Abstract
To determine the population structure and mechanisms of molecular evolution of Apple stem pitting virus (ASPV) isolates from pear in China, we compared 48 coat protein (CP) sequences from 31 ASPV pear isolates and 66 Triple Gene Block (TGB) sequences from 44 ASPV pear isolates. Phylogenetic analysis based on these sequences and corresponding sequences from GenBank showed that ASPV grouping in phylogenetic trees was correlated to the host of origin (apple, pear and Korla pear), regardless of gene sequences examined. The ASPV isolates from pear could be divided into six evolutionary divergent subgroups (A–F) based on their CP sequences, and two new subgroups (B and F) were identified in this study. The ASPV isolates could be divided into five evolutionarily divergent groups based on their TGB sequences. Multiple alignment analysis indicated continuous nucleotide insertions or deletions were present in CP of ASPV pear isolates in China. Recombination events were detected in CP and TGB sequences in our study. These results suggest that ASPV CP and TGB genes were under negative selection. Our study suggests that insertion or deletion mutation, selection pressure and recombination play important roles in genetic diversity of ASPV pear isolates in China.
Résumé
Afin de déterminer la structure de la population et les mécanismes de l’évolution moléculaire d’isolats du virus du bois strié du pommier (VBSP) chez le poirier en Chine, nous avons comparé 48 séquences de protéine capsidiaire (PC) de 31 isolats de VBSP collectés sur des poiriers et 66 séquences de blocs de trois gènes (BTG) de 44 isolats de VBSP collectés également sur des poiriers. L’analyse phylogénétique basée sur ces séquences et sur les séquences correspondantes de la GenBank a montré que le groupement de VBSP en arbres phylogénétiques était corrélé à l’hôte d’origine (pommier, poirier et poirier de Korla), indépendamment des séquences de gènes étudiées. En se basant sur leurs séquences de PC, les isolats du VBSP collectés sur les poiriers pourraient être divisés en six différents sous-groupes évolutifs (A–F); de plus, deux nouveaux sous-groupes (B et F) ont été identifiés dans le cadre de cette étude. En se basant sur leurs séquences de BTG, les isolats du VBSP pourraient être divisés en cinq différents groupes évolutifs. L’analyse des alignements multiples a indiqué qu’il y avait, dans les PC des isolats de VBSP en Chine, des séquences nucléotidiques continues sujettes à des insertions ou à des délétions. Au cours de notre étude, des évènements de recombinaison ont été détectés dans les séquences de PC et de BTG. Ces résultats suggèrent que les gènes des CP du VBSP et des BTG étaient soumis à la sélection négative. Notre étude suggère que la mutation par insertion ou délétion, la pression sélective et la recombinaison jouent un rôle important dans la diversité génétique des isolats du VBSP chez le poirier en Chine.
Introduction
Apple stem pitting virus (ASPV) is distributed worldwide and its natural hosts are largely restricted to Maloideae species, including pear and apple. There are also recent reports of ASPV infecting cherry and sour cherry in India (Dhir et al. Citation2010) and wild Rosaceae (Cydonia japonica, Pyrus calleryana and P. amygdaliformis) in Greece (Mathioudakis & Katis Citation2006) as well as grapevine in South Africa (GenBank No.: EU247940.1–EU247950.1; HM460716.1–HM460735.1; EU753978.1–EU753981.1). ASPV infected apple trees often remain symptomless, although ASPV causes visible symptoms on susceptible rootstocks (Jelkmann Citation1994; Jelkmann & Keim-Konrad Citation1997), xylem pits in the stem of Malus pumila ‘Virginia Crab’, as well as epinasty and decline of M. domestica ‘Spy227' (Jelkmann Citation1994; Komorowska et al. Citation2010). In many pear cultivars, ASPV infection results in vein yellowing (Wu et al. Citation2010), red mottling (Komorowska et al. Citation2011) or pear necrotic spot (PNS) or stony pits (Mathioudakis et al. Citation2009). ASPV infection frequently occurs in combination with other viruses, including Apple chlorotic leaf spot virus (ACLSV), Apple stem grooving virus (ASGV) and Apple mosaic virus (ApMV). Mix infections by these viruses cause significant decreases in the quality and quantity of fruit. There are no insect vectors reported for ASPV (Martelli & Jelkmann Citation1998).
ASPV is the type species of the genus Foveavirus in the family Betaflexiviridae (Carstens 2009). The flexible filamentous ASPV particles are approximately 12–15 nm in width and 800 nm in length, and can form end-to-end aggregates in host cells. The single-stranded positive-sense RNA (+ssRNA) genome of ASPV is approximately 9300 nucleotides (nts) long, and encodes five open reading frames (ORFs, ORF1–ORF5) as well as the 5ʹ untranslated region (UTR) and 3ʹ UTR. ORF1 encodes the viral replicase polyprotein, ORF2–ORF4 encode triple gene block proteins (TGBps) and ORF5 encodes the viral coat protein (CP) (Jelkmann Citation1994).
RNA viruses have high mutation rates, which result in the accumulation of abundant genetic variation in viral populations (Holmes Citation2010). Liu et al. (Citation2012) reported that all five genes of ASPV had large genetic variability, especially in the CP-encoding ORF, which provided a strong motive to study the diversity of ASPV based on the CP (Liu et al. Citation2012). Additional studies have showed that the CP consists of a conserved C-terminal region and a variable N-terminal region (Wu et al. Citation2010; Yoon et al. Citation2014). Indeed, the CP of different ASPV isolates is highly variable in sequence and in length, resulting in different CP sequence lengths, ranging from 1125 to 1245 nt (Komorowska et al. Citation2011; Yoon et al. Citation2014). Significant genetic variation in ASPV isolates was also reported by analysing a portion of the replicase-encoding ORF (Mathioudakis et al. Citation2010). In addition, previous results indicated that ASPV genetic variation did not correlate to geographic origin, but rather to the host plant (Liu et al. Citation2012). To date, 12 ASPV genomes have been sequenced, including nine isolates from apple and three isolates from Korla pear (Liu et al. Citation2012). In addition, almost 50 complete ASPV CP sequences are available in GenBank (about 33 sequences were from apple isolates, four from Korla pear and 10 from pear isolates) (Wu et al. Citation2010; Komorowska et al. Citation2011; Yoon et al. Citation2014). Most studies on ASPV genetic diversity conducted so far have involved the ASPV CP ORF, or portion thereof, from apple isolates (Komorowska et al. Citation2011; Yoon et al. Citation2014); however, sequence characteristics of pear isolates have not been extensively documented.
China has one of the largest pear cultivation areas in the world, and ASPV causes serious damage to pear trees in this country (Wang et al. Citation1994; Liu et al. Citation2012), and is considered to be a major threat to the Chinese pear industry. Currently, planting virus-free pear materials is the main method used to prevent the losses caused by pear viruses, which makes virus detection important for certified plant materials. However, conventional reverse transcription PCR (RT-PCR) detection of most pear viruses still has problems, including the lack of available primers for detecting all virus variants and very low detection sensitivity, particularly given the high degree of genetic diversity of ASPV (Wu et al. Citation2010; Komorowska et al. Citation2011; Yoon et al. Citation2014). To date, the extent of sequence variation in ASPV pear isolates from China is unknown, which prevents the development of nucleotide sequence-based detection methods. An expansion of the data set of available ASPV sequences could allow for the development of reliable diagnostic methods for certifying virus-free pear plant material.
The objectives of the present study were to: (i) investigate ASPV incidence on pear in China; (ii) analyse the genetic diversity of ASPV isolates obtained from pear by sequencing functionally important genes (TGB and CP); and (iii) infer ASPV evolutionary mechanisms.
Materials and methods
Sample collection A field survey of ASPV occurrence was conducted from August 2009 to June 2012 in 17 orchards located in 12 provinces in China. A total of 465 samples (451 pear samples and 14 apple samples) were randomly collected to test for ASPV (). The pear samples included leaves or fruits of pear (Pyrus spp.), most of which were symptomless although some showed leaf vein yellowing in leaves or stony pits in the fruit. Virus-free leaves from seedlings of Pyrus betulifolia Bge were used as a negative control.
Table 1. ASPV incidence on apple and pear trees surveyed in this study in different areas.
RT-PCR, cloning and sequencing
Total RNA was extracted from 0.1 g of leaf tissue using the cetyl-triethylammonium bromide (CTAB) method (Li et al. Citation2008) and was subsequently used as a template for ASPV detection and for the amplification of fragments containing the ASPV CP and TGB genes by RT-PCR. The primer sequences used for ASPV detection were 370-F/370-R (Menzel et al. Citation2002): 370-F, 5ʹ-ATGTCTGGAACCTCATGCTGCAA-3ʹ and 370-R, 5ʹ-TTGGGATCAACTTTACTAAAAAGCATAA-3ʹ. The primer pairs used for amplifying fragments containing complete CP were D-F/A-R (Jelkmann & Keim-Konrad Citation1997): D-F, 5ʹ-GTACATGAGTAACTCGAGCC-3ʹ and A-R, 5ʹ-GTACATGAGTAACTCGAGCC-3ʹ. Fragment sizes amplified by D-F/A-R ranged from 1430 to 1552 bp, including the entire CP ORF as well as 221 and 78 bp upstream and downstream of the CP, respectively. Primer pairs for amplifying the TGB genes were MP-F/MP-R, which were designed based on TGB genes sequences available in GenBank (Accession No: D21829.1, AB045371.1 and EU095327.1). The primers MP-F, 5ʹ-GTGTGTAAGCATATTAGG-3ʹ and MP-R, 5ʹ-CTACACCCTAACCTAATG-3ʹ amplified a fragment with the expected size of 1203 bp. First-strand cDNA synthesis was performed using 0.5 mM of random hexamers (TaKaRa, Dalian, China) and M-MLV reverse transcriptase (Promega, Madison, USA) at 37°C for 1.5 h. PCR reactions were performed in a 25 μL volume with reaction mixtures containing 2.5 μL 10×PCR buffer, 0.5 mM dNTP, 1 mM specific primer, 0.15 μL of 5 U L−1 rTaq DNA polymerase (TaKaRa, Dalian, China), and corresponding templates (3 μL first-strand cDNA). The PCR cycling parameters were set as follows: pre-activation at 94°C for 5 min, followed by 35 cycles of denaturation (94°C for 30 s), annealing (30 s; annealing temperature for D-F/A-R is 54°C; 370-F/370-R, 56°C; and MP-F/MP-R, 50°C), and extension (1 min at 72°C), final extension (1 cycle of 72°C for 10 min). The PCR reaction was conducted in a 96-well PCR Thermal Cycler (T-100TM, BIO-RAD, USA). The PCR products were analysed on 1% agarose gels and visualized in a UV transilluminator by ethidium bromide (1 μL mL−1) staining. All amplified PCR products were gel purified and cloned individually into the pMD18-T simple vector (TaKaRa, Dalian, China) according to the manufacturer’s instructions. To exclude in vitro RT-PCR errors, at least three clones from each ASPV isolate were sequenced in forward and reverse orientations. If the three independent clones showed ≥98% similarity, a consensus sequence was obtained. If there was <98% nucleotide similarity between the three initially sequenced clones, additional clones were sequenced to rule out the possible occurrence of molecular variant mixtures of isolates within individual samples. The GenBank accession numbers of sequences from our study are JX673781–JX673830 for the CP gene (Table S2) and JX673831–JX673905 for TGB genes (Table S3).
Sequence alignments, phylogenetic, genetic distance and recombination analyses
Nucleotide sequence alignments were carried out using ClustalX 1.81 (Thompson et al. Citation1997) with default settings and adjusted manually for the correct open reading frames. The corresponding sequences of ASPV isolates available in GenBank were also included for analysis (Table S1). Corresponding genes of Apple green crinkle associated virus (AGCaV), which is a member of the genus Foveavirus (James et al. Citation2013), were used as an outgroup for phylogenetic analysis. Phylogenetic and virus molecular evolutionary analyses were conducted using MEGA 6.06 using the neighbour-joining method with 1000 bootstrap replicates and bootstrap values <60% were omitted (Tamura et al. Citation2013). The genetic distance (the average number of nucleotide substitutions between two randomly selected sequences in a population) within and between phylogenetic groups and subgroups of different ASPV genes was calculated using MEGA 6.06 (Tamura et al. Citation2013). The CP and TGB sequences of each isolate were also subjected to recombination analysis. Possible recombination events were detected by using the following programs: RDP (Heath et al. Citation2006), GENECONV (Padidam et al. Citation1999), BOOTSCAN (Martin et al. Citation2005), Maximum Chi Square (MAXCHI) (Smith Citation1992), CHIMAERA (Posada & Crandall Citation2001), 3SEQ (Boni et al. Citation2007) and Sister Scanning (SISCAN) (Posada & Crandall Citation2001).
Selection pressure and neutrality test analysis
Selection pressure was estimated by the dN/dS ratio, where dN/dS represents the average number of nucleotide substitutions per site between two sequences. The values of dN and dS were estimated separately by using the software DnaSP 5.10 (Librado & Rozas Citation2009). Genes are considered under positive (or diversifying) selection when the dN/dS ratio is >1, neutral selection when the dN/dS ratio = 1 and negative (or purifying) selection when the dN/dS ratio is <1. DnaSP 5.10 was also used to estimate Tajima’s D, Fu & Li’s D and F statistical tests, haplotype (gene) diversity (Hd) and nucleotide diversity (Pi) (Rozas et al. Citation2004; Librado & Rozas Citation2009). Tajima’s D, Fu & Li’s D and F statistical tests hypothesize that all mutations are selectively neutral. Haplotype diversity refers to the frequency and number of haplotypes in the population. Nucleotide diversity estimates the average pairwise differences among sequences.
Results
Genetic diversity and phylogenetic analysis of ASPV pear isolates from China
Of the 451 pear tissue samples analysed, 145 (32.3%) tested positive for ASPV by RT-PCR. Apple samples were found to be infected at a much higher rate (78.6%; 11/14) compared with pear, although considering the lower number of apple samples, future work will be required to determine if apples are more susceptible (). CP sequences from 32 ASPV isolates (31 from pear and one from apple) and TGB sequences from 52 ASPV isolates (44 from pear and eight from apple) were selected for ASPV genetic diversity analysis. For most isolates, the three primary sequenced clones from a single sample showed ≥98.0% similarity, with the exception of the CP clones from 14 isolates (Table S2) and the TGB clones from 16 isolates (Table S3). In total, 50 CP and 75 TGB sequences obtained in this study have been deposited in GenBank (Table S2, Table S3).
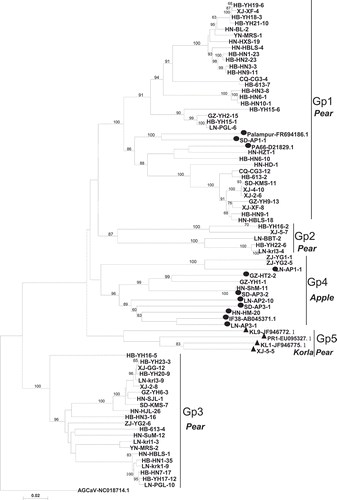
Fifty CP sequences from our study and related sequences obtained from GenBank (Table S1) were used to reconstruct a phylogenic tree using the neighbour-joining method (). This analysis revealed that all CP sequences in our study clustered into either Gp1 or Gp2, two of the three well-defined groups (Gp1, Gp2 and Gp3) reported in previous studies (Wu et al. Citation2010; Liu et al. Citation2012). Gp1 consisted of isolates only from pear as well as a German isolate PA66 (D21829.1), and was further divided into five subgroups (named A-E); Gp2 consisted of isolates largely from apple, but also included 10 isolates from pear, which formed a subgroup named F. Five previously reported isolates (KRL1, KL1, KL2, KL9 and Y2) from Korla pear (Pyrus sinkiangensis Yü) (Liu et al. Citation2012), formed a group named Gp3. Gp2 has the highest genetic distance (0.2005 ± 0.0109), followed by Gp1 (0.1822 ± 0.0096) and Gp3 (0.1602 ± 0.0105). The genetic distance between the three groups ranged from 0.2824 ± 0.0164 to 0.3821 ± 0.0234. Subgroup D had the highest genetic distance (0.1475 ± 0.0064), followed by subgroup C (0.0754 ± 0.0065), and lowest in subgroup F (0.0370 ± 0.0032). The genetic distances between subgroups ranged from 0.1482 ± 0.0129 to 0.3281 ± 0.0178 ().
Table 2. Genetic distance between and within groups clustered in phylogenetic trees based on ASPV CP and TGB sequences.
Fig. 1 Phylogenetic trees of complete CP (a) and TGB (b) sequences of ASPV isolates in this study and global isolates available in GenBank. Reference isolates are named according to their isolate name with GenBank accession numbers. Isolates reported herein are indicated by isolate abbreviations and number followed by the sequenced CP or TGB clone number. Sequences isolated from apple are indicated with the symbol ●, whereas sequences from Korla pear are denoted with the symbol ▲ and all other sequences are from pear isolates. The tree was constructed by the neighbour joining method implemented by MEGA6. Bootstrap analysis with 1000 replicates was performed. Only ≥50% bootstrap values are shown, and branch lengths are proportional to the genetic distances. Bar, 0.05 substitutions per site.
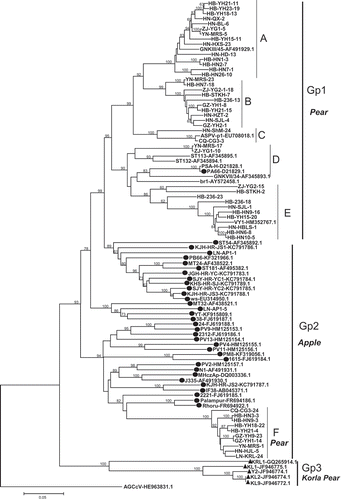
For TGB, 75 sequences from our study and related sequences obtained from GenBank were used to reconstruct phylogenetic trees (tree topologies based on TGB1, TGB2 and TGB3 were highly similar; therefore, only the phylogenetic tree based on the whole TGB is shown) (). Results revealed that all TGB sequences clustered into five groups, designated as Gp1-Gp5. Gp1, Gp2 and Gp3 mainly consisted of isolates from pear (except for the Indian isolate Palampur and the SD-AP1-1 isolate from our study, both from apple). Most of the isolates in Gp4 were from apple (except for pear isolates ZJ-YG2-5 and ZJ-YG1-1 from our study); Gp5 consisted of four isolates from Korla pear (KL1, KL9, PR1 and XJ-5–5, the latter from our study, isolated from Korla pear). The genetic distance of ASPV TGB genes within the variant groups was highest in Gp4 (0.1941 ± 0.011), followed by Gp5 (0.1690 ± 0.0104), Gp2 (0.1653 ± 0.0105), Gp1 (0.1404 ± 0.0086) and Gp3 (0.0906 ± 0.0063). The genetic distance between variant groups ranged from 0.2398 ± 0.0137 to 0.2721 ± 0.0149.
With respect to CP, mixed infections of divergent ASPV variants were detected in 13 out of 31 pear isolates (HB-HN9, HB-HN7, HB-YH21, HB-YH18, HB-YH15, GZ-YH1, CQ-CG3, HB-236, HB-STHK, HN-SJL, ZJ-YG2, ZJ-YG1 and YN-MRS). These 13 isolates consisted of different numbers of divergent variants: two (11 isolates), three (one isolate) or four variants (one isolate) (Table S4). The occurrence of mixed infections with divergent ASPV variants was further confirmed with TGB sequencing and this approach identified 12 out of 44 pear isolates as being mixed infections, all 12 of which were found to be double infections (Table S4). To estimate genetic variability for CP and TGB, we calculated population genetic parameters of all available sequences on the basis of variant groups and geographic distributions (). The parameters of TGB were calculated only based on Chinese isolates due to the lack of sufficient TGB sequences from other countries. Based on variant groups, the overall haplotype diversity (Hd) values were around 0.900–1.000, which indicated high genetic diversity within groups. However, their nucleotide sequence diversity (Pi) values varied among different groups and subgroups. The relatively higher Pi value (high value indicates higher sequence diversity) in Gp1 (0.1487) than Gp2 (0.1396) and Gp3 (0.1450) indicated higher sequence diversity of CP sequences from pear isolates; however, for TGB, the Pi values showed the opposite pattern. Pi values of sequences from apple in Gp4 (0.1624) and from Korla pear in Gp5 (0.1504) were higher than sequences from pear in Gp1–Gp3. Based on geographic origin, higher Pi values were observed in sequences from Poland (0.1915) and China (0.16616) compared with those from South Korea (0.15123) and Germany (0.00261).
Table 3. List of putative recombination events involving ASPV CP and TGB sequences.
Table 4. Analysing the selection pressures (dN/dS) of different ASPV genes.
Table 5. Population genetic parameters and neutrality tests calculated for ASPV CP and TGB based on geographic origins or variant groups.
A new type of continuous insertion in the 5ʹ region of the CP
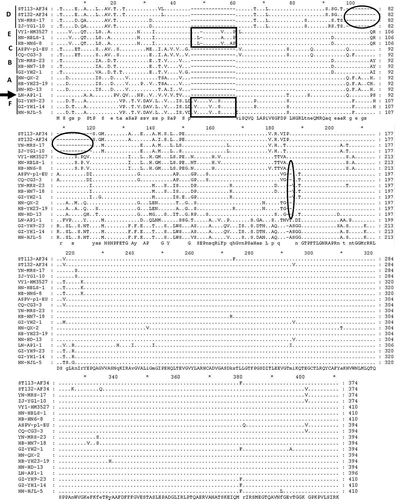
The CP size of different ASPV isolates fluctuated from 1125 nt to 1245 nt (Wu et al. Citation2010). Normally, the CP ORF is either 1194 nt or 1191 nt long for Korla pear (Liu et al. Citation2012) and apple isolates (Liu et al. Citation2012; Yoon et al. Citation2014), respectively, although there are some exceptions (for example, it is 1188 nt in the apple isolate PV7). Our results revealed that CP sizes for ASPV pear isolates varied according to the subgroups in the phylogenetic tree (): 1185 nt long for sequences in subgroups A–C; 1125 nt for sequences in subgroup D; 1233 nt for sequences in subgroups E and F. Multiple alignments with LN-AP1-1 from apple (1191 nt) as a reference sequence showed that amino acid insertions or deletions or mutations occurred in the 5ʹ terminal portion of the CP; however, the 3ʹ terminal portion of the CP was relatively conserved (), which is consistent with previous reports (Wu et al. Citation2010; Yoon et al. Citation2014). In different subgroups, different amino acid insertions or deletions were present at different positions in the 5ʹ terminal region: two amino acids deleted after aa position 169 for sequences in subgroups A–C; 22 amino acids deleted after aa position 82 for sequences in subgroup D; 14 amino acids inserted after aa position 45 for sequences in subgroup E; 15 amino acids inserted after aa position 45; and one amino acid deleted after aa position 169 for sequences in subgroup F. Although CP size and insertion position were the same for sequences in subgroups E and F, the inserted amino acids were not the same. The type of insertion seen in subgroup E has been reported once (isolate VY1, HM352767.1) (Wu et al. Citation2010). However, the insertion type seen in subgroup F is reported for the first time in our study. Surprisingly, there were no similar kinds of deletions or insertions in the TGB region.
Fig. 2 Multiple alignments of amino acids of 16 representative ASPV CP sequences. 2–3 sequences from each subgroup in the phylogenetic tree in were selected. LN-AP1-1 (arrow) from an apple isolate was used as a reference sequence. The deleted regions are represented by rectangular boxes and the inserted regions are represented by oval boxes.
Novel recombination events in the ASPV CP and TGB
Phylogenetic trees based on CP and TGB showed some discordant clustering of sequences, suggesting the possiblity of recombination events. A total of five putative recombinant events was detected using seven programs in RDP software with default settings (). Two CP recombinants, HB-HN7-18 and YN-MRS-23, had the same parental variants, originating from subgroup A (YN-MRS-3) and subgroup B (HN-HZT-2), as well as the same statistical values and crossing-over sites (). For TGB, several putative recombinants were identified: HB-YH15-6 (from group Gp2) was a recombinant of variants in Gp3 (HB-HN6-10) and Gp2 (LN-PGL-6); HN-BL-2 (Gp1) was a recombinant of variants in Gp5 (HN-SJL-1) and Gp1 (HB-HN2-23); the previously reported sequence PR1 (EU095327) (Gp7) was a recombinant of variants in Gp7 (XJ-5–5) and Gp3 (PA66, D21829). These three recombinant sequences had different break points: HB-YH15-6 crossed over the entire TGB, whereas that of HN-BL-2 was located in TGB1 and that of PR1 was located at TGB3.
Selection pressure and neutrality tests analysis
Ratios of dN/dS are commonly used to estimate the selection pressure to which viral genes have been subjected (Zhang et al. 2011; Liu et al. Citation2012; Farooq et al. Citation2013). To estimate selection pressure for the CP and TGB regions, we calculated the dN/dS ratios of ASPV CP, TGB1, TGB2 and TGB3 on the basis of all available sequences. The dN/dS ratios for all the tested genes were <1, indicating that TGB and CP were under negative selection (). The higher dN/dS ratio of CP and TGB3 compared with that of TGB1 and TGB2 suggested that the evolution constraints on TGB1 and TGB2 are higher than CP and TGB3. In neutrality tests, the Tajima’s D, Fu and Li’s D and F values for subpopulations from Groups Gp1, Gp2, Gp3, based on the CP, and groups Gp1, Gp3 and Gp5 based on TGB, were negative (), suggesting these subpopulations are in a state of increase. However, these results were not statistically significant (P-value >0.05), which indicated the result was not conclusive. The Tajima’s D, Fu and Li’s D and F values of subpopulations from groups Gp2 and Gp4 based on TGB were positive, which seemed to suggest these two subpopulations are in a state of decrease, although these results were also not statistically significant (P-value >0.05). As such, neutrality tests were not conclusive.
Discussion
To investigate ASPV incidence and to provide a clear account of the disease status of pear trees in China, 451 pear samples from different geographic areas of China were collected and assessed for the presence of ASPV. ASPV incidence detected by RT-PCR in pear was 32.3%, which is higher than reports of the disease in the Czech Republic and Egypt (Kundu Citation2003; Youssef et al. Citation2010), but lower than those in Greece, Turkey and northern China (Wang et al. 1994; Aglayan et al. Citation2006; Mathioudakis et al. Citation2010). Possible reasons that could account for the different ASPV detection rates between the different studies include the use of different primer pairs corresponding to different regions of the ASPV genome, or the use of different detection methodologies (Komorowska et al. Citation2010). At the same time, viral titres might differ at different times of the year (Mathioudakis et al. Citation2009) or in different tissues of the plant (Klerks et al. Citation2001). Therefore, more reliable and sensitive methods such as RT-nPCR, real-time PCR or RT loop-mediated isothermal amplification (RT-LAMP) are required for ASPV detection. Differences in detection rates between studies are also likely due to real differences in ASPV incidences in different countries. It is interesting to note that in our study, ASPV incidence in apple trees (78.6%) was significantly higher than that in pear trees (32.3%), which is similar to results from two previous surveys in Greece (Syrgianidis Citation1989; Mathioudakis et al. Citation2010).
A previous study showed that the phylogenetic grouping of ASPV isolates appeared to correlate with the hosts from which they were isolated (Liu et al. Citation2012); however, these results were not supported by enough sequence data. Since previous studies mainly focused on CP molecular characteristics of ASPV isolates from apple and Korla pear (Komorowska et al. Citation2010; Wu et al. Citation2010; Liu et al. Citation2012; Yoon et al. Citation2014), we confirmed the relationship between phylogenetic groups and hosts by sequencing large numbers of CP and TGB isolates from pear. Our results showed that no matter which ASPV genes (CP or TGB) were used to construct the phylogenetic trees, ASPV isolate groupings were always related with their hosts (apple, pear and Korla pear). Here, we consider pear and Korla pear (the latter specifically grown in Xinjiang province, north-western China) to be distinct hosts for ASPV, since ASPV isolates from Korla pear always formed a separate group in the phylogenetic trees. For example, CP sequences from ASPV isolates in the Korla pear-specific Gp3 (CP) shared a similarity of 89.9–100% at the nucleotide level and 92.7–100% at the amino acid level; however, they had relatively low levels of identity with CP sequences from isolates in other groups, ranging from 66.4 to 72.2% (nt) and 68.6 to 78.20% (aa). These values were below the limits of species demarcation criteria for the family BetaFlexiviridae, wherein isolates sharing greater than 72% nt or 80% aa sequence identities between their CP are considered one species (Adams et al. Citation2014). This suggests that previously reported ASPV isolates from Korla pear could be a new species, closely related to ASPV. However, some exceptions to the relationship between phylogeny and host were noted. For example, subgroup F, consisting of 10 pear isolates, clustered in the Gp3 group, whereas all other Gp3 isolates originated from apple (). The 10 isolates in subgroup F shared a similarity of 94.8–99.7% at nt level and 94.9–100% at the amino acid (aa) level, and the genetic distance in subgroup F was the lowest (0.0370 ± 0.0032) among the six subgroups (A–F), indicating that sequences in this subgroup do not cluster by chance or by mistakes during sequencing progress. Previous studies have shown that when a virus adapts to a new host, variation is primarily manifested as amino acid substitutions, which could allow more efficient virus entry into the new host, block interactions with host proteins or promote escape from both the new and the old host defence responses (Moya et al. Citation2004; Boulila Citation2010; Bandín & Dopazo Citation2011). However, interactions between ASPV and its hosts have not been studied at the molecular level and further studies of these interactions are required to understand the relationship between sequence variation and host preference.
Six subgroups (A–F) for the CP gene were identified in Chinese ASPV pear isolates. Two subgroups, B and F, did not correspond to any previously reported variant groups. Likewise, three groups (Gp1–Gp3) were identified for the TGB genes in Chinese ASPV pear isolates. We also identified mixed infections with different ASPV pear isolates in our study. Numerous pear varieties are cultivated in China, at least including Pyrus ussuriensis Maxim, P. bretschneideri Rehd, P. pyrifolia, P. communis L. and P. sinkiangensis T. T. Yu. The use of top grafting frequently employed in the introduction of new pear varieties, combined with frequent regional and international exchange of propagating materials, may have played a major role in the great genetic diversity of ASPV pear isolates and mixed infections detected in our study.
Recombination represents an evolutionary phenomenon (Glasa et al. Citation2004) that plays a role in the ability of various viruses to acquire sequence diversity, allowing faster adaptation to new hosts, environmental changes or overcoming host resistance (Valli et al. Citation2007; Martín et al. Citation2009; Seo et al. Citation2009). Indeed, there has been a recent increase in the reports of recombination events in RNA viruses from fruit trees (Alabi et al. Citation2010; Zhang et al. 2011; Farooq et al. Citation2013). Recombination events within the ASPV CP from apple isolates have been reported in two previous studies (Komorowska et al. Citation2011; Yoon et al. Citation2014) and here we report recombination events within Chinese ASPV pear isolates. Recombination events were detected in both the CP (two out of 50 unique CP sequences) and the TGB (two out of 75 unique TGB sequences) in our study. These recombination events were unlikely to be experimental errors for two reasons: first, the breaking sites of these recombination events are located within CP and TGB ORFs region; second, the parents of these recombination events originated from different geographic regions. The two recombinants in CP shared exactly the same recombination site, which implied that they were probably the progeny of the same successful recombination. Nevertheless, these results indicated that recombination has played a role in ASPV genetic diversity.
The dN/dS ratios of four ASPV genes indicated that all of them were under negative selection. In comparison, TGB1 was under the strongest negative selection pressure, followed by TGB2. TGB1 proteins from other Potexviruses have been reported to play many roles in interactions between viruses and plants, including cell-to-cell movement and the suppression of RNA silencing (Voinnet et al. Citation2000; Senshu et al. Citation2009; Solovyev et al. Citation2012), although the functions of ASPV TGB proteins have not been studied. The localization of the TGB1 encoded protein to plasmodesmata is generally thought to require the functions of the TGB2 and TGB3 proteins (Verchot-Lubicz et al. Citation2010; Solovyev et al. Citation2012). Thus, the requirement to maintain their tertiary and quaternary structure to function may explain the high degree of constraint on these proteins.
In conclusion, our study showed that mutations (including insertions or deletions), negative selection and recombination were important factors driving ASPV evolution in China and probably in the rest of the world, although further research is required to confirm this.
Supplemental Material
Download MS Word (202.4 KB)Acknowledgements
We are grateful to Professor Shifang Li for critical reading of the manuscript.
Supplemental material
Supplemental data for this article can be accessed online here: http://dx.doi.org/10.1080/07060661.2016.1158741.
Additional information
Funding
References
- Adams MJ, Lefkowitz EJ, King AM, Carstens EB. 2014. Ratification vote on taxonomic proposals to the international committee on taxonomy of viruses (2014). Arch Virol. 159:2831–2841.
- Aglayan K, Sere IU, Gazel M, Jelkmann W. 2006. Detection of four apple viruses by ELISA and RT-PCR assays in Turkey. Turk J Agric For. 4:241–246.
- Alabi OJ, Martin RR, Naidu RA. 2010. Sequence diversity, population genetics and potential recombination events in Grapevine rupestris stem pitting-associated virus in Pacific North-West vineyards. J Gen Virol. 91:265–276.
- Bandín I, Dopazo CP. 2011. Host range, host specificity and hypothesized host shift events among viruses of lower vertebrates. Veter Res. 42:544.
- Boni MF, Posada D, Feldman MW. 2007. An exact nonparametric method for inferring mosaic structure in sequence triplets. Genetics. 176:1035–1047.
- Boulila M. 2010. Putative recombination events and evolutionary history of five economically important viruses of fruit trees based on coat protein-encoding gene sequence analysis. Biochem Genet. 48:357–375.
- Dhir S, Tomar M, Thakur PD, Ram R, Hallan V, Zaidi AA. 2010. Molecular evidence for Apple stem pitting virus infection in India. Plant Pathol. 59:393.
- Farooq AB, Ma YX, Wang ZQ, Zhuo N, Xu W, Wang GP, Hong N. 2013. Genetic diversity analyses reveal novel recombination events in Grapevine leafroll-associated virus 3 in China. Virus Res. 171:15–21.
- Glasa M, Palkovics L, Komínek P, Labonne G, Pittnerová S, Kúdela O, Candresse T, Subr Z. 2004. Geographically and temporally distant natural recombinant isolates of Plum pox virus (PPV) are genetically very similar and form a unique PPV subgroup. J Gen Virol. 85:2671–2681.
- Heath L, Van der Walt E, Varsani A, Martin DP. 2006. Recombination patterns in aphthoviruses mirror those found in other picornaviruses. J Virol. 80:11827–11832.
- Holmes EC. 2010. The evolution and emergence of RNA viruses. New York: Oxford University Press.
- James D, Varga A, Jesperson GD, Navratil M, Safarova D, Constable F, Horner M, Eastwell K, Jelkmann W. 2013. Identification and complete genome analysis of a virus variant or putative new foveavirus associated with apple green crinkle disease. Arch Virol. 158:1877–1887.
- Jelkmann W. 1994. Nucleotide sequences of Apple stem pitting virus and of the coat protein gene of a similar virus from pear associated with vein yellows disease and their relationship with potex- and carlaviruses. J Gen Virol. 75:1535–1542.
- Jelkmann W, Keim-Konrad R. 1997. Immuno-capture polymerase chain reaction and plate-trapped ELISA for the detection of Apple stem pitting virus. J Phytopathol. 145:499–503.
- Klerks MM, Leone G, Lindner JL, Schoen CD, Van den Heuvel JF. 2001. Rapid and sensitive detection of Apple stem pitting virus in apple trees through RNA amplification and probing with fluorescent molecular beacons. Phytopathology. 91:1085–1091.
- Komorowska B, Malinowski T, Michalczuk L. 2010. Evaluation of several RT-PCR primer pairs for the detection of Apple stem pitting virus. J Virol Meth. 168:242–247.
- Komorowska B, Siedlecki P, Kaczanowski S, Hasiów-Jaroszewska B, Malinowski T. 2011. Sequence diversity and potential recombination events in the coat protein gene of Apple stem pitting virus. Virus Res. 158:263–267.
- Kundu JK. 2003. The occurrence of Apple stem pitting virus and Apple stem grooving virus within field-grown apple cultivars evaluated by RT-PCR. Plant Prot Sci. 88–92.
- Li R, Mock R, Huang Q, Abad J, Hartung J, Kinard G. 2008. A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. J Virol Meth. 154:48–55.
- Librado P, Rozas J. 2009. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 25:1451–1452.
- Liu N, Niu J, Zhao Y. 2012. Complete genomic sequence analyses of Apple stem pitting virus isolates from China. Virus Genes. 44:124–130.
- Martelli GP, Jelkmann W. 1998. Foveavirus, a new plant virus genus. Arch Virol. 143:1245–1249.
- Martin DP, Posada D, Crandall KA, Williamson C. 2005. A modified bootscan algorithm for automated identification of recombinant sequences and recombination breakpoints. Aids Res Human Retrov. 21:98–102.
- Martín S, Sambade A, Rubio L, Vives MC, Moya P, Guerri J, Elena SF, Moreno P. 2009. Contribution of recombination and selection to molecular evolution of Citrus tristeza virus. J Gen Virol. 90:1527–1538.
- Mathioudakis MM, Katis NI. 2006. First record of the Apple stem pitting virus (ASPV) in quince in Greece. J Plant Pathol. 88:221.
- Mathioudakis MM, Maliogka VI, Dovas CI, Paunović S, Katis NI. 2009. Reliable RT-PCR detection of Apple stem pitting virus in pome fruits and its association with quince fruit deformation disease. Plant Pathol. 58:228–236.
- Mathioudakis MM, Maliogka VI, Katsiani AT, Katis NI. 2010. Incidence and molecular variability of Apple stem pitting and Apple chlorotic leaf spot viruses in apple and pear orchards in Greece. J Plant Pathol. 92:139–147.
- Menzel W, Jelkmann W, Maiss E. 2002. Detection of four apple viruses by multiplex RT-PCR assays with coamplification of plant mRNA as internal control. J Virol Meth. 99:81–92.
- Moya A, Holmes EC, González-Candelas F. 2004. The population genetics and evolutionary epidemiology of RNA viruses. Nat Rev Microbiol. 2:279–288.
- Padidam M, Sawyer S, Fauquet CM. 1999. Possible emergence of new geminiviruses by frequent recombination. Virology. 265:218–225.
- Posada D, Crandall KA. 2001. Evaluation of methods for detecting recombination from DNA sequences: Computer simulations. Proc Natl Acad Sci USA. 98:13757–13762.
- Rozas J, Sánchez-DelBarrio JC, Messeguer X, Rozas R. 2004. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 19:2496–2497.
- Senshu H, Ozeki J, Komatsu K, Hashimoto M, Hatada K, Aoyama M, Kagiwada S, Yamaji Y, Namba S. 2009. Variability in the level of RNA silencing suppression caused by triple gene block protein 1 (TGBp1) from various potexviruses during infection. J Gen Virol. 90:1014–1024.
- Seo JK, Ohshima K, Lee HG, Son M, Choi HS. 2009. Molecular variability and genetic structure of the population of Soybean mosaic virus based on the analysis of complete genome sequences. Virology. 393:91–103.
- Smith JM. 1992. Analyzing the mosaic structure of genes. J Mol Evol. 34:126–129.
- Solovyev AG, Kalinina NO, Morozov SY. 2012. Recent advances in research of plant virus movement mediated by triple gene block. Front Plant Sci. 3:276.
- Syrgianidis GD. 1989. Problems of virus diseases of deciduous fruit trees in Greece. Acta Horticul. 21–26.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729.
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882.
- Valli A, López-Moya JJ, García JA. 2007. Recombination and gene duplication in the evolutionary diversification of P1 proteins in the family Potyviridae. J Gen Virol. 88:1016–1028.
- Verchot-Lubicz J, Torrance L, Solovyev AG, Morozov SY, Jackson AO, Gilmer D. 2010. Varied movement strategies employed by triple gene block-encoding viruses. Mol Plant-Microbe Inter. 23:1231–1247.
- Voinnet O, Cederer C, Baulcombe DC. 2000. A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell. 103:157–167.
- Wang PG, Hong N, Zhong PZ, Hu CS, Dong ZY. 1994. Identification of kinds of viruses for main pear cultivars in pear growing areas in northern China. China Fruits. 5:1–4.
- Wu ZB, Ku HM, Su CC, Chen IZ, Jan FJ. 2010. Molecular and biological characterization of an isolate of Apple stem pitting virus causing pear vein yellows disease in Taiwan. J Plant Pathol. 92:721–728.
- Yoon JY, Joa JH, Choi KS, Do KS, Lim HC, Chung BN. 2014. Genetic diversity of a natural population of Apple stem pitting virus isolated from apple in Korea. Plant Pathol J. 30:195–199.
- Youssef SA, Moawad SM, Nosseir FM, Shalaby AA. 2010. Detection and identification of Apple stem pitting virus and Apple stem grooving virus affecting apple and pear trees in Egypt. Julius-Kühn-Archiv. 427:248.
- Zhang CL, Gao R, Wang J, Zhang GM, Li XD, Liu HT. 2011. Molecular variability of Tobacco vein banding mosaic virus populations. Virus Res, 158:188–198.