific molecular tool for detecting Xanthomonas campestris pv. musacearum. Plant Pathol. 60:443–452.
- Beccari F. 1967. Contributo alla conoscenza del Cosmopolites sordidus (Germ.) (Coleopt., Curcul.). Riv Agr Subtrop Trop. 61:131–150.
- Budenberg WJ, Ndiege JO, Karago FW, Hansson BS. 1993. Behavioural and electro-physiological responses of the banana weevil Cosmopolites sordidus to host plant volatiles. J Chem Ecol. 19:267–277.
- Castrillon C. 2000. Distribution de las Especies de picudo del platano Evalucion de sus Entomopatogenous Nativos en el Departemento de Risaralda CORPOICA. Manizales (Columbia). Manizales. 1–3.
- FAO. 2013 Mar. Core production data. Rome (Italy): FAOSTAT, Food and Agriculture Organization of the United Nations. http://faostat.fao.org/site/340/default.aspx
- Gold CS, Bagabe MI. 1997. Banana weevil, Cosmopolites sordidus Germar (Coleoptera: Curculionidae), infestations of cooking- and beer-bananas in adjacent plantations in Uganda. Afr Entomol. 5:103–108.
- Gold CS, Kagezi G, Nemeye P, Ragama P. 2002. Density effects of the banana weevil Cosmopolites sordidus (Germar) on its oviposition performance and egg and larval survivorship. Insect Sci Appl. 22:205–213.
- Gold CS, Messiaen S. 2000. The banana weevil Cosmopolites sordidus. Musa Pest Fact Sheet No 4. Montpellier (France): INIBAP.
- Gold CS, Ogenga-Latigo MW, Tushemereirwe W, Kashaija I, Nankinga CM. 1993. Farmer perceptions of banana pest constraints in Uganda. In: Gold CS, Gemmil B, editors. Results from a rapid rural appraisal. Biological and integrated control of highland banana and plantain, Proceeding of Research Co-ordination meeting. Ibadan (Nigeria); International Institute of Tropical Agriculture (IITA). p. 3–25.
- Gold CS, Pena JE, Karamura EB. 2001. Biology and integrated pest management for the banana weevil Cosmopolites sordidus (Germar) (Coleoptera: Curculionidae). Int Pest Manag Rev. 6:79–155.
- Gold CS, Rukazambuga NDTM, Karamura EB, Nemeye P, Night G. 1999. Recent advances in banana weevil biology, population dynamics and pest status with emphasis on East Africa. In: Frison EA, Gold CS, Karamura EB, Sikora RA, editors. Mobilizing IPM for sustainable banana production in Africa. Montpellier (France): INIBAP; p. 35–50.
- Kiggundu A. 2000. Host-plant interactions and resistance mechanisms to banana weevil Cosmopolites sordidus (Germar) in Ugandan Musa germplasm [ M.Sc. Thesis]. Bloemfontain (South Africa): University of the Orange Free State.
- Luckmann WH. 1963. Observations on the biology and control of Glischrochilus quadrisignatus. J Econ Entomol. 56:681–686.
- Mahuku GS. 2004. A simple extraction method suitable for PCR-based analysis of plant, fungal and bacterial DNA. Plant Mol Bio Rep. 22:71–81.
- McCoy CE, Brindley TA. 1961. Biology of the four-spotted fungus beetle, Glischrochilus quadrisignatus, and its effect on European corn borer populations. Plant Mol Bio Rep. 54:713–717.
- Meki S, Addis T, Mekonen S, De Waele D, Blomme G. 2010. Nematode infection predisposes banana to soil-borne Xanthomonas campestris pv. musacearum transmission. Tree For Sci Biotech. 4:63–64.
- Messiaen S. 2002. Components of a strategy for the integrated management of the banana weevil Cosmopolites sordidus (Germar) (Coleoptera: Curculionidae). Dissertationes de Agriculture No. 540 [ PhD thesis]. Belgium: Faculty of Agricultural and Applied Biological Sciences, Catholic University Leuven.
- Mwangi M, Mwebaze M, Bandyopadhyay R, Aritua V, Eden-Green S, Tushemereirwe WK, Smith J. 2007. Development of a semi-selective medium for isolating Xanthomonas campestris pv. musacaerum from infected vectors, infected plant materials and soil. Plant Pathol. 56:383–390.
- Sharrock S, Frison E. 1999. Musa production around the world-trends, varieties and regional importance. In: Networking Banana and Plantain: INIBAP Annual Report 1998. International Network for the Improvement of Banana and Plantain, Montepellier, France, pp 42–47.
- Tinzaara W, Dicke M, Van Huis A, Van Loon JJA, Gold CS. 2003. Different bioassays for investigating orientation responses of the banana weevil, Cosmopolites sordidus, show additive effects of host plant volatiles and a synthetic male- produced aggregation pheromone. Entom Exp Appl. 106:169–175.
- Tinzaara W, Gold CS, Ssekiwoko F, Tushemereirwe WK, Bandyopadhyay R, Abera A, Eden-Green SJ. 2006. Role of insects in the transmission of banana bacterial wilt. Banana bacterial wilt in Uganda: a disease that threatens livelihoods. Afr Crop Sci J. 14:105–110.
- Tushemereirwe WK, Kangire A, Ssekiwoko F. 2004. First report of Xanthomonas campestris pv. musacearum on banana in Uganda. Plant Pathol. 53:802.
- Tushemereirwe WK, Okaasai O, Kubiriba J, Nankinga C, Muhangi J, Odoi N, Opio F. 2006. Status of banana bacterial wilt in Uganda. Special issue. Banana bacterial wilt in Uganda: a disease that threatens livelihoods. Afr Crop Sci J. 14:73–82.
- Van Asten PJA, Gold CS, Wendt J, De Waele D, Okech SHO, Ssali H, Tushmereirwe WK. 2004. The contribution of soil quality to yield and its relation with other banana yield loss factors in Uganda. In: Blomme G, Gold CS, Karamura E, editors. Proceedings of the Workshop held on farmer participatory testing of IPM options for sustainable banana production in eastern Africa. Montpellier: IPGRI; p. 100–115.
- Windels CE, Mark B, Kommedahl T. 1976. Association of Fusarium species with picnic beetles on corn ears. Phytopathology. 66:328–331.
- Windels CE, Windels MB. 1974. Nitidulid beetles as vectors for Fusarium species on corn. Annu Proc Am Phytopathol Soc. 1:131 (Abstr).