Abstract
Wild eggplant (Solanum torvum) has been used as a rootstock for eggplant propagation in China for decades. In the winter of 2011–2012, a severe disease with typical symptoms of small brown spots was observed on the leaves of wild eggplant in Chengmai County, Hainan Province, China. This disease had an incidence of about 30–65% among all the plants in the region (about 2 hectares in total). The causal pathogen was identified as Stemphylium solani based on morphological features, cultural characteristics and molecular identification. The pathogenicity was confirmed by Koch’s postulates. This is the first report of natural infection by S. solani causing leaf spot on wild eggplant in China.
Résumé
L’aubergine sauvage (Solanum torvum) a été utilisée comme porte-greffe pour la propagation de l’aubergine en Chine depuis des décennies. Au cours de l’hiver 2011-2012, une grave maladie, affichant comme symptômes de petites taches brunes, a été observée sur les feuilles de l’aubergine sauvage dans le comté de Chengmai, dans la province du Hainan, en Chine. Cette maladie avait une incidence d’environ 30 à 65% chez tous les plants de la région (approximativement 2 ha en tout). En se basant sur les traits morphologiques, les caractéristiques culturales et l’identification moléculaire, il a été établi que l’agent pathogène causal était Stemphylium solani. La pathogénicité a été confirmée grâce aux postulats de Koch. Il s’agit de la première mention d’une infection naturelle produisant des taches foliaires, causées par S. solani, chez l’aubergine sauvage en Chine.
Introduction
Wild eggplant (Solanum torvum SW.), also known as wild tomato, belongs to the family Solanaceae (Zhang et al. Citation2015). It is a bushy, erect and spiny perennial plant used as a rootstock for propagating eggplant. Disease resistance, stress tolerance, yield and quality can be increased through grafting in protected and field areas (Li et al. Citation2007). Therefore, the demand for wild eggplant is growing rapidly. The ethanolic extract of wild eggplant showed an inhibitory effect on Verticillium dahliae (Li et al. Citation2007). In recent years, with the continuous cropping of eggplant in protected areas and greenhouses, various diseases have appeared (Zhang et al. Citation1998). During 2011 and 2012, a severe disease on leaves of wild eggplant with dark brown, subcircular or V-shaped lesions and abundant spores was observed in the main production regions in Chengmai county (110°0′E, 19°73′N), Hainan province of China, with disease incidence varying from 30% to 65% of all the plants. The purpose of our study was to ascertain the causal agent and describe the pathogen using morphological and molecular approaches and to confirm pathogenicity.
Material and methods
Sample collection
During 2011–2012, diseased leaves of wild eggplant with typical symptoms were collected from Chengmai, Hainan Province, China. Three diseased samples were collected from each plant, and 10 plants were selected randomly. The leaf tissues were cut into approximately 3 mm2 pieces and surface-sterilized in 70% ethanol for 30 s, rinsed in sterile water, plated on potato dextrose agar (PDA) and incubated at 25 ± 0.5°C for 7 days. Mycelia generated from the pieces of tissue were transferred to PDA plates or slant tubes for continuous growth and identification. Among the tissue pieces plated, 100% gave rise to fungal colonies. A total of 30 isolates were recovered. They were subcultured and purified by transferring 6-mm diameter mycelium plugs from the margin of older colonies to PDA plates.
Pathogenicity testing
Pathogenicity testing of 30 isolates was carried out in a greenhouse at the Institute of Vegetables and Flowers of CAAS in Beijing (116°33′E, 39°73′N). Thirty healthy wild eggplant plants (‘Tuo Lu Ba Mu’) at the 4-true-leaf stage were inoculated by spraying with mycelia (1 × 106 CFU mL−1), as the fungus did not sporulate in culture. Mycelia from each isolate were grown in 50 mL potato dextrose broth (PDB) for 7 days at 25°C. Another five wild eggplant plants were sprayed with sterilized water to served as controls. The experiment was repeated three times, and each replicate included 10 potted plants of wild eggplant (Solanum torvum) for each isolate. All of these plants were kept in a glass cabinet for 48 h (temperature 25°C; RH 90%), and then incubated in the greenhouse at 15–18°C (night)/25–28°C (day) under natural daylight conditions. After inoculation, the development of symptoms was observed and recorded every day. To satisfy Koch′s postulates, the causal fungus was re-isolated from the lesions on inoculated plants, and compared with the original isolates in morphological and cultural characteristics.
Molecular identification
DNA of all 30 isolates was extracted by the CTAB method from harvested mycelial (Lee Citation1990). The internal transcribed spacer region (ITS) of ribosomal DNA (rDNA) was amplified using the universal primers ITS1 (5ʹ-TCCGTAGGTGAACCTGCGC-3ʹ) and ITS4 (5ʹ-TCCTCCGCTTATTGATATGC-3ʹ) (White et al. Citation1990). The amplification of beta-tubulin gene fragment was carried out using the primers of β-tubf1 (5ʹ-CAGCTCGAGCGTATGAACGTCT-3ʹ) and β-tubr1 (5ʹ-TGTACCAATGCAAGAAAGCCTT-3ʹ), which were designed within conserved regions of the beta-tubulin gene from filamentous fungi (McKay et al. Citation1999). The PCR amplification procedure was 94°C for 4 min, 35 cycles of denaturation at 94°C for 1 min, annealing at 56°C for 1 min and extension at 72°C for 1 min, with final extension at 72°C for 10 min. The resulting ITS and beta-tubulin gene sequences were blasted in the GenBank database of NCBI (http://www.ncbi.nlm.nih.gov). The generated sequences and downloaded sequences were edited and aligned using MEGA version 5.1. Phylogenetic analysis was performed using the neighbour-joining method with a bootstrap of 1000 replicates (Tamura et al. Citation2007). Sequences of Curvularia aeria were used as an out-group.
Results
Lesions on leaves of naturally infected plants were subcircular or V-shaped, medium brown, with abundant spores, 0.5–2.0 cm in diameter (,). The 30 strains of the isolated fungus used in these experiments had the same cultural characteristics and were kept in the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences (CAAS) for future reference. Colonies were subhyaline initially, greyish green, gradually becoming dark brown (,). Conidiophores were subhyaline to light fawn, single or several clusters, 2–12 septa, 28.5–197.5 μm in length, and 3.8–6.3 μm in width, with an apical cell slightly to distinctly swollen, bearing a single spore at the apex (). Conidia were muriform, mostly ovoid, subhyaline to brown, mostly constricted at the median septum, 31.3–55.0 × 13.8–21.5 μm, 1–8 transverse septa, and 0–5 longitudinal septa (Fig. 1f). Shape, length and width of 80 conidia and 40 conidiophores in total were examined by microscopy (NIKON ECLIPSE 80i, 220 V) from leaves of naturally infected plants. Morphological characteristics of the fungus were consistent with the description of Stemphylium solani Weber (Weber Citation1930).
Fig. 1 (Colour online) Symptoms and morphological characters of Stemphylium solani on wild eggplant. a, Small brown spots developing on the leaves. b, Larger V-shaped blight on leaves of naturally infected wild eggplant (Solanum torvum) in the field. c, Colony of Stemphylium solani on PDA. (7-day-old), surface view. d, Colony of Stemphylium solani on PDA (7-day-old), bottom view. e, Microscopic structures of conidiophores (×400) and (f) conidia (×400). Symptoms observed in pathogenicity tests 10 days postinoculation (g) small brown spots and (h) big V-shaped blight on leaves.
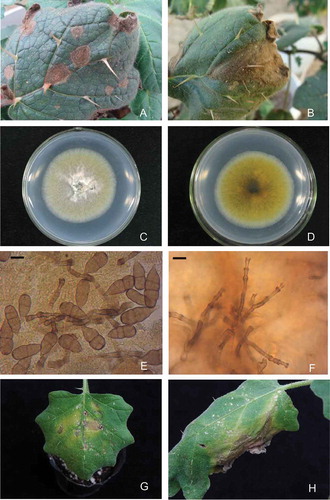
The pathogenicity test was repeated with similar results on all of the inoculated plants 10 days after inoculation, but not on control plants. The disease incidence of inoculated plants was 100%. The symptoms on the inoculated plants were similar to those observed in the field. Small brown spots developed on the leaves (Fig. 1g,h). The same pathogen was re-isolated from the symptomatic tissue on the inoculated plants. Plants inoculated with sterile water remained asymptomatic and the fungus was not isolated from these leaves.
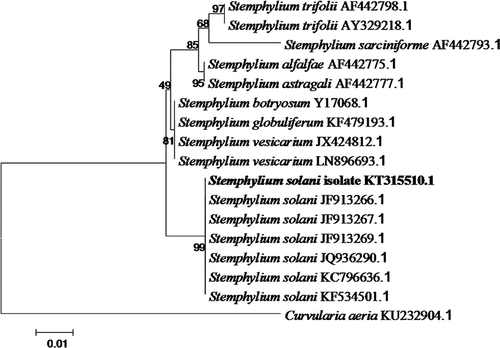
Fragments of approximately 583 bp and 1064 bp were obtained from ITS and beta-tubulin gene, respectively, of all 30 isolates. The ITS and beta-tubulin sequences of isolate YQZ11020901 have been submitted to the NCBI database (GenBank Accession No. KT315510 and KT315511, respectively). BLAST analysis confirmed the identity of the fungus, showing 100% identity to the ITS sequence of strain TL1 of S. solani isolate (KC581686.1) and 99% identity to the beta-tubulin sequence of S. solani isolate (JN105109.1). In the phylogenetic tree established on the basis of ITS sequence, the representative isolate was placed within a clade comprising reference isolates of S. solani (). Thus the pathogen was identified as S. solani based on morphological, molecular characteristics and pathogenicity to the host plants.
Fig. 2 Phylogenetic tree constructed with the ITS rDNA sequence of Stemphylium solani isolates and related species, including the isolate in this study (isolate KT315510.1 in bold), and the sequences of 15 isolates retrieved from GenBank. Bootstrap values resulting from 1000 replicates are shown at the branch points.
Discussion
Eggplant is very popular in the diet of the Chinese population. Verticillium wilt caused by Verticillium dahliae is a vascular disease causing severe yield and quality losses in eggplant (Çürük et al. Citation2009). As wild eggplant is robust and can resist Verticillium wilt, it is used as a rootstock for eggplant (Zhang et al. Citation1998). Stemphylium leaf spot is a new disease that has arisen rapidly in recent years, which was more severe following periods of wet weather or sprinkler irrigation. In spite of the control measures applied, economic losses may be between 1 and 10% of total production. Levels of disease (5–10%) one year may be followed by up to 90% infected fruit in the next year (Llorente & Montesinos Citation2006). The genus Stemphylium contains numerous important plant pathogenic fungi from a diverse range of hosts throughout the world. Stemphylium solani as a pathogen has been reported previously on several hosts, such as garlic, jioquil, water spinach and sweet potato in China (Sawada Citation1959; Shen et al. Citation2012; Zheng et al. Citation2012; Chai et al. Citation2015), pear and sugar beet in Netherlands (Llorente Citation1997; Hanse et al. Citation2015), cucumber in Greece (Vakalounakis & Markakis Citation2013), tomato, pepper, eggplant and lettuce in Malaysia (Nasehi et al. Citation2012, Citation2013a, Citation2013b). To the best of our knowledge, this is the first report of leaf spot caused by S. solani on wild eggplant in China.
Additional information
Funding
References
- Chai AL, Du GF, Shi YX, Xie XW, Li BJ. 2015. Leaf spot on sweet potato (Ipomoea batatas) caused by Stemphylium solani, a new disease in China. J Phytopathol. 163:1046–1049.
- Çürük S, Dasgan HY, Mansuroğlu S, Kurt Ş, Mazmanoğlu M, Antaklı Ö, Tarla G. 2009. Grafted eggplant yield, quality and growth in infested soil with Verticillium dahliae and Meloidogyne incognita. Pesq Agropec Bras. 44:1673–1681.
- Hanse B, Raaijmakers EEM, Schoone AHL, Van Oorschot PMS. 2015. Stemphylium sp., the cause of yellow leaf spot disease in sugar beet (Beta vulgaris L.) in the Netherlands. Eur J Plant Pathol. 142:319–330.
- Lee SB. 1990. Isolation of DNA from fungal mycelia and single spore. In: Innis MA, Gelfand DH, Sninsky TJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York, NY: Academic Press; p. 282–287.
- Li YP, Zhou BL, Li ZP, Yin YL, Fu YW. 2007. Allelopathic effects of wild anti-disease eggplant decomposing products on verticillium wilt (Verticillium dahliae). J Shenyang Agric Univ. 38:500–503.
- Llorente I. 1997. Development of an infection forecasting model for Stemphylium vesicarium. Evaluation, validation and implementation on experimental plots in pear commercial orchards [Ph.D. thesis]. Girona: University of Girona.
- Llorente I, Montesinos E. 2006. Brown spot of pear: an emerging disease of economic importance in Europe. Plant Dis. 90:1368–1375.
- McKay GJ, Brown AE, Bjourson AJ, Mercer PC. 1999. Molecular characterisation of Alternaria linicola and its detection in linseed. Eur J Plant Pathol. 105:157–166.
- Nasehi A, Kadir JB, Esfahani MN, Mahmodi F, Ghadirian H, Ashtiani FA, Golkhandan E. 2013a. An outbreak of leaf spot caused by Stemphylium solani on eggplant in Malaysia. Plant Dis. 97:689.
- Nasehi A, Kadir JB, Esfahani MN, Mahmodi F, Ghadirian H, Ashtiani FA, Soleimani N. 2013b. Leaf spot on lettuce (Lactuca sativa) caused by Stemphylium solani, a new disease in Malaysia. Plant Dis. 97:689.
- Nasehi A, Kadir JB, Zainal Abidin MA, Wong MY, Ashtiani FA. 2012. First report of gray leaf spot on pepper caused by Stemphylium solani in Malaysia. Plant Dis. 96:1227.
- Sawada K. 1959. Descriptive catalogue of Taiwan (Formosan) fungi. Taipei: College of Agriculture, National Taiwan University Press.
- Shen YM, Yang YC, Fu YJ, Hung TH. 2012. First report of Stemphylium solani causing leaf spot of Kalanchoe blossfeldiana in Taiwan. New Dis Rep. 25:10.
- Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA 4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 24:1596–1599.
- Vakalounakis DJ, Markakis EA. 2013. First report of Stemphylium solani as the causal agent of a leaf spot on greenhouse cucumber. Plant Dis. 97:287–288.
- Weber GF. 1930. Gray leaf spot of tomato caused by Stemphylium solani sp. nov. Phytopathology. 20:513–518.
- White TJ, Bruns TD, Lee SB, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York, NY: Academic Press; p. 315–322.
- Zhang SY, Yu NT, Wang X, Liang J, Xie HM, Wang JH, Zhang YL, Liu ZX. 2015. Natural occurrence of wild tomato mosaic virus in wild eggplant in China. J Phytopathol. 163:1023–1026.
- Zhang WC, He M, Wang LN. 1998. Eggplant root stock and grafting cultivation research progress in China. Liaoning Agric Sci. 6:30–32.
- Zheng L, Lv RJ, Huang JB, Jiang DH, Liu XH, Hsiang T. 2012. Integrated control of garlic leaf blight caused by Stemphylium solani in China. Can J Plant Pathol. 32:135–145.
