Abstract
Two hundred and thirty-three single pustule isolates of Puccinia triticina were recovered from leaf rust infected wheat leaves from across Canada in 2012. These were tested for virulence on 16 standard differential lines and two additional lines containing Lr21 and LrCen, respectively. There were 49 different virulence phenotypes found, the most common of which were TBBG (18.9%), TNBG (15.0%), TDBJ (7.7%) and MBDS (6.4%). From Manitoba and Saskatchewan, 28 virulence phenotypes were found among 177 isolates, with the most common being TBBG (22.0%), TNBG (18.6%), TDBJ (10.2%) and MBDS (7.3%). There were 13 virulence phenotypes among 22 isolates from Ontario – MBTN (34.8%), MCGJ and TDPN (9.1% each) were the most common. Thirteen virulence phenotypes were found from 20 isolates in Quebec, with TBBG (25%), TNBG, MBDS, MBTN (each at 10.0%) being the most frequent. Two virulence phenotypes were found from among 14 isolates from Prince Edward Island, 12 isolates were TBRK, and two were TBRJ. There were some changes in virulence frequencies in 2012 compared with 2011, including increases for Lr2a, Lr2c, Lr9, Lr18 and Lr21, while there were decreases to Lr24, Lr26, Lr3ka, Lr17, LrB and Lr14a. Virulence to Lr21 was first found at 5.2% in Canada in 2011, and this increased to 7.7% in 2012. Since many Canadian wheat cultivars have Lr21, this could lead to greater susceptibility to leaf rust. However, all the Lr21 virulent isolates were found only in western Canada and in just four virulence phenotypes.
Résumé
Au Canada en 2012, 233 isolats obtenus d’une pustule unique de Puccinia triticina ont été collectés sur des feuilles de blé infectées par la rouille brune. Ces derniers ont été testés pour leur virulence sur 16 lignées différentielles standards et 2 lignées additionnelles contenant respectivement les gènes Lr21 et LrCen. On a décelé 49 phénotypes de virulence différents, dont les plus courants étaient TBBG (18.9%), TNBG (15,0 %), TDBJ (7.7%) et MBDS (6.4%). Du Manitoba et de la Saskatchewan, 28 phénotypes de virulence ont été trouvés parmi 177 isolats, les plus courants étant TBBG (22.0%), TNBG (18.6%), TDBJ (10.2%) et MBDS (7.3%). Il y avait 13 phénotypes de virulence parmi les 22 isolats de l’Ontario, dont MBTN (34.8%), MCGJ et TDPN (9.1%). Du Québec, 13 phénotypes de virulence ont été trouvés parmi 20 isolats, dont TBBG (25%), TNBG, MBDS, MBTN (à 10.0% chacun) étant les plus courants. Deux phénotypes de virulence ont été trouvés parmi 14 isolats de l’Île-du-Prince-Édouard, 12 isolats étant TBRK et 2, TBRJ. Il y a eu quelques changements quant à la fréquence de la virulence en 2012 comparativement à 2011, y compris des augmentations à l’égard de Lr2a, Lr2c, Lr9, Lr18 et Lr21, tandis qu’il y a eu des diminutions quant à Lr24, Lr26, Lr3ka, Lr17, LrB et Lr14a. Au Canada en 2011, la virulence à Lr21 a d’abord été établie à 5.2% et, en 2012, elle s’était accrue à 7.7%. Étant donné que plusieurs cultivars de blé canadien possèdent le gène Lr21, cela pourrait engendrer une plus grande sensibilité à la rouille brune. Toutefois, tous les isolats virulents à l’égard de Lr21 ont été détectés dans l’Ouest canadien, et ce, chez seulement quatre phénotypes de virulence.
Introduction
Wheat leaf rust, caused by Puccinia triticina Eriks. (Anikster et al., Citation1997) (syn. P. recondita Rob. ex Desmaz. f. sp. tritici) is an annual production concern for wheat growers in Canada (McCallum et al., Citation2016a) and is one of the most common and damaging diseases of wheat worldwide (Huerta-Espino, Citation2011). Severity of leaf rust varies in Canada annually, although susceptible cultivars would sustain severe damaging yield losses in most years without protection from fungicides. Disease severity is affected by several variables, including the amount of inoculum, in the form of urediniospores, blowing into Canada from the USA, temperature, rainfall, relative humidity, the level of resistance of the prevalent cultivars, and foliar fungicide applications (McCallum et al., Citation2007).
Virulence surveys for P. triticina began in Canada in 1931 to understand the virulence spectrum in the P. triticina populations, and have been conducted annually since then. This continuous record has provided valuable information on the evolution of virulence and dynamics in this pathogen (McCallum et al., Citation2016a). The P. triticina population has changed and diversified continuously over this time period (Wang et al., Citation2010; McCallum et al., Citation2016a), characterized by a large number of unique virulence phenotypes each year, with a few predominating for a number of years, then being replaced by other predominant virulence phenotypes. Understanding the constant evolution in this pathogen is critical to developing wheat cultivars with genetic resistance effective in the near and longer term.
The objective of the present research was to sample the Canadian P. triticina population in 2012 and determine the frequency of virulence phenotypes and virulence to key resistance genes. Results were compared with previous years to detect trends in virulence changes and the predominant virulence phenotypes.
Materials and methods
Virulence on the standard seedling differential lines
Infected wheat leaves were collected from individual fields and nurseries from June to September in 2012 in various locations throughout Saskatchewan, Manitoba, Ontario, Quebec and Prince Edward Island (PEI). The leaves were air dried at 20–27°C for ~12–24 h then stored separately for 2–4 months at 5°C. Puccinia triticina urediniospores from individual collections were then scraped off from infected leaves using a metal spatula and a small amount of water, and inoculated onto the susceptible wheat cultivar ‘Little Club’ by rubbing the leaves with urediniospores, water, and Tween 20 mixture as described previously (McCallum & Seto-Goh, Citation2005). Each pot of ‘Little Club’ seedlings was pretreated with 50 mL maleic hydrazide solution (0.36 g L−1 concentration) ~5 d after seeding to prevent the emergence of secondary leaves and to produce larger uredinia with abundant sporulation. A plastic cone ~25 cm in height with an open top was placed over each pot to minimize cross-contamination. Inoculated plants were placed into a dew chamber (Model I-60D, Percival Scientific, Perry, IA) with nearly 100% relative humidity for ~17 h to allow the urediniospores to germinate and initiate the infection process, then placed into a greenhouse at 20 ± 4°C with supplemental high-pressure sodium lighting, resulting in a photoperiod of ~16 h. Approximately 7 d after inoculation, chlorotic spots appeared, indicating areas of infection. The leaves were then trimmed so that a single isolated uredinium remained on the upper edge of each trimmed leaf. Cross-contamination was minimized by removing all extra leaves. At ~14 d after inoculation, urediniospores were collected from a single isolated uredinium into a 00 gelatin capsule using a vacuum suction micro-collector, mixed with a light mineral oil (Bayol, Esso Canada, Toronto, ON), and sprayed onto a 7-d-old set of wheat seedlings which included a flat of ‘Thatcher’ and 16 single resistance gene ‘Thatcher’ near-isogenic lines to test virulence, and a pot of ‘Thatcher’ plants for urediniospore increase. Two single uredinial isolates were typically evaluated from each rust collection, although sometimes one or three isolates per collection were analysed. The inoculated pot of ‘Thatcher’ increase was kept isolated with a plastic cone on top of the pot, and urediniospores were vacuum collected for subsequent inoculations. Approximately 12 seeds of each ‘Thatcher’ near-isogenic line were planted in a clump, and the clumps were evenly spaced in a fibre flat (25 × 15 cm). Plants were pretreated with maleic hydrazide as described above. After inoculation, the plants were allowed to dry for at least 1 h to allow the oil to evaporate and then incubated and maintained as described above. Infection types produced on the 12 standard leaf rust (Lr) differential lines (Set 1: Lr1 (RL6003a), Lr2a (RL6016), Lr2c (RL6047), Lr3 (RL6002); Set 2: Lr9 (RL6010), Lr16 (RL6005), Lr24 (RL6064), Lr26 (RL6078); Set 3: Lr3ka (RL6007), Lr11 (RL6053), Lr17 (RL6008), Lr30 (RL6049)) were used to determine the three letter code according to the virulence phenotype nomenclature (Long & Kolmer, Citation1989). Four supplemental differential lines (Set 4: LrB (RL6051), Lr10 (RL6004), Lr14a (RL6013), Lr18 (RL6009)) were added to provide additional virulence information about the isolates, resulting in a four letter code. All isolates were also inoculated onto ‘Thatcher’, Thatcher-Lr21 (RL6043) and LrCen (RL6003b). The resistance gene temporarily named LrCen was previously identified in the Thatcher-Lr1 near-isogenic line RL6003 (McCallum & Seto-Goh, Citation2006b). Infection types to all the differential near-isogenic lines were rated 12 d after inoculation. Isolates that produced infection types ‘;’ (hypersensitive flecks), ‘1’ (small uredinia with necrosis) and ‘2’ (small- to medium-sized uredinia with chlorosis) were considered avirulent to the differential line, and those that produced infection types ‘3’ (medium-sized uredinia without chlorosis or necrosis) and ‘4’ (large uredinia without chlorosis or necrosis) were considered virulent to the line (Long & Kolmer, Citation1989). Inoculations were repeated if the infection response was not clear.
Virulence on adult plant differential lines and additional seedling differential lines
At least one isolate from most of the unique virulence phenotypes identified was inoculated onto adult plants of ‘Thatcher’ and six ‘Thatcher’ near-isogenic lines (Lr12 (RL6011), Lr13 (RL4031), Lr21 (RL6043), Lr22a (RL6044), Lr35 (RL6082) or Lr37 (RL6081)), using urediniospores that were increased as described previously. The Thatcher-Lr21 line was inoculated at the adult plant stage to confirm the seedling results since the adult plant reaction is more definitive. Single plants of each ‘Thatcher’ near-isogenic line and ‘Thatcher’ were grown together in a 15-cm-diameter pot in a greenhouse as described above. Plants were trimmed so that only two or three culms per plant remained. Flag leaves of all the plants within a pot were inoculated with a single pustule isolate, as described previously for the seedling inoculation. Inoculated plants were dried for over 1 h to prevent cross-contamination and then incubated overnight in a dew chamber and grown in the greenhouse as described previously for seedling inoculation. Infection types were evaluated 14 d after inoculation. This subset of isolates was also tested on 12 additional ‘Thatcher’ near-isogenic lines at the seedling stage (Lr2b (RL6019), Lr3bg (RL6042), Lr14b (RL6006), Lr15 (RL6052), Lr19 (RL6040), Lr20 (RL6092), Lr23 (RL6012), Lr25 (RL6084), Lr28 (RL6079), Lr29 (RL6080), Lr32 (RL6086) and Lr52) and retested on Lr21 (RL6043). These isolates were also retested on seedling plants of the set of 16 ‘Thatcher’ near-isogenic lines mentioned previously to confirm their infection types, since many of these resistance genes (particularly Lr18 and LrB) are sensitive to temperature and other conditions. Inoculation, incubation and rating were as described previously for seedling evaluation.
Results and discussion
Virulence on the standard seedling differential lines
Leaf rust was first observed in early June of 2012 in southern Manitoba. The disease was found at lower than normal levels in Manitoba and Saskatchewan due to very dry and hot conditions which persisted throughout June, July and August (McCallum & Seto-Goh, Citation2013). Average severity levels were 8.0% in Manitoba and 2.0% in eastern Saskatchewan in plots of susceptible cultivars. Wheat leaf rust was also found at low levels in Ontario in 12 of 22 fields surveyed, with an average severity of 2.4% (Xue & Chen, Citation2013). There were 233 single pustule isolates from diseased leaf samples collected throughout Canada in 2012, 177 from Manitoba and Saskatchewan, 22 from Ontario, 20 from Quebec and 14 from PEI; there were no isolates from Alberta due to low disease pressure in that province. When these isolates were analysed for virulence on 16 standard differentials, 49 unique virulence phenotypes were found, the most common being TBBG (18.9%), TNBG (15.0%), TDBJ (7.7%) and MBDS (6.4%) (). Of these, TDBJ and TBBG were also common in 2011. MBDS was one of the most common virulence phenotypes from 2000–2004 but had been less frequent in recent years, and TNBG was not common previously ().
Table 1. Frequency and distribution of virulence phenotypes of Puccinia triticina identified in 2012 by infection types to selected resistance genes.
Table 2. Frequency (%) of predominant P. triticina virulence phenotypes in Canada from 2001 to 2012.
In Manitoba and Saskatchewan, 28 virulence phenotypes were found among the 177 isolates tested, with TBBG (22.0%), TNBG (18.6%), TDBJ (10.2%) and MBDS (7.3%) being most frequent. In 2011, the most common virulence phenotypes from this region were TDBJ (16.7%), TDBG (13.9%), MLDS and TBBG (both at 9.3%) (McCallum et al., Citation2017). In the north-central region of the USA, bordering Manitoba and Saskatchewan, the predominant virulence phenotypes in 2012 were TDBGQ (14.7%), TBBGJ (11.8%) and TNBGJ (10.3%), similar to those found in Manitoba and Saskatchewan (Kolmer & Hughes, Citation2014).
There were 13 virulence phenotypes found among 22 isolates from Ontario (). The most frequent phenotypes were MBTN (36.4%) and MCGJ and TDPN (each 9.1%), while all other virulence phenotypes were represented by a single isolate. In 2011, the most frequently isolated phenotypes were MBTN (25.5%), MCTN (8.5%) and then MLDS, TBRK, TBJS and TCRK (each at 4.3%) (McCallum et al., Citation2017). In the bordering region of the USA, the most common virulence phenotypes in 2012 were MBTNB (33.8%), TBRKG (16.2%) and TCRKG (12.2%) (Kolmer & Hughes, Citation2014).
In Quebec, 13 virulence phenotypes were identified from among 20 isolates. TBBG was represented by five isolates, while TNBG, MBDS and MBTN were each represented by two isolates, and all other virulence phenotypes with a single isolate. In 2011, MCGS was found from two isolates and all other virulence phenotypes were represented by one isolate each (McCallum et al., Citation2017).
There were two virulence phenotypes found among 14 isolates from Prince Edward Island (PEI). TBRK was the most common, representing 12 isolates, while TBRJ was found from two isolates. In 2011, there were six virulence phenotypes found among 10 isolates, with MCNQ (three isolates), MBPS and MHNQ (two isolates each) being the most common (McCallum et al., Citation2017). TBRK has been found at a low frequency in recent years (), but mostly from Ontario and Quebec. In 2012, most isolates of TBRK were found in PEI, but there were also single isolates found in Ontario and Quebec.
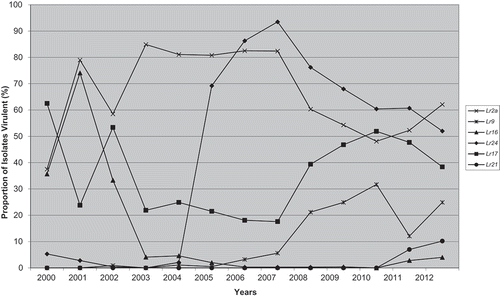
Virulence on the standard 16 differential lines, and Lr21, changed from 2011 to 2012 (). In Canada, there were increases in the frequencies of virulence for Lr2a, Lr2c, Lr9, Lr18 and Lr21, while there were decreases to Lr24, Lr26, Lr3ka, Lr17, LrB and Lr14a. documents the longer term (2000–2012) changes in virulence for a few key resistance genes in the Manitoba and Saskatchewan region, since the majority of isolates came from this region. There was an increase in the frequency of virulence to Lr21, and while it is still relatively low at 10.2%, it increased from 2011 when it was first detected in Canada. This is important since many Canadian wheat cultivars have Lr21 (McCallum et al., Citation2016a) and therefore they could become more susceptible to leaf rust as the frequency of virulence shifts higher to this gene. The Lr21 virulent isolates were found in just a few of the virulence phenotypes in 2012 (TBBG, TDBG, TDBJ and TDBS) and similarly in 2011 (TBBJ, TDBG, TDBJ and TDBQ). Avirulence to LrCen was also found in nine (TBBG, TBBH, TBBJ, TBBR, TDBG, TDBJ, TGBG, TLBG, TNBG) of the 49 virulence phenotypes found in 2012. There was an increase in the frequency of isolates virulent to Lr2a and Lr2c, as T_ _ _ isolates became relatively more frequent than M _ _ _ isolates ( and ).
Table 3. Frequencies of virulence of Puccinia triticina in Canada in 2012 and 2011 to lines of wheat with single Lr genes for leaf rust resistance.
Fig. 1 Frequency of virulence (%) from 2000–2012a in the Manitoba and Saskatchewan population of P. triticina to near-isogenic lines containing Lr2a, Lr9, Lr16, Lr24, Lr17, or Lr21.
a Data from McCallum and Seto-Goh (Citation2003, Citation2004, Citation2005, Citation2006a, Citation2006b, Citation2008, Citation2009) and McCallum et al. (Citation2010, Citation2011, Citation2013, Citation2016b, Citation2017).
Virulence on adult plant differential lines and additional seedling differential lines
When 96 isolates, representing nearly every unique virulence phenotype found in each region, were tested at the adult plant stage on differential lines containing the adult plant resistance genes, Lr12, Lr13, Lr22a, Lr35 and Lr37, all isolates were avirulent to Lr22a and virulent to Lr13. All isolates tested were virulent to Lr12 except 12–160-1 MBSS and 12–172-2 MCGJ and avirulent to Lr35 except 12–44-2 MBDS, 12–123-1 MBPS, 12–160-1 MBSS, 12–172-1 MBTN, 12–179-2 MBTN, 12–29-1 MLDS, 12–125-2 MLDS and 12–78-1 MMPS. Isolates reacted in the same manner to Lr21 at the adult plant stage as they did in the seedling stage, confirming the previous seedling results. The isolates differed in their reaction to Lr37. provides the results for 59 of these 96 isolates, as a single isolate was chosen to represent those of the same virulence phenotype and region that had a similar reaction on these differential lines. These results are similar to previous years, with a high frequency of virulence to Lr12, Lr13 and Lr35, no virulence on Lr22a and an intermediate level of virulence on Lr37.
Table 4. Infection types of 59 representative isolates of Puccinia triticina from Canada in 2012 tested on near-isogenic lines of ‘Thatcher’ at the seedling (Lr3bg, Lr20, Lr23, Lr28) or adult-plant stage (Lr37).
When this same group of 96 isolates were tested at the seedling stage on 12 additional ‘Thatcher’ differential lines at the seedling stage (Lr2b, Lr3bg, Lr14b, Lr15, Lr19, Lr20, Lr23, Lr25, Lr28, Lr29, Lr32 and Lr52), all isolates were avirulent to Lr19, Lr29, Lr32 and Lr52 while the reaction on Lr2b was similar to Lr2a. All isolates were virulent to Lr15 except five (12–123-1 MBPS, 12–108-2 PBDQ, 12–115-1 PBDQ, 12–37-2 TDBJ, and 12–93-1 TDBJ). All isolates were avirulent to Lr25 except eight (12–112-2 PBDJ, 12–107-1 PBDQ, 12–105-1 PBDQ, 12–108-2 PBDQ, 12–115-1 PBDQ, 12–101-1 PCDG, 12–160-1 MBSS and 12–172-2 MCGJ). All isolates were virulent to Lr14b except 10 (12–200-2 MBDN, 12–172-1 MBTN, 12–191-2 MCSQ, 12–191-1 MCTN, 12–112-2 PBDJ, 12–107-1 PBDQ, 12–105-1 PBDQ, 12–108-2 PBDQ, 12–115-1 PBDQ and 12–101-1 PCDG). There were intermediate frequencies of virulence to Lr3bg, Lr20, Lr23 and Lr28 which differentiated the isolates ().
Overall, the virulence phenotypes found in Canada during 2012 were similar to previous years; however, there were important changes in the predominant virulence phenotypes and the frequency of virulence to some resistance genes. The most frequently found virulence phenotype in 2012, TBBG, has been increasing in frequency since 2009, but it was also the second-most frequent phenotype found in 2004 (). The second-most frequent phenotype in 2012 was TNBG, which was found at low levels in 2010 and 2011 but very rarely before that. Some phenotypes such as MBTN and TBRK were only found in eastern Canada, although these were the most frequently found phenotypes in Ontario and PEI, respectively. Similarly, virulence to Lr21 was only found in western Canada, and has been limited to a small number of virulence phenotypes to date. The increase in Lr21 virulence frequency has important implications for breeding programmes in Canada since this gene is found fairly often in Canadian cultivars (McCallum et al., Citation2016a); therefore, Lr21 could lose some of its effectiveness in controlling leaf rust. Many genes are still completely effective in controlling leaf rust as no virulence has been detected, including Lr19, Lr22a, Lr29, Lr32 and Lr52. The resistance gene Lr16 is interesting since the frequency of virulence is low (3.4%), although most isolates have an intermediate level of virulence, and the ‘Thatcher’ isoline with Lr16 (RL6005) had leaf rust infection in field trials of ~65% (B. D. McCallum, unpublished). However, when combined with Lr34, it still provides some protection (B. D. McCallum unpublished). This could also be the case for Lr21, which could still provide some degree of protection in combination with other resistance genes, even if the frequency of virulence in the pathogen continues to increase since it was first detected in 2011.
The P. triticina populations in Canada in 2012 were similar to those in the USA states south of the border. This is consistent with previous years and indicates the Canadian populations are largely derived from the incoming inoculum from these states each year, as the inoculum moves north up the Puccinia pathway from the southern USA (Roelfs, Citation1985). There is likely also some selection within Canada for virulence to some of the common resistance genes in Canadian wheat, such as Lr1, Lr2a, Lr10, Lr12, Lr13, Lr16, Lr14a, Lr16 and Lr21 (McCallum et al., Citation2016a). The wheat cultivars grown in eastern and western Canada are different, but so is the inoculum coming into western and eastern Canada from the adjacent states. The populations from western Canada were often different in previous years from the eastern Canadian populations (Wang et al., Citation2010), as was the case in 2012.
Acknowledgements
We thank André Comeau, Harpinder Randhawa and Richard Martin who sent samples for analysis, and other cooperators who grew trap rust nurseries.
References
- Anikster Y, Bushnell WR, Eilam T, Manisterski J, Roelfs AP. 1997. Puccinia recondita causing leaf rust on cultivated wheats, wild wheats, and rye. Can J Bot. 75:2082–2096.
- Huerta-Espino J, Singh RP, Germán S, McCallum BD, Park RF, Chen WQ, Bhardwaj SC, Goyeau H. 2011. Global status of wheat leaf rust caused by Puccinia triticina. Euphytica. 179:143–160.
- Kolmer JA, Hughes ME. 2014. Physiologic specialization of Puccinia triticina on wheat in the United States in 2012. Plant Dis. 98:1145–1150.
- Long DL, Kolmer JA. 1989. A North American system of nomenclature for Puccinia recondita f.sp tritici. Phytopathology. 79:525–529.
- McCallum BD, Fetch T, Chong J. 2007. Cereal rust control in Canada. Aust J Agri Res. 58:639–647.
- McCallum BD, Hiebert CW, Cloutier S, Bakkeren G, Rosa SB, Humphreys DG, Marais GF, McCartney CA, Panwar V, Rampitsch C, et al. 2016a. A review of wheat leaf rust research and the development of resistant cultivars in Canada. Can J Plant Pathol. 38:1–18.
- McCallum BD, Seto-Goh P. 2003. Physiologic specialization of wheat leaf rust (Puccinia triticina) in Canada in 2000. Can J Plant Pathol. 25:91–97.
- McCallum BD, Seto-Goh P. 2004. Physiologic specialization of Puccinia triticina, the cause of wheat leaf rust, in Canada in 2001. Can J Plant Pathol. 26:109–120.
- McCallum BD, Seto-Goh P. 2005. Physiologic specialization of wheat leaf rust (Puccinia triticina) in Canada in 2002. Can J Plant Pathol. 27:90–99.
- McCallum BD, Seto-Goh P. 2006a. Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2003. Can J Plant Pathol. 28:208–213.
- McCallum BD, Seto-Goh P. 2006b. Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf, in Canada in 2004. Can J Plant Pathol. 28:566–576.
- McCallum BD, Seto-Goh P. 2008. Physiologic specialization of Puccinia triticina in Canada in 2005. Can J Plant Pathol. 30:124–132.
- McCallum BD, Seto-Goh P. 2009. Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2006. Can J Plant Pathol. 31:80–87.
- McCallum BD, Seto-Goh P. 2013. Leaf rust and stripe rust of wheat in Manitoba and eastern Saskatchewan in 2012. Can Plant Dis Surv. 93: 134. http://www.cps-scp.ca/cpds.shtml.
- McCallum BD, Seto-Goh P, Xue A. 2010. Physiological specialization of Puccinia triticina in Canada in 2007. Can J Plant Pathol. 32:229–236.
- McCallum BD, Seto-Goh P, Xue A. 2011. Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2008. Can J Plant Pathol. 33:541–549.
- McCallum BD, Seto-Goh P, Xue A. 2013. Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2009. Can J Plant Pathol. 35:338–345.
- McCallum BD, Seto-Goh P, Xue A. 2016b. Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2010. Can J Plant Pathol. 35:338–345.
- McCallum BD, Seto-Goh P, Xue A. 2017. Physiological specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2011. Can J Plant Pathol. 39:454–463.
- Roelfs AP. 1985. Epidemiology in North America. In: Roelfs AP, Bushnell WR, editors. The cereal rusts: volume II diseases, distribution, epidemiology, and control. Orlando (FL): Academic Press; p. 403–434.
- Wang X, Bakkeren G, McCallum B. 2010. Virulence and molecular polymorphisms of the wheat leaf rust fungus Puccinia triticina in Canada from 1997 to 2007. Botany. 88:575–589.
- Xue AG, Chen Y. 2013. Diseases of spring wheat in central and eastern Ontario in 2012. Can Plant Dis Surv. 93: 139–141. http://www.cps-scp.ca/cpds.shtml.
