Abstract
The frequency of strains of Monilinia fructicola that are resistant to the demethylation inhibiting fungicide tebuconazole in Brazil was determined by three methods. The first involved testing 295 isolates for relative growth under a discriminatory dose of 0.3 µg mL−1. The second and the third methods involved testing 120 isolates for the effective concentration for 50% population inhibition (EC50); one method employed the discriminatory dose of 0.3 µg mL−1 and the other method utilized the baseline dose of 0.046 µg mL−1 to separate the strains based on sensitivity. The frequencies of resistance among the isolates based on the three methods were determined by measuring mycelial growth in fungicide-amended media. The shifts in M. fructicola sensitivities were determined by comparing the EC50 density distributions of the baseline (2000–2004, n=31), intermediate (2005–2008, n=88), and current (2009–2011, n=120) isolate populations. The frequency of resistance to tebuconazole varied according to the assessment method; the resistance frequencies were 18.3% for the relative growth method, 40% for the baseline EC50 method, and 8.33% for the EC50 method segregated by the dose of 0.3 µg mL−1. The application of the EC50 method involving the discriminatory dose of 0.3 µg mL−1 revealed a decrease in the frequency of resistant genotypes. However, the baseline EC50 method is recommended for future resistance surveys because this method was originally used to determine the mean sensitivity prior to the use of tebuconazole in Brazil. Additionally, a shift of the current population toward sensitivity was observed compared with the intermediate population. Finally, São Paulo was found to be the state with the highest EC50 values (0.186 µg mL−1) compared with Paraná (0.053 µg mL−1) and Rio Grande do Sul (0.026 µg mL−1). These results suggest that the build-up of fungicide resistance in Monilinia fructicola has been prevented by the use of anti-resistance strategies in the main peach production areas of Brazil; however, additional studies are required to demonstrate how resistance instability and fitness are affecting isolate sensitivity.
Résumé
La fréquence des souches de Monilinia fructicola qui, au Brésil, sont résistantes au tébuconazole, un fongicide inhibiteur de la déméthylation, a été déterminée par trois méthodes. La première consistait à analyser la croissance relative de 295 isolats à une dose discriminatoire de 0.3 µg mL−1. La deuxième et la troisième méthode consistaient à analyser 120 isolats afin d’évaluer la concentration efficace permettant d’inhiber 50% de la population (CE50); une méthode reposait sur la dose discriminatoire de 0.3 µg mL−1 et l’autre, sur la dose de référence de 0.046 µg mL−1 pour séparer les souches en se basant sur la sensibilité. Les fréquences de résistance chez les isolats, basées sur les trois méthodes, ont été déterminées en mesurant la croissance mycélienne dans un milieu de culture amendé avec le fongicide. Les changements de sensibilité chez M. fructicola ont été déterminés en comparant les distributions de densité de la CE50 chez les populations d’isolats de référence (2000–2004, n = 31), intermédiaires (2005–2008, n = 88) et courantes (2009–2011, n = 120). La fréquence de résistance au tébuconazole variait selon la méthode d’analyse utilisée. Les fréquences de résistance étaient de 18.3% avec la méthode de croissance relative, de 40% avec la méthode faisant appel à la CE50 impliquant la dose de référence et 8.33% avec la méthode faisant appel à la CE50 impliquant la dose discriminatoire de 0.3 µg mL−1. Cette dernière a révélé une réduction de la fréquence des génotypes résistants. Toutefois, la méthode de référence de la CE50 est recommandée pour les futures études sur la résistance parce qu’elle a été utilisée initialement pour déterminer la sensibilité moyenne préalablement à l’utilisation du tébuconazole au Brésil. De plus, un changement de la population actuelle à l’avantage de la sensibilité a été observé comparativement à la population intermédiaire. Finalement, l’État de São Paulo s’est révélé celui où les valeurs de la CE50 étaient les plus élevées (0.186 µg mL−1), comparativement au Paraná (0.053 µg mL−1) et au Rio Grande do Sul (0.026 µg mL−1). Ces résultats suggèrent que le développement de la résistance au fongicide chez M. fructicola a été évité grâce à la mise en place de stratégies antirésistance dans les principales régions productrices de pêches au Brésil. Toutefois, il faut procéder à des études supplémentaires pour démontrer comment l’instabilité de la résistance et l’aptitude pour celle-ci influencent la sensibilité de l’isolat.
Introduction
Monilinia fructicola (G. Wint.) Honey is the only causal agent of brown rot disease of peaches in Brazil (Bleicher Citation1997; May De Mio et al. Citation2004). The control of M. fructicola is necessary during the blooming, pre- and post-harvest stages to prevent blossom blight and fruit rot (Bleicher Citation1997). Control of this disease requires multiple fungicide applications (May De Mio et al. Citation2008), including methyl benzimidazole carbamates, dicarboximides, quinone outside inhibitors, and demethylation inhibitor (DMI) (May De Mio et al. Citation2004), combined with cultural practices that begin in the winter and seek to reduce the source of the inoculum (Fortes & Martins Citation1998). Such cultural practices include the removal of mummified fruits and the pruning of diseased shoots and limbs (Harvey et al. Citation1972). According to May De Mio et al. (Citation2011), protective fungicides applications are performed during flowering and later in the season near harvesting. The number of applications per season can vary between locations due to climate conditions (May De Mio et al. Citation2011) and involve up to eight applications in Rio Grande do Sul (Tibola et al. Citation2005), 10 in Paraná (May De Mio et al. Citation2008) and up to 15 in São Paulo (May De Mio et al. Citation2011). With so many sprays, it is difficult to determine how many times DMI tebuconazole is applied; however, it is known that tebuconazole is among the most widely used DMI in Brazil for controlling brown rot disease (Silva et al. Citation2011). Consequently, the selection of tebuconazole resistant isolates of M. fructicola is mainly occurring in regions where frequent fungicide applications are needed (May De Mio et al. Citation2011). During the time at which May De Mio et al. (Citation2011) has observed the resistance to tebuconazole, Silva et al. (Citation2011) reported that 60% of peach growers from Rio Grande do Sul used tebuconazole sprays to control M. fructicola. Considering the above-mentioned findings of May De Mio et al. (Citation2011) and Silva et al. (Citation2011), it is likely that the sensitivity of M. fructicola to tebuconazole has decreased in Brazil. Therefore, it is critical to monitor the development of resistance to establish an effective fungicide management programme that will help to maintain low frequencies of resistant genotypes and prolong the efficacy of tebuconazole.
Fungal isolates that are kept in storage are useful for laboratory fungicide resistance studies because they provide sensitivity information from before and after the introduction of a fungicide (Penrose & Senn Citation1995; Zhu et al. Citation2012). However, the effects of laboratory storage of M. fructicola have been demonstrated by Zhu et al. (Citation2012) to cause a reversion in DMI sensitivity that cannot be prevented via the use of different storage methods and temperatures. These effects are extremely important due to their potential interference with the interpretation of data collected from isolates that are maintained under storage conditions.
The objectives of this study were to: (i) determine the frequency of M. fructicola-resistant isolates using three different methods for the determination of the sensitivity of this pathogen to tebuconazole; (ii) examine the differences in M. fructicola tebuconazole sensitivity distributions among populations collected within the last 11 years; (iii) verify the differences in M. fructicola tebuconazole sensitivity distributions between the origins from which the current populations were collected; and (iv) investigate the effects of storage on the stabilities of the sensitivities of M. fructicola isolates.
Materials and methods
Isolate set and fungicide stock solution
Studies were performed at the Laboratory of Epidemiology for Integrated Plant Disease Management (LEMID) of the Federal University of Paraná (UFPR). A total of 295 isolates of M. fructicola were obtained from symptomatic and mummified peach fruits collected in the states of São Paulo (SP), Paraná (PR) and Rio Grande do Sul (RS) from 2009 to 2011 (). Before any isolate preparation, isolates from 2009 and 2010 recovered from stored mummies were resurrected on peach fruit to regain their vigour. Single-spore isolates were cultured in full-strength acidified potato dextrose agar (APDA with 2.5 mL of 25% [w/v] lactic acid; Hi-Media Laboratories, Mumbai, India) and incubated for 5 days at 22°C under a 12-h light regime. The mycelial inoculum used in the experiments was produced on this medium. Tebuconazole stock solution was prepared by dissolving the commercial product (Folicur 200 EC 200 g L−1, Bayer S/A) in 100% ethanol and then diluting it to concentrations of 10 and 100 mg a.i. mL−1. The baseline (2000–2004, BL) and intermediate (2005–2008, IP) sensitivities of M. fructicola populations to tebuconazole that were used for comparison with the current population (CP) are those described by May De Mio et al. (Citation2011). In the present study, the current (2009–2011), and intermediate (2005–2008) isolate populations were collected from the same surveyed orchards located in the Rio Grande do Sul, Paraná and São Paulo states in Brazil. The baseline (2000–2004) population excluded isolates from São Paulo state.
Table 1. Location, year and Monilinia fructicola isolate codes sampled for this study.
Frequencies of resistance
The frequencies of resistance to tebuconazole of the isolates from the current population (2009–2011) were determined with three different sensitivity methods. The first method tested was the relative growth under the tebuconazole discriminatory dose (RG) method that was suggested by Russell (Citation2004) and was applied to 295 isolates. The RG method has previously been used in M. fructicola propiconazole resistance studies (Cox et al. Citation2007; Amiri et al. Citation2009). This method consists of the addition of tebuconazole stock solution to APDA cooled to 45°C after sterilization to produce a final discriminatory concentration of 0.3 µg mL−1. Four-millimetre mycelial plugs from 5-day old cultures were placed at the centre of supplemented and non-supplemented media while allowing for direct contact between the mycelia and media. Three repetitions were prepared per sample. Colony growth was recorded by averaging two perpendicular colony diameters after 4 days of incubation at 24°C under a 12-h light regime. The growth in the supplemented media was expressed as a percentage relative to the growth in the non-supplemented media. Resistant isolates were defined as those that obtained at least 50% of the colony growth size observed in the non-supplemented media (Russell Citation2004).
The second and third methods utilized the EC50 values for the determinations of the sensitivities of 120 randomly selected isolates. Stock solution was added to APDA and cooled to 45°C after sterilization to produce final concentrations of 0, 0.01, 0.04, 0.16, 0.64, 2.56 and 10.24 µg mL−1. For each isolate, a 4-mm mycelial plug was transferred from a 5-day-old colony and placed onto APDA media supplemented with one of the above fungicide concentrations. Two repetitions were prepared for each concentration, and plugs were placed upside down to allow contact between the mycelia and the media. The incubation parameters and colony measurement were consistent with previous descriptions. For each concentration, the inhibition of colony growth (Li) of isolate i was calculated as Li = (Cck–Ci)/Cck·100, where Cck is the mean colony diameter of the control with no fungicide, and Ci is the mean colony diameter of the isolate i on the supplemented medium (May De Mio et al. Citation2011).
The differences between the second and third methods were related to how the sensitivity frequencies were inferred. For the second method, the M. fructicola isolates were considered resistant when their EC50 values were greater than 0.3 µg mL−1 as defined by May De Mio et al. (Citation2011). The third method employed the baseline value for tebuconazole (0.046 µg mL−1) to separate the resistant from sensitive isolates. The M. fructicola baseline (BL) value was retrieved from May De Mio et al. (Citation2011) EC50 results for populations collected from 2000–2004 in Paraná and Rio Grande do Sul state, prior to the use of tebuconazole in Brazil.
Sensitivity density distributions
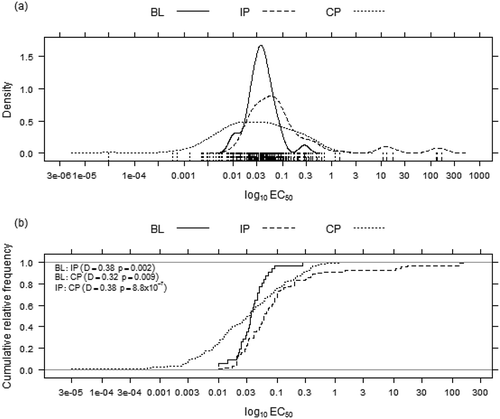
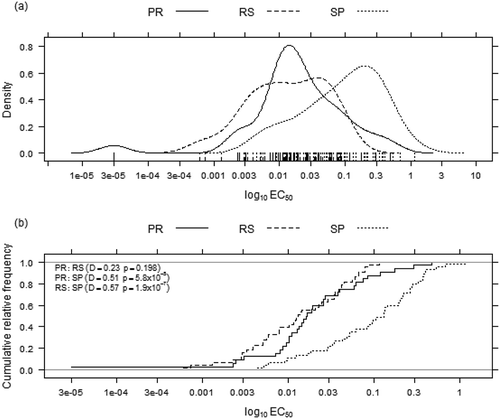
The distributions of the M. fructicola sensitivities to tebuconazole were analysed by plotting the densities and cumulative frequencies of the log-transformed EC50 values. First, three different populations were compared, i.e. 2000–2004 (baseline – BL), 2005–2008 (intermediate population – IP), and 2009–2011 (current population – CP). Next, comparisons of the origins (i.e. SP, PR and RS) were performed within the current population (). The BL and IP EC50 values were retrieved from May De Mio et al. (Citation2011).
Sensitivity instability due to storage
To evaluate the effects of isolate storage on the resistance of M. fructicola to tebuconazole, 32 isolates were randomly selected from the IP (17 isolates) and CP (15 isolates) populations. The isolates from the IP population were first evaluated for their EC50 values in 2010 by May De Mio et al. (Citation2011). The second EC50 evaluation was performed after 4 years of long-term storage in 25% glycerol at −80°C. The 15 isolates from the CP populations were evaluated for their EC50 values for the first time in 2012. Later in 2013, a second EC50 evaluation was performed after 1 year of storage on sterile filter paper at 4°C. The M. fructicola EC50 values for tebuconazole were calculated according to May De Mio et al. (Citation2011).
Data analysis
The EC50 values were analysed by logarithm (log10)-transforming the fungicide concentrations and then performing linear regressions of the colony inhibition values (L) by the log10 concentrations. The tebuconazole EC50 values were calculated from the corresponding linear regression equations for each isolate for which the regression coefficient was significant at P < 0.05. Changes in the sensitivity density curves were analysed with two-sample Kolmogorov–Smirnov tests. The non-parametric Kolmogorov–Smirnov test compares the cumulative frequency distributions of two datasets at a time and reports the maximum difference between the distributions (Kirkman Citation1996). The arithmetic means of the EC50 values for the populations, origins, storage methods and dates were calculated separately. Significant differences in population sensitivities were verified with Welch two-sample t-tests, and differences in the storage methods were verified with paired t-tests; both tests were considered significant at P < 0.05. The statistical software R (version 3.1.1) was used for data analyses and graphical presentation.
Results
Frequency of resistance
The frequency of the resistance of M. fructicola to tebuconazole varied according to the evaluation method used. The relative growth method produced an 18.3% frequency of resistant isolates within the surveyed population from the peach orchards in Brazil. In the SP, PR and RS regions, the frequencies of resistant isolates were 37.3%, 5.3% and 2.7%, respectively (). The EC50 method used by May De Mio et al. (Citation2011) yielded the lowest resistance frequencies among the three evaluation methods (the methods were not statistically compared) with a total of 8.3% resistant isolates in the surveyed population. This latter method revealed a 20% resistance in SP, 3.1% in PR and no resistance was observed in RS (). Finally, the baseline EC50 method indicated an overall resistance frequency of 40%. In SP, PR and RS, the baseline EC50 method revealed resistance frequencies of 71.1%, 25% and 18.6%, respectively ().
Fig. 1 Frequency of Monilinia fructicola sensitivity to tebuconazole among the isolates collected between 2009 and 2011 based on the relative growth under the discriminatory dose of 0.3 µg mL−1 method (a), the EC50 value using the 0.3 µg mL−1 as the discriminatory dose (b) and the EC50 value using the baseline value of 0.046 µg mL−1 as the discriminatory dose (c). SP = São Paulo, PR = Paraná, RS = Rio Grande do Sul, R = resistant, and S = sensitive. The entire values correspond to the sample size, and values in brackets correspond to the percentages [n (%)].
![Fig. 1 Frequency of Monilinia fructicola sensitivity to tebuconazole among the isolates collected between 2009 and 2011 based on the relative growth under the discriminatory dose of 0.3 µg mL−1 method (a), the EC50 value using the 0.3 µg mL−1 as the discriminatory dose (b) and the EC50 value using the baseline value of 0.046 µg mL−1 as the discriminatory dose (c). SP = São Paulo, PR = Paraná, RS = Rio Grande do Sul, R = resistant, and S = sensitive. The entire values correspond to the sample size, and values in brackets correspond to the percentages [n (%)].](/cms/asset/1a4b8195-876c-4978-876d-16798a06d40a/tcjp_a_1147496_f0001_b.gif)
Sensitivity density distribution
The tebuconazole sensitivity distributions revealed that all of the tested populations differed from each other according to the D statistics and the corresponding P-values (). The BL population differed from the IP (P = 0.002, D = 0.38) and the CP populations (P = 0.009, D = 0.32; ), and the IP and CP populations exhibited significance in their sensitivity distributions (P = 8.8 × 10–7, D = 0.38; ). The mean EC50 values obtained for the BL, IP and CP populations were 0.046, 5.593 and 0.093 µg mL−1, respectively (). Differences at 10% of significance in the mean EC50 values were observed between the BL and IP (P = 0.052), and IP and CP populations (P = 0.054; ). While BL and CP were different at 5% significance (P = 0.006).
Table 2. Comparison of the effective concentrations of tebuconazole required for 50% population inhibition (EC50 value) of the Monilinia fructicola isolates from three different populations in Brazil.
Fig. 2 Monilinia fructicola density distributions (a) and cumulative relative frequencies (b) for the tebuconazole effective concentration for 50% population inhibition (EC50) in Brazil comparing the baseline (BL, 2000–2004), intermediate (IP, 2005–2008) and current (CP, 2009–2011) populations.
Within the CP, the sensitivity distribution of the SP isolates differed significantly from those of the PR isolates (P = 5.8 × 10–5, D = 0.51, ) and RS isolates (P = 1.9 × 10–7, D = 0.57, ). Additionally, a similar difference in distributions was observed between the PR and RS isolates (P = 0.198, D = 0.23; ). The mean EC50 values obtained for the SP, PR and RS isolates were 0.186, 0.053 and 0.026 µg mL−1, respectively (). Significant differences in the mean EC50 values were observed between the SP and PR (P = 0.001) and between the SP and RS isolates (P = 1.24 × 10–5) but not between the PR and RS isolates (P = 0.143; ).
Table 3. Comparisons of effective concentrations of tebuconazole required for 50% population inhibitions (EC50 value) of Monilinia fructicola isolates collected between 2009–2011 in São Paulo (SP), Paraná (PR) and Rio Grande dos Sul (RS).
Fig. 3 Monilinia fructicola density distributions (a) and cumulative relative frequencies (b) of the tebuconazole effective concentrations required for 50% population inhibition (EC50) in Brazil comparing the origins within the actual population (2009–2011). SP = São Paulo, PR = Paraná, RS = Rio Grande do Sul.
Sensitivity instability during storage
Storage effects on the sensitivity of M. fructicola to tebuconazole were observed in the IP (P = 1.8 × 10–6) and CP (P = 1.4 × 10–5) populations after 4 years of storage at −80°C in 25% glycerol and 1 year of storage at 4°C on filter paper, respectively (). For both storage methods, the mean EC50 values decreased from the first to the second evaluations.
Table 4. Mean tebuconazole sensitivity differences due to Monilinia fructicola storage.
Discussion
In Brazil, M. fructicola isolates that are resistant to tebuconazole accounted for 40% of the surveyed population. The baseline EC50 method was suitable for surveying the resistance of isolates to tebuconazole because it allowed for comparisons with similar data collected from M. fructicola prior to the use of tebuconazole in Brazil. The relative growth method involving the use of the discriminatory dose of 0.3 µg mL−1 is a useful method for the initial screening of resistant isolates because its performance demands less time and material. However, the relative growth method should be avoided for determining tebuconazole resistance frequencies in isolates from Brazil because some resistant isolates may be misclassified as sensitive. Different methods for calculating the relative growths of M. fructicola isolates in the presence of propiconazole based on the discriminatory dose have been reported in efforts to separate the isolates by sensitivity (Russell Citation2004; Amiri et al. Citation2008; Villani & Cox Citation2011). The discriminatory dose for tebuconazole in M. fructicola isolates from Brazil remains unknown and difficult to determine due to the continuous resistance pattern against this fungicide and the absence of an isolate collection from fields in which tebuconazole failed to control brown rot. To refine the relative growth method for resistance surveys, adjustments of the relative growth percentages that are used for sensitivity segregation are needed so that the obtained frequencies match with those obtained with the baseline EC50 method.
In a previous study, May De Mio et al. (Citation2011) reported a total resistance frequency of 15.8% for an isolate population that was surveyed between 2005 and 2008 (IP). By the reproduction of the sensitivity separation method used by May De Mio et al. (Citation2011), an overall resistance frequency of 8.33% was determined for the CP. Therefore, a shift away from resistance in the CP was observed as demonstrated by the EC50 density distribution analysis and the cumulative relative frequency. If the previous tebuconazole survey performed by May De Mio et al. (Citation2011) had used the mean EC50 baseline value to segregate the resistant and sensitive isolate frequencies, a reduction would also have been observed in the population examined in that study (data not shown).
The observed decrease in the resistance frequency between the current and previous surveys, demonstrated by the density and cumulative resistance frequency plots, can be explained by changes in brown rot fungicide management programmes that peach growers have implemented in the surveyed areas. In SP, the use of azoxystrobin fungicide sprays has increased to prevent peach rust caused by Tranzschelia discolor, while brown rot control has been given secondary importance (Alves & May De Mio Citation2008). In PR and RS, the usage of iminoctadine tris (albesilate) and iprodione has resulted in satisfactory control of brown rot disease (Moreira & May De Mio Citation2009), and therefore, these agents have been included in the spray programmes. Those changes in fungicide management may reduce the selection for resistant isolates and affect disease incidence and consequently reduce the frequency of DMI-resistant isolates as demonstrated by Staub (Citation1991) and Brannen et al. (Citation2006) and suggested by Brent and Hollomon (Citation2007).
The instability in the sensitivities observed in the reductions of the EC50 values for the isolates recovered from both storage methods may have affected the degree of resistance reported in this paper, but not the validity of results here presented. In contrast, this survey and that of May De Mio et al. (Citation2011) would have been similarly affected by the storage instability despite different methods of storage that were used. This conclusion is supported by Zhu et al. (Citation2012), who found similarities between the propiconazole EC50 values of M. fructicola after 36 weeks of storage in glycerol and filter paper as well. Moreover, Zhu et al. (Citation2012) suggested that the changes that occurred due to cold storage at −80°C likely resulted from cellular damage rather than deleterious effects on the DNA because mutations related to resistance were identified before and after the storage experiments. Regarding the changes observed following filter paper storage, the differences may be attributable to the continuous growth that has been observed at 4°C (Ogawa & English Citation1991; Moreira & May De Mio Citation2007) and has been shown to cause an effect similar to that observed following successive colony transfers in the absence of fungicide (Cox et al. Citation2007). Similar storage effects have previously been reported in other DMI studies of different pathogens (Köller et al. Citation1991; Karaoglanidis & Thanassoulopoulos Citation2002).
Similar to the study of May De Mio et al. (Citation2011), the SP population exhibited highest mean EC50 value and a significantly different sensitivity distribution relative to the isolates collected from the peach production areas of PR and RS. These differences were attributed to the higher and homogeneous yearly mean temperatures, rainfall and humidity levels that commonly occur in SP. These characteristics have been described by Bergamin Filho & Amorim (Citation1996) to favour inocula continuity across seasons and to reduce the interval between pathogen sporulation and host infection; such conditions increase the need for pathogen control. Therefore, the EC50 values from SP most likely resulted from intensive spraying to control peach rust and brown rot as mentioned by Alves and May De Mio (Citation2008). In PR and RS, peach rust is not as problematic as it is in SP.
In summary, this study demonstrated results for three different approaches to segregate tebuconazole sensitivity of M. fructicola already used by different authors. The baseline EC50 value of 0.046 µg mL−1 was more useful for determining the frequency of M. fructicola isolates resistant to tebuconazole, and the application of this method is recommended in Brazil. The relative growth method with the discriminatory dose of 0.3 µg mL−1 can be used for the rapid screening of resistant isolates, but additional studies are needed to verify the efficacy of the application of this method in resistance frequency studies. Additionally, the sensitivity distribution of resistant M. fructicola isolates indicated a significant shift away from resistance as indicated by the cumulative relative frequency plot. This finding provided us an important insight into anti-resistance strategies that can prevent or delay practical resistance to tebuconazole in Brazilian peach orchards. Studies, including those that investigate the instability of sensitivity following successive isolate transfers in the absence of fungicide, and those that investigate differences in the competitive abilities and fitness between resistant and sensitive isolates, are essential for supporting new anti-resistance strategies that can prolong the fungicide usage to benefit peach and other stone fruits growers.
Acknowledgements
We thank Karla Kudlawiek and Fernando Ramos for their technical assistance, Ryan Puckett for his editing, and the Capes/Reuni doctoral fellowship.
Additional information
Funding
References
- Alves G, May De Mio LL. 2008. Efeito da desfolha causada pela ferrugem na floração e produtividade do pessegueiro. Rev Bras Frutic. 30:907–912.
- Amiri A, Brannen PM, Schnabel G. 2008. Monitoring resistance in Monilinia fructicola populations of the southeastern United States for enhanced brown rot control in peach. Proceedings of the 100th APS Centennial Meeting; 2008 Jul 26–30; Minneapolis, US; p. S12.
- Amiri A, Brannen PM, Schnabel G. 2009. Validation of the lipbalm tube assay for evaluation of fungicide sensitivity in field isolates of Monilinia fructicola. Plant Health Prog. doi:10.1094/PHP-2009-1118-01-RS
- Bergamin Filho A, Amorim L. 1996. Doenças de plantas tropicais: Epidemiologia e controle econômico. São Paulo, Brazil: Ceres.
- Bleicher J. 1997. Doenças de rosáceas de caroço. In: Kimati H, Amorim L, Bergamin Filho A, Camargo L, Rezende J, editors. Manual de fitopatologia: Vol 2. Doenças das plantas cultivadas. São Paulo, Brazil: Ceres; p. 621–627.
- Brannen P, Hotchkiss M, Reilly C, Schnabel G. 2006. Evaluation of fungicide programs to manage a DMI-resistant Monilinia fructicola population in a Georgia peach research block, 2005. Fungicide &Nematicide Tests. 61.
- Brent KJ, Hollomon DW. 2007. Fungicide resistance in crop pathogens: How can it be managed? Brussels, Belgium: Fungicide Resistance Action Commitee.
- Cox K, Bryson P, Schnabel G. 2007. Instability of propiconazole resistance and fitness in Monilinia fructicola. Phytopathology. 97:448–453.
- Fortes J, Martins O. 1998. Sintomatologia e controle das principais doenças. In: Mederos C, Raseira MdC, editors. A cultura do pessegueiro. Pelotas, Brazil: Embrapa-CPACT/Embrapa-SPI; p. 243–264.
- Harvey JM, Smith WL, Kaufman J. 1972. Market diseases of stone fruits: Cherries, peaches, nectarines, apricots, and plums. Washington: United States Department of Agriculture.
- Karaoglanidis G, Thanassoulopoulos C. 2002. Phenotypic instability of Cercospora beticola Sacc. strains expressing resistance to the sterol demethylation‐inhibiting (DMI) fungicide flutriafol after cold exposure. J Phytopathol. 150:692–696.
- Kirkman T. 1996. Statistics to use [Internet]. [ updated 2015 Jan 3; cited 2015 Jan 3]. Available from: http://www.physics.csbsju.edu/stats/
- Köller W, Smith F, Reynolds K. 1991. Phenotypic instability of flusilazole sensitivity in Venturia inaequalis. Plant Pathol. 40:608–611.
- May De Mio L, Garrido L, Ueno B. 2004. Doenças de Fruteiras de Caroço. In: Monte Serrat B, Motta A, Cuquel F, editors. Fruteiras de caroço: Uma visão ecológica. Curitiba, Brazil: UFPR; p. 169–185.
- May De Mio L, Luo Y, Michailides TJ. 2011. Sensitivity of Monilinia fructicola from Brazil to tebuconazole, azoxystrobin, and thiophanate-methyl and implications for disease management. Plant Dis. 95:821–827.
- May De Mio L, Moreira L, Monteiro LB, Justiniano P. 2008. Infecção de Monilinia fructicola no período da floração e incidência de podridão parda em frutos de pessegueiro em dois sistemas de produção. Trop Plant Pathol. 33:227–234.
- Moreira L, May De Mio LL. 2007. Crescimento Micelial de Monilinia fructicola e Trichothecium roseum em diferentes temperaturas e sensibilidade do antagonista a fungicidas e fosfitos. Scientia Agraria. 8:337–341.
- Moreira L, May De Mio LL. 2009. Controle da podridão parda do pessegueiro com fungicidas e fosfitos avaliados em pré e pós-colheita; Control of peach tree brown rot by fungicides and phosphites evaluated during preharvest and postharvest. Ciência Agrotec. 33:405–411.
- Ogawa JM, English H. 1991. Diseases of temperate zone tree fruit and nut crops. Oakland, USA: University of California, Division of Agriculture and Natural Resources.
- Penrose L, Senn A. 1995. Baseline sensitivities of preserved isolates of Sclerotinia fructicola from various host species to propiconazole and iprodione. Australas Plant Pathol. 24:9–14.
- Russell P. 2004. Sensitivity baselines in fungicide resistance research and management. Cambridge, UK: Fungicide Resistance Action Commitee.
- Silva S, Kohls V, Manica-Berto R, Rigatto P, Rombaldi C. 2011. Apropriação tecnológica da produção integrada de pêssegos na região de Pelotas no Estado do Rio Grande do Sul. Ciência Rural. 41:1667–1673.
- Staub T. 1991. Fungicide resistance: practical experience with antiresistance strategies and the role of integrated use. Annu Rev of Phytopathol. 29:421–442.
- Tibola CS, Fachinello JC, Grützmacher AD, Picolotto L, Krüger L. 2005. Manejo de pragas e doenças na produção integrada e convencional de pêssegos. Rev Bras Frutic. 27:215–218.
- Villani SM, Cox KD. 2011. Characterizing fenbuconazole and propiconazole sensitivity and prevalence of ‘Mona’ in isolates of Monilinia fructicola from New York. Plant Dis. 95:828–834.
- Zhu F, Bryson PK, Schnabel G. 2012. Influence of storage approaches on instability of propiconazole resistance in Monilinia fructicola. Pest Manag Sci. 68:1003–1009.
