Abstract
Unusual blight-like symptoms appeared on highbush blueberry plants in Serbia during August 2015 and infected plants showed browning and reddening of leaves, drying of foliage and brown discolouration of internal vascular stem tissues. The objective of this study was to isolate and confirm a causal agent of the disease. Five diseased blueberry plants (2-year-old), with visible brown discolouration in the wood, were collected for isolation on potato dextrose agar (PDA). Morphological analysis of the selected fungal isolates showed the presence of abundant black, round to oblong, or irregularly shaped microsclerotia immersed in the PDA. Dark, globose pycnidia formed on water agar with an initially hyaline, granular content and single-celled conidia, indicating the presence of plant pathogenic fungus Macrophomina phaseolina associated with symptomatic plant tissues. Pathogenicity was confirmed on potted blueberry plants based on the initial symptoms of leaves turning yellowish to brown at the leaf edges, followed by the defoliation of leaves of the inoculated stems. Discolouration of vascular tissues was also observed on transverse sections of inoculated stems. The pathogen M. phaseolina was confirmed using molecular analysis of the ITS1-5.8S-ITS2 region of rDNA and a part of the TEF-1α gene region. This is the first report of M. phaseolina causing a blight disease on highbush blueberry in Serbia. The study should help in elucidating disease symptomatology and provide information on the risk which this fungus could pose in blueberry production.
Résumé
En août 2015, des symptômes inhabituels ressemblant à ceux causés par la brûlure sont apparus sur des plants de bleuets en corymbe en Serbie. Les feuilles des plants infectés rougissaient ou brunissaient, le feuillage se desséchait et les tissus vasculaires internes des tiges affichaient une coloration brunâtre. Le but de cette étude était d’isoler et d’identifier l’agent causal de la maladie. Cinq plants de bleuets infectés (vieux de deux ans), dont le bois présentait une coloration brunâtre, ont été collectés afin d’isoler l’agent pathogène sur de la gélose dextrosée à la pomme de terre. L’analyse morphologique des isolats fongiques sélectionnés a révélé de nombreux microsclérotes noirs, de forme ronde à oblongue ou irrégulière, ancrés dans le milieu de croissance. Des pycnides foncées et globulaires avec, initialement, un contenu hyalin et granulaire ainsi que des conidies unicellulaires, se sont développées sur de l’eau gélosée. Cela indiquait que le champignon pathogène Macrophomina phaseolina était responsable des symptômes associés aux tissus. La pathogénicité a été confirmée sur des plants de bleuets cultivés en pots en se basant sur les symptômes initiaux relatifs au jaunissement et au brunissement de la bordure des feuilles, suivis de la défoliation des tiges inoculées. Une coloration des tissus vasculaires a également été observée sur des sections transversales des tiges inoculées. L’identité de l’agent pathogène a été confirmée par analyse moléculaire de la région de l’ITS1-5.8S-ITS2 de l’ADNr et d’une partie de la région du gène du TEF-1α. Il s’agit de la première mention de M. phaseolina causant la brûlure sur des plants de bleuets en corymbe en Serbie. L’étude devrait aider à élucider la symptomatologie de la maladie et fournir de l’information quant au risque que ce champignon pourrait poser pour la production de bleuets.
Introduction
Blueberries (Vaccinium spp.) have gained popularity worldwide due to their economic value and nutritional and health benefits i.e. high antioxidant compounds such as anthocyanins, phenols and flavonols in fruit (Moyer et al., Citation2002). There are three types of blueberry in commercial production: lowbush (Vaccinium angustifolium Ait.), highbush (V. corymbosum L.) and rabbiteye (V. ashei Reade). Highbush is the most common, with different cultivars which vary in their response to exposure to low temperature periods and differ in their normal flowering and fruiting periods (DeFrancesco & Murray, Citation2011).
Blueberries are cultivated commercially worldwide (Evans & Ballen, Citation2014), mostly in the USA and Canada, but some production also occurs in a few European countries. In Serbia, blueberries are cultivated on an area of around 200 ha, with the biggest producers being located near urban areas. There has been a notable increase in blueberry production in Serbia over the last few years (Nikolić & Milivojević, Citation2015), and ‘Duke’ is the most common cultivar grown in the region.
Blueberries are grown for 20–30 years, and are thus exposed to various fungal, oomycete, bacterial or viral diseases (Schilder & Miles, Citation2008; Pscheidt & Weiland, Citation2015). Botrytis blight, anthracnose and septoria leaf spot are prevalent diseases of blueberries in Serbia. In the summer of 2015 (at the end of August), in Pridvorica (Mačva region) in Serbia, unusual foliar blight symptoms () were observed on a 3-year-old highbush blueberry ‘Duke’ (1-year-old planted highbush blueberry from 2-year-old propagation material). The observed symptoms consisted of browning and reddening of leaves and drying of foliage, and brown discolouration of internal vascular tissues at the basal part of the bush. The disease incidence ranged from 2 to 5%. The affected blueberry field had a long crop-rotation history, with corn and wheat being the last crops sown in the season prior to the planting of blueberries. Following the planting of blueberries, the field was put under the conditions of intensive irrigation and fertilization. The disease symptoms were observed only on the part of the field where corn was the previous crop in the rotation system and where there was water retention in the soil. This study was carried out to identify and characterize the causative agent associated with the blight symptoms, and confirm its pathogenicity on highbush blueberry.
Materials and methods
Sampling and isolation of pathogens
A field survey, which included the 3-year-old highbush blueberry ‘Duke’ (i.e. one year after planting), was carried out during 2015 to determine the incidence of blight symptoms in a 2 ha commercial field located in Pridvorica (Mačva region), Serbia. Five diseased plants with visible brown discolouration in the wood tissue at the basal part of bush were randomly sampled ( and c) and taken to the laboratory. Fungal isolation from the 2-year-old stems was carried out as previously described by Xu et al. (Citation2015) for blight disease on blueberry. Sections of vascular tissues (phloem and xylem) were cut with a sterile scalpel into 1 mm thick sections. These thin sections were surface-sterilized by immersing them in a 3% (v/v) bleach solution for 3 min, followed by dipping in 70% ethanol for 30 s and rinsing in sterile distilled water (SDW) three times, each time for 1 min. Five pieces were taken from each stem and placed onto potato dextrose agar, PDA (Difco, USA), amended with streptomycin sulfate (50 mg L−1). Petri plates were incubated for 7 days at 24°C in the dark. The plates were examined daily to record the fungal colony growth. During the incubation process, with the exception of contaminations, only one fungal colony type, suspected to be pathogenic, was developed. Fungal colonies were purified by transferring them onto new PDA plates. In total, 20 fungal isolates, TP1b–TP20b (four isolates representing one plant) were selected for further morphological study. Fungal isolates were maintained as stock cultures on PDA slants at 4°C.
Morphological characterization
All 20 purified fungal isolates were analysed for colony morphology and conidial characteristics (Beas-Fernandez et al., Citation2006; Gupta et al., Citation2012; Kaur et al., Citation2012). The fungal isolates were incubated on PDA under a photoperiod of 10 h/14 h of light/dark, and cool, white fluorescent light, at 24°C for a period of 14 days. The colour and shape of the colonies were recorded on the seventh day. The shape, colour and size of at least 50 microsclerotia were observed under the optical microscope (Euinstruments WF10/20; Amscope FMA50 camera).
To observe the pycnidia, they were induced on 2% water agar (WA) with autoclaved pine needles as a substrate, after a 3-week incubation at 25°C (Slippers et al., Citation2004). For each of the tested fungal isolates, length and width of 50 pycnidia and 100 conidia were measured using a compound microscope (Olympus BX51TF; Imaging Software; Olympus digital camera E-620). The mean value, standard deviation and 95% confidence limits were calculated for each fungal isolate. The colour of pycnidia and conidia was also recorded.
Based on the similar morphological results obtained for all of the tested fungal isolates, three representative fungal isolates, coded as TP1b, TP2b and TP3b, were selected for the purpose of the pathogenicity test, PCR and DNA sequencing.
Pathogenicity
Pathogenicity was conducted on 3-year-old potted blueberry ‘Duke’, according to previous pathogenic studies of M. phaseolina on other host plants (Almomani et al., Citation2013; Cummings & Bergstrom, Citation2013; Pavlović et al., Citation2015). Two-year-old stems of potted plants were first surface-disinfected with 70% ethanol for 1 min, and cuts (5–8 mm in length, 2–3 mm in width) were made with a sterile scalpel through the bark to the cambium. Mycelial discs (5 mm in diameter) from 4-day-old fungal isolates (PDA) were applied behind a bark flap of the wounded site. To avoid drying, inoculation sites were wrapped with moist cotton and covered with aluminium foil. In the control plants, the wounds were treated with PDA discs. Three replicates were used for each of the three representative fungal isolates, TP1b, TP2b and TP3b. Inoculated plants were kept in a growth chamber at a temperature of 27 ± 2°C, 12 h/12 h light/dark photoperiod and watered as necessary. The experiment was repeated twice.
The symptoms on inoculated plants were assessed over a period of 2 months. Two months after the inoculation, plants were transverse sectioned from the point of inoculation to observe the discolouration of the vascular tissue. To fulfil Koch’s postulates, inoculated stem tissue with discolouration symptoms was used to reisolate the fungal pathogen onto PDA, as described above for the pathogen isolation. Three obtained re-isolates (RTP1b, RTP2b, RTP3b) were further identified based on morphological characteristics to confirm if they were the same as the original. Likewise, re-isolation from control blueberry stems was done in order to show that the healthy plants used for the pathogenicity test were not already infected.
Polymerase chain reaction (PCR) and DNA sequencing
Three fungal isolates selected for further molecular characterization (TP1b, TP2b and TP3b) were grown on PDA for 7 days at 24°C. Approximately 50 mg of dry weight of the mycelium were collected and used for DNA extraction, following the manufacturer’s instructions of Plant DNeasy Mini Kit (Qiagen, Valencia, CA, USA). For the identification of these isolates, amplification of internal transcribed spacer (ITS1-5.8S-ITS2) of ribosomal DNA (rDNA), using universal primer pair ITS1/ITS4 (White et al., Citation1990), and a fragment of the TEF-1α gene region, using primer pair EF1-728F and EF1-986R (Carbone & Kohn, Citation1999) were performed by polymerase chain reaction (PCR), following the procedures of White et al. (Citation1990) for the ITS and Carbone & Kohn (Citation1999) for the TEF-1α gene region. The resulting PCR products were separated by electrophoresis on a 1% agarose gel, and the bands were excised and purified (Purification Kit, Qiagen, USA) for sequencing (Macrogen, Seoul, Korea). The obtained fungal sequences were deposited into the NCBI GenBank database.
Phylogenetic analysis
Phylogenetic analysis was performed to determine the evolutionary relationship between the isolates from blueberry (TP1b, TP2b, TP3b) and the most closely related strains originating from other host plants. For the ITS-5.8S rDNA analysis, the isolates were identified as Macrophomina phaseolina based on sequences of M. phaseolina originating from different hosts from NCBI database (GenBank numbers for these strains are shown in ): Helichrysum arenarium (strain MP 1), hemp (strain MAC 81), Phaseolussp. (strain MP 74), sesame (strain MP 219), soybean (strain MP 105), Sorghumsp. (strain MP 122), Staevia rebaudiana (strain 39R(3)), sugar beet (strain PM 19), sunflower (strain MAC 25) and Zea mays (strain CBS 227.33). Botryosphaeria dothidea strain PI565 was used as an out-group taxon.
Fig. 2 a, Fungal colony of M. phaseolina isolates grown on PDA after 5 days (left), 10 days (middle), 21 days (right). b, Microsclerotia immersed in agar surface. c, Pycnidia (left), protruding conidia from pycnidia (middle), conidia (right).
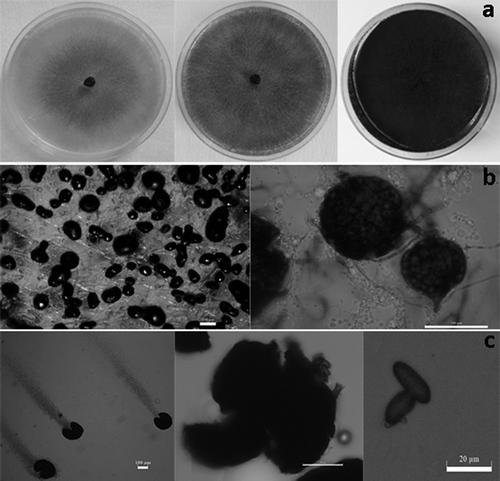
Fig. 3 Phylogenetic tree constructed with the ITS-5.8S rDNA a, and TEF-1α b, sequences of three blueberry isolates of M. phaseolina (TP1b, TP2b, TP3b) and M. phaseolina sequences retrieved from NCBI GenBank originating from different hosts. The out-group taxon was Botryosphaeria dothidea.
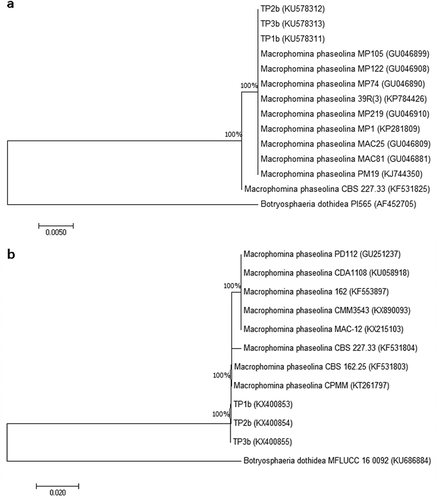
The obtained TEF-1α sequences were compared with eight M. phaseolina sequences deposited into the NCBI database (GenBank numbers for these strains are shown in ), originating from almond (strain PD112), Eucalyptussp. (strain CBS 162.25), Jatropha curcas (strain 162), Opuntia ficus-indica (strain CPMM), Phaseolus lunatus (strain CPMM3543), Ricinus communis (strain CDA1108), strawberry (strain MAC-12) and Zea mays (strain 227.33). Botryosphaeria dothidea strain MFLUCC 16 0092 was used as an out-group taxon.
All sequences were aligned using ClustalW multiple sequence alignment implemented in BioEdit software (version 7.1.3), and phylogenetic trees were constructed in MEGA 7 (Kumar et al., Citation2016), using the Neighbour-joining method. Evolutionary distances were computed using the Kimura two-parameter nucleotide substitution model (Kimura, Citation1980).
Results and discussion
Cultural and morphological characteristics
All fungal isolates from blueberry plants initially produced whitish colonies with concentric growth on PDA, which turned grey after 5 days, dark after 10 days, and black after 21 days (). Black, round to oblong or irregularly shaped microsclerotia also formed on PDA (), and the size of microsclerotia in the 7-day-old culture ranged between 93.8–179 ± 2.56 (length) × 54.3–111.36 ± 2.8 (width) μm. Pycnidia which formed on pine needles WA medium were typically dark, globose, measuring 98.0–224 ± 3.88 × 67.5–170 ± 3.1 μm in size. The conidia were initially hyaline, with a granular content and single-celled, measuring 13.7–26.6 ± 1.2 (length) × 6.9–14.2 ± 0.9 (width) μm (). Based on these cultural and morphological characteristics, all fungal isolates were identified as Macrophomina phaseolina.
Pathogenicity
All tested M. phaseolina isolates produced disease symptoms on inoculated highbush blueberry ‘Duke’ plants (–g). The first symptom appeared 3 weeks after inoculation, when the edges of leaves turned yellowish to brown; later, the yellowish areas enlarged and turned brown and started to fall off, and almost all leaves dried out and had fallen after 60 days. The vascular tissues of the inoculated stems also showed internal discolouration. Following plating of vascular tissue sections from the inoculated stems, the developing fungal colonies on PDA were identical in colony morphology to the original M. phaseolina isolates from blueberry. Non-inoculated control plants developed no disease symptoms and were negative for fungal reisolation.
In this study, the fungus M. phaseolina was consistently isolated from infected blueberry plants showing blight symptoms and its pathogenicity was confirmed on potted healthy blueberry plants. Therefore, this study reports a new host for this pathogen from Serbia. Previously, only a short note, published by Zuckerman (Citation1960), included Macrophoma sp. among the fungi isolated from diseased blueberry stems collected in Massachusetts. Following that initial report, there were no further data pertaining to this pathogen’s specificity to this host. Macrophomina phaseolina affects more than 500 cultivated and wild plant species belonging to 75 families (Su et al., Citation2001) and has a wide geographic distribution. Cultivating blueberry mainly after susceptible hosts, as was the case with corn in the observed Serbian field, can further increase the importance of this pathogen for blueberry production. Macrophomina phaseolina causes a broad spectrum of symptoms, such as stem canker, seedling blight, charcoal rot, dry root rot, wilt, leaf and stem blight, and pre-emergence and post-emergence damping-off, root and stem rot (Babu et al., Citation2007; Kaur et al., Citation2012). Gupta et al. (Citation2012) indicated that the reddish-brown discolouration of the vascular elements of roots and lower stem precedes the premature yellowing, as the fungus spreads up the stem during the season. Similarly, these symptoms could be observed for Serbian blueberries infected with M. phaseolina. Wounding was conducted prior to inoculation of blueberry stems, which has been shown to be required for infection by M. phaseolina. Different methods including tissue wounding such as the toothpick inoculation and stem tape inoculation have been described to evaluate M. phaseolina, but these techniques do not remotely simulate the natural infection processes, in contrast to the soil inoculation method (Grezes-Besset et al., Citation1996). It has been reported that M. phaseolina indirectly penetrates through natural openings or wounds (Mayek-Pérez et al., Citation2002) and spreads over a larger area via conidia (Kaur et al., Citation2012). Macrophomina phaseolina is a primarily soilborne pathogen known to produce microsclerotia in the root and stem tissues of its infected hosts (Kaur et al., Citation2012).
Molecular characterization
ITS1 and ITS4 primers yielded a single 600-bp fragment from all three fungal isolates TP1b, TP2b, TP3b. The BLASTn analysis of these representative isolates showed a 100% identity with M. phaseolina isolate MAC17 from GenBank (accession no. GU046807). The sequences of blueberry M. phaseolina isolates were deposited in GenBank (accession nos. KU578311–KU578313). All isolates of M. phaseolina were grouped into the same cluster showing 100% similarity ().
EF1-728F and EF1-986R primers amplified a 300-bp fragment from the three tested fungal isolates and BLASTn analysis showed a 99% identity with M. phaseolina strain MAC12 from GenBank (accession no. KX215103). The sequences of three representative blueberry M. phaseolina isolates were deposited in GenBank (accession nos. KX400853–KX400855). The blueberry isolates were grouped together with the other compared M. phaseolina strains, showing 100% bootstrap value ().
Although thus far no formae speciales, subspecies or physiological races of M. phaseolina have been reported, some researchers have found diversity among M. phaseolina isolates based on morphological, pathogenic and genetic studies (Vandemark et al. Citation2000; Mayék-Pérez et al. Citation2001; Su et al. Citation2001; Almeida et al. Citation2003; Beas-Fernandez et al. Citation2006; Purkayastha et al. Citation2006; Babu et al. Citation2007). It could be speculated that the genetic diversity of M. phaseolina could favour its survival and/or adaptation to variable environments or different hosts. Molecular tools used in this work were able to precisely identify the fungal isolates, but differences were not detected between the tested Serbian blueberry isolates and 10 comparative M. phaseolina strains originating from different hosts. Su et al. (Citation2001) and Babu et al. (Citation2007) reported high homogeneity in the ITS conserved sequences among the M. phaseolina isolates irrespective of their host specificity. Jana et al. (Citation2003, Citation2005a, Citation2005b) reported random amplified polymorphic DNA (RAPD), simple sequence repeats (SSRs) or universal rice primer PCR (URP-PCR) for differentiation of M. phaseolina isolates on the basis of host or geographic origin. According to some studies (Vandemark et al., Citation2000; Almeida et al., Citation2003; Reyes-Franco et al., Citation2006; Sarr et al., Citation2014), there was no correlation between DNA polymorphisms and geographic locations or hosts.
In conclusion, this is the first report of M. phaseolina causing a blight disease on highbush blueberry in Serbia. The study should help in recognizing the disease symptomatology of M. phaseolina in blueberries, thus providing the basis for accurately predicting the risk which this fungus poses and could also help with a strategy for devising future disease management.
Additional information
Funding
References
- Almeida AMR, Abdelnoor RV, Arrabal Arias CA, Carvalho VP, Jacoud Filho DS, Marin SRR, Benato LC, Pinto MC, Carvalho CGP. 2003. Genotypic diversity among Brazilian isolates of Macrophomina phaseolina revealed by RAPD. Fitopatol Bras. 28:279–285.
- Almomani F, Alhawatema M, Hameed K. 2013. Detection, identification and morphological characteristic of Macrophomina phaseolina: the charcoal rot disease pathogens isolated from infected plants in Northern Jordan. Arch Phytopathology Plant Protect. 46:1005–1014.
- Babu BK, Saxena AK, Srivastava AK, Arora DK. 2007. Identification and detection of Macrophomina phaseolina by using species-specific oligonucleotide primers and probe. Mycologia. 99:797–803.
- Beas-Fernandez R, De Santiago A, Hernandez-Delgado S, Mayek-Perez N. 2006. Characterization of Mexican and non-Mexican isolates of Macrophomina phaseolina based on morphological characteristics, pathogenicity on bean seeds and endoglucanase genes. J Plant Path. 88:53–60.
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 91:553–556.
- Cummings JA, Bergstrom GC. 2013. First report of charcoal rot caused by Macrophomina phaseolina in soybean in New York. Plant Dis. 97:1506.
- DeFrancesco J, Murray K. 2011. Pest management strategic plan for blueberries in Oregon and Washington. [Accessed 15 June 2011]. http://www.ipmcenters.org/pmsp/pdf/ORWABlueberry.pdf.
- Evans EA, Ballen FH. 2014. An overview of US blueberry production, trade, and consumption, with special reference to Florida. IFAS Extension, University of Florida. [Accessed May 2014]. http://edis.ifas.ufl.edu/pdffiles/FE/FE95200.pdf.
- Grezes-Besset B, Lucante N, Kelechian V, Dargent R, Muller H. 1996. Evaluation of castor bean resistance to sclerotial wilt disease caused by Macrophomina phaseolina. Plant Dis. 80:842–846.
- Gupta GK, Sharma SK, Ramteke R. 2012. Biology, epidemiology and management of the pathogenic fungus Macrophomina phaseolina (Tassi) Goid with special reference to charcoal rot of soybean (Glycine max (L.) Merrill). J Phytopathol. 160:167–180.
- Jana T, Sharma TR, Prasad RD, Arora DK. 2003. Molecular characterization of Macrophomina phaseolina and Fusarium species by a single primer RAPD technique. Microbiol Res. 158:249–257.
- Jana T, Sharma TR, Singh NK. 2005a. SSR-based detection of genetic variability in the charcoal root rot pathogen Macrophomina phaseolina. Mycol Res. 109:81–86.
- Jana TK, Singh NK, Koundal KR, Sharma TR. 2005b. Genetic differentiation of charcoal rot pathogen, Macrophomina phaseolina, into specific groups using URP-PCR. Can J Microbiol. 51:159–164.
- Kaur S, Dhillon GS, Brar SK, Vallad GE, Chand R, Chauhan VB. 2012. Emerging phytopathogen Macrophomina phaseolina: biology, economic importance and current diagnostic trends. Crit Rev Microbiol. 38:136–151.
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 16:111–120.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33:1870–1874.
- Mayek-Pérez N, García-Espinosa R, López-Castañeda C, Acosta-Gallegos JA, Simpson J. 2002. Water relations, histopathology and growth of common bean (Phaseolus vulgaris L.) during pathogenesis of Macrophomina phaseolina under drought stress. Physiol Mol Plant Pathol. 60:185–195.
- Mayék-Pérez N, López-Castañeda C, González-Chavira M, Garcia-Espinosa R, Acosta-Gallegos J, de la Vega OM, Simpson J. 2001. Variability of Mexican isolates of Macrophomina phaseolina based on pathogenesis and AFLP genotype. Physiol Mol Plant Pathol. 59:257–264.
- Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. 2002. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J Agric Food Chem. 50:519–525.
- Nikolić M, Milivojević J. 2015. Jagodaste voćke. Tehnologija gajenja. Belgrade: University of Belgrade, Faculty of Agriculture. Serbian.
- Pavlović S, Ristić D, Aleksić G, Milošević D, Stević T, Starović M. 2015. The first report of Macrophomina phaseolina of Immortelle (Helichrysum italicum) in Serbia. Plant Dis. 99:1279.
- Pscheidt J, Weiland J. 2015. Blueberry bacterial and fungal diseases. Proceedings of OSU blueberry school, Mar 16–17. Oregon State University, Corvallis, Oregon, p. 107–113.
- Purkayastha S, Kaur B, Dilbaghi N, Chaudhury A. 2006. Characterization of Macrophomina phaseolina, the charcoal rot pathogen of cluster bean, using conventional techniques and PCR based molecular markers. J Plant Pathol. 55:106–116.
- Reyes-Franco MC, Hernandez-Delgado S, Beas-Fernandez R, Medina-Fernandez M, Simpson J, Mayek-Perez N. 2006. Pathogenic and genetic variability within Macrophomina phaseolina from Mexico and other countries. J Phytopathol. 154:447–453.
- Sarr MP, Ndiaye M, Groenewald JZ, Crous PW. 2014. Genetic diversity in Macrophomina phaseolina, the causal agent of charcoal rot. Phytopathol Mediterr. 53:250–268.
- Schilder AC, Miles TD. 2008. Virus and viruslike diseases of blueberries. Michigan Blueberry Facts. Extension Bulletin E-3048. [Accessed December 2008]. http://msue.anr.msu.edu/uploads/files/e3048.pdf.
- Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ. 2004. Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia. 96:83–101.
- Su G, Suh S-O, Schneider RW, Russin JS. 2001. Host specialization in the charcoal rot fungus, Macrophomina phaseolina. Phytopathology. 91:120–126.
- Vandemark G, Martinez O, Pecina V, Alvarado MJ. 2000. Assessment of genetic relationships among isolates of Macrophomina phaseolina, using a simplified AFLP technique and two different methods of analyses. Mycology. 92:656–664.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press; p. 315–322.
- Xu C, Zhang H, Zhou Z, Hu T, Wang S, Wang Y, Cao K. 2015. Identification and distribution of Botryosphaeriaceae species associated with blueberry stem blight in China. Eur J Plant Pathol. 143:737–752.
- Zuckerman BM. 1960. Fungi collected from blueberry stems in Massachusetts. Plant Dis Rep. 44:416.

