Abstract
The resistance of 10 lettuce cultivars to bacterial leaf spot caused by Xanthomonas campestris pv. vitians (Xcv) was evaluated to establish the relationship between stomatal density, variations in pathogen population size, and the presence of epiphytic Bacillus sp. On the basis of average disease severity index ranked on a scale from 0 to 6, the cultivars were divided into three groups: tolerant, with 2.09; intermediate, with 3.59; and susceptible, with 4.92. The initial inoculum population of the pathogen was slightly increased, by less than 1 logCFU g−1 after 14 d, on only the tolerant cultivars ‘Batavia Reine des Glaces’ and ‘Little Gem’. In contrast to Xcv, the initial inoculum population of Bacillus sp. decreased on all the cultivars, by 1 to more than 2 logCFU g−1, without any significant relationship with the resistance groups. The tolerant cultivars exhibited averages of 40 and 57 stomata mm−2 on the adaxial and abaxial sides of the leaves, respectively; those averages were significantly lower than those of the susceptible cultivars, with 67 and 111 stomata mm−2, respectively. However, the stomatal area of the cultivars varied from 110 to 263 µm2/stomata and 126 to 232 µm2/stomata on the adaxial and abaxial sides of the leaves, respectively. Therefore, no relationship could be established between stomatal area and lettuce cultivar susceptibility. The density of stomata seems to be a good criterion for predicting the degree of tolerance of cultivars, especially when the adaxial side of the leaves is considered.
Résumé
La résistance de 10 cultivars de laitue à la tache bactérienne causée par le Xanthomonas campestris pv. vitians (Xcv) a été évaluée en vue d’établir la relation entre la densité stomatique, les variations de la taille de la population pathogène, et la présence du Bacillus sp. Sur la base de l’indice moyen de sévérité de la maladie classée sur une échelle de 0 à 6, les cultivars ont été divisés en trois groupes: tolérant, avec un indice de 2.09; intermédiaire: 3.59; et sensible: 4.92. La population initiale de l’agent pathogène a augmenté légèrement, de moins de 1 logCFU g−1 après 14 jours de croissance, seulement pour les cultivars tolérants ‘Batavia Reine des Glaces’ et ‘Little Gem’. Contrairement à celle du Xcv, la population initiale du Bacillus sp. a diminué pour tous les cultivars, de 1 à plus de 2 logCFU g−1, sans relation significative avec les groupes de résistance. Les cultivars tolérants présentaient des moyennes de 40 et 57 stomates/mm2 sur les faces adaxiales et abaxiales des feuilles, respectivement. Ces moyennes étaient significativement inférieures à celles des cultivars sensibles, avec respectivement 67 et 111 stomates mm−2. Cependant, la surface stomatique des cultivars variait de 110 à 263 μm2 et de 126 à 232 μm2 sur les faces adaxiales et abaxiales des feuilles, respectivement. Par conséquent, aucune relation n’a pu être établie entre la surface stomatique et la sensibilité des cultivars de laitue. La densité des stomates semble être un bon critère pour prédire le degré de tolérance des cultivars, surtout quand on considère la face adaxiale des feuilles.
Introduction
Lettuce (Lactuca sativa L.) is susceptible to various diseases caused by fungi, viruses and bacteria. Bacterial leaf spot (BLS) caused by Xanthomonas campestris pv. vitians (Xcv) is one of the economically important diseases of lettuce (Lu & Raid, Citation2013; Hayes et al., Citation2014). The first report of this disease in Quebec, Canada, was in 1994, and a severe outbreak occurred in 1996, causing losses of up to 100% in some lettuce fields (Toussaint, Citation1999). In most cases, Xcv enters lettuce leaves via stomata (Underwood et al., Citation2007). The first symptoms of BLS generally appear at the leaf margin and consist of water-soaked lesions that become necrotic and confluent later in the season (Carisse et al., Citation2000). Thus, the symptoms of BLS affect the market value of lettuce, reducing quality and increasing post-harvest losses (Barak et al., Citation2001). Controlling BLS of lettuce is quite difficult because there are few pesticides registered for treatment (Lu & Raid, Citation2013). The use of resistant lettuce cultivars remains the most viable strategy for BLS control (Bull & Koike, Citation2005; Lu & Raid, Citation2013; Hayes et al., Citation2014). Lu & Raid (Citation2013) evaluated 69 lettuce germplasm lines for resistance to BLS, and most of them (67) were rated as susceptible to moderately susceptible. Only one resistance gene has been identified in lettuce genomes. The dominant gene Xar1 (Xanthomonas resistance 1) was found on chromosome 2 in the lettuce cultivar ‘La Brillante’ and confers a high level of resistance to specific strains of Xcv (Hayes et al., Citation2014). The cultivar ‘Little Gem’ is also considered to be resistant to a few Xcv strains, with a resistance gene that is either allelic or closely linked to Xar1 (Hayes et al., Citation2014). Bull et al. (Citation2015) further demonstrated that Xcv population growth on lettuce varied among cultivars and was usually higher on susceptible ones. Those authors proposed that a hypersensitive response was responsible for the weak growth rate of Xcv on the cultivar ‘Little Gem’ (Bull et al., Citation2015). Another determinant for the development of bacterial diseases in lettuce is the bacterial community composition of the leaves. Rastogi et al. (Citation2012) established that the presence of some bacterial genera, including Bacillus, Erwinia and Pantoe, was negatively correlated with the presence of Xcv. However, few studies have looked at the effect of cultivar on the populations of epiphytic bacteria.
Stomata are present in large numbers on lettuce leaf surfaces and serve as a major route of pathogen entry into plant tissues (Melotto et al., Citation2006). Both the morphology and structure of stomata can play a role in host resistance by limiting the penetration of the pathogen (Huang, Citation1986). Some studies have already referred to this relationship between stomatal density and disease resistance in plants. Ramos & Volin (Citation1987) showed that stomatal frequency on the adaxial and abaxial surfaces of tomato (Solanum lycopersicum) leaves was correlated with the number of spot lesions produced after infection with X. campestris pv. vesicatoria. Given the weakness of monogenic resistance and the availability of few BLS-resistant genotypes in lettuce, breeding efforts must focus on various alternative resistance factors, including morphoanatomical characteristics.
In light of the role that stomata play in plant–pathogen interactions, the objectives of this study were to: (i) evaluate the resistance to BLS of 10 lettuce cultivars; (ii) determine if BLS severity in lettuce is modulated by the stomatal characteristics of leaves and by the population dynamics of Xcv in a genotype-dependent manner; and (iii) assess the population densities of epiphytic Bacillus sp. on lettuce leaves in relation to Xcv.
Materials and methods
Bacterial strains and inoculum preparation
Both the rifampicin-resistant Xcv strain B07-007 and the streptomycin-resistant Bacillus sp. LS2014 were isolated from field-grown lettuce in Montérégie, Quebec, Canada (this study). The isolates were stored at −80°C in a solution of glycerol and nutrient broth (50/50, vol/vol). The bacterial suspensions used for lettuce inoculation were prepared as follows. First, Xcv strain B07-007 or Bacillus sp. was grown for 72 h at 28°C on yeast extract-dextrose-calcium carbonate agar or on nutrient agar, respectively. Bacteria were then recovered by flooding the plates with saline (NaCl 0.85%), and the inoculum was prepared by adjusting the bacterial concentration using a spectrophotometer, to 108 CFU mL−1.
Plant production
The morphotype and the identification number of the 10 lettuce cultivars ‘Batavia Reine des glaces’ (BRG), ‘Chief’ (CHI), ‘Estival’ (EST), ‘Gorilla’ (GOR), ‘Hochelaga’ (HOC), ‘Little Gem’ (LIG), ‘Parris Island Cos’ (PIC), ‘Romora’ (ROM), ‘Turbo’ (TUR) and ‘Vista Verde’ (VIV) used in our study are listed in . Lettuce seeds were sowed in seedling trays containing peat moss (Promix; Saint-Remi, Quebec, Canada) as the growth substrate. The trays were placed in a growth chamber for 3 wk at 18°C/16°C (day/night) and 70% relative humidity with a 16-h photoperiod. After emergence, the plants were watered daily and fertilized weekly with a 20-8-20 (N-P-K) soluble fertilizer. When the seedlings were 3 wk old, they were transplanted into 15-cm-diameter plastic pots filled with the same growth substrate and then transferred to a greenhouse and grown under the same conditions as in the growth chamber except for the temperature conditions, which were set at 28°C/14°C (day/night), while relative humidity and photoperiod remained unchanged.
Table 1. Average disease severity index (ADSI) values for 10 lettuce cultivars inoculated with Xanthomonas campestris pv. vitians.
Lettuce inoculation
The Xanthomonas or Bacillus suspension was applied to 4-wk-old lettuce plants by misting both the adaxial and abaxial leaf surfaces to run-off using a handheld sprayer. The control plants were sprayed with saline only. The plants were then incubated in a greenhouse misting room with a mister set to spray fine water droplets for 30 s every 30 min over a 12-h period for 14 d.
Assessment of susceptibility of lettuce to BLS
To determine an estimate of disease severity and susceptibility for lettuce cultivars inoculated with Xcv, 20 treatments consisting of 10 cultivars inoculated with Xcv or uninoculated were arranged as a completely randomized block design. The experiment was carried out in five replicates and was repeated twice. The plants were evaluated for BLS symptoms at 14 d post inoculation (dpi). Disease severity was determined on the most affected leaf of each plant as described in Bull et al. (Citation2015) with some modifications. Ratings were given as follows: 0 for leaves with no symptoms; 1 for leaves with small spots measuring less than 2 mm in diameter; 2 for leaves with distinct lesions measuring 2–5 mm in diameter; 3 for leaves exhibiting coalesced lesions measuring 5–10 mm in diameter; 4 for leaves with coalesced lesions measuring 10–20 mm in diameter; 5 for leaves with coalesced lesions measuring more than 20 mm in diameter; and 6 for dead leaves. A decimal was added to the score to reflect the total area of diseased tissue. No decimal was added if only one-third of the leaf exhibited symptoms. When lesions covered less than half of the leaf surface, 0.33 was added to the score, while 0.66 was added when more than half of the leaf presented symptoms.
Evaluation of population dynamics
Population dynamics were evaluated on the lettuce plants inoculated with Bacillus or infected with Xcv. The plants were sampled at five different times: 1 d before infection (T−1), immediately after infection (T0), and at 2, 8 and 14 dpi (T1, T2 and T3, respectively). At each sampling time, four lettuce plants per cultivar were collected at random. Whole plants were harvested without roots, weighed, and placed individually in polyethylene Stomacher® bags. The plants were then macerated in 100–200 mL of saline, by means of a Stomacher® 400 Circulator machine (Seward Ltd, Bohemia, NY, USA) at 250 rpm for 2 min. Serial dilutions of the macerating solution were plated in duplicate either onto plates of 10% tryptic soy agar supplemented with rifampicin (50 mg L−1) and cycloheximide (50 mg L−1) for Xcv enumeration or onto plates of nutrient agar supplemented with cycloheximide (50 mg L−1) for Bacillus enumeration. The plates were incubated at 28°C for 3 d before typical colonies of each inoculated strain were counted.
Characterization of lettuce stomata
Five-wk-old lettuce plants were used for determination of stomatal characteristics (density and area occupied on the leaves). For five plants of each cultivar, epidermal imprints were made on the second fully expanded leaf. The stomatal imprints were obtained by applying clear nail polish on two-thirds of both the adaxial and abaxial leaf surfaces. After a 5-min drying period, the polymer was peeled off the leaf with pliers and then examined under a Zeiss Imager M1 optical microscope. The stomatal density (number of stomata per unit area) was obtained from microscopy images (five images per cultivar) by counting the stomata on the adaxial and abaxial sides of the leaves, and the stomatal area of each cultivar was determined using the CellProfiler software. CellProfiler is an open-source software that automatically identifies and measures a variety of biological objects in digital images. This software records a full spectrum of measurements for each object, including localization within the image, size, shape, colour, intensity, degree of correlation between colours, texture and number of neighbours (Lamprecht et al., Citation2007).
Statistical analyses
Statistical analyses were carried out using the Statistix 9 software. All sets of data requiring statistical analyses were performed with one-way or two-way analysis of variance (ANOVA). Stomatal density, stomatal area and population dynamics (variation of initial inoculum) were analysed with one-way ANOVA, while disease severity index was analysed using two-way ANOVA with cultivar and repetition as main factors. A posteriori comparisons of cultivars were made using least significant difference (LSD) tests.
Results
Evaluation of lettuce susceptibility to BLS
The 10 lettuce cultivars were tested for their susceptibility to BLS in two independent trials. The data from both experiments were combined because the variations for severity index in the interactions between cultivars and trials were not significant (P = 0.5519 for repetition and P = 0.1645 for the interaction repetition × cultivar). No typical symptoms of BLS were observed on the non-inoculated lettuce plants (controls), whereas the inoculated cultivars could be differentiated according to their average disease severity index (ADSI) (P < 0.05; ). Figure 1 shows representative plants (one plant inoculated with Xcv and the other not) for each cultivar.
The 10 cultivars infected with Xcv strain B07-007 were classified into three groups according to symptom severity (). The cultivars exhibiting an ADSI below 3.0 were classified as tolerant, and those with an ADSI above 4.0 were classified as susceptible. The cultivars associated with an ADSI between 3.0 and 4.0 were considered to have an intermediate level of susceptibility. However, it was not determined here whether tolerance abilities to BLS shown by cultivars were due to vertical or horizontal resistance (or both). The tolerant group contained the cultivars LIG, BRG, HOC and EST, which had ADSIs ranging from 1.63 to 2.63 (). The cultivars ROM and TUR were classified in the intermediate group, with ADSIs of 3.52 and 3.66, respectively. Lastly, the susceptible cultivars PIC, GOR, CHI and VIV had ADSIs between 4.46 and 5.16 (). The most tolerant cultivar was LIG (ADSI of 1.63), whereas GOR was the most susceptible cultivar (ADSI of 5.16). All the romaine morphology-type cultivars belong to the susceptible or intermediary levels of susceptibility groups (, ).
Fig. 1 (Colour online) Photographs of the 10 lettuce cultivars infected with Xanthomonas campestris pv. vitians and their corresponding controls sprayed with saline. The photographs were taken 7 d post inoculation. The cultivars used were as follows: BRG, ‘Batavia Reine des glaces’ (a); CHI, ‘Chief’ (b); EST, ‘Estival’ (c); GOR, ‘Gorilla’ (d); HOC, ‘Hochelaga’ (e); LIG, ‘Little Gem’ (f); PIC, ‘Parris Island Cos’ (g); ROM, ‘Romora’ (h); TUR, ‘Turbo’ (i); VIV, ‘Vista Verde’ (j). Red tags are shown in front of the experimental plants sprayed with Xcv and white tags are shown in front of the controls sprayed with saline only.

Characteristics of stomata of lettuce cultivars
Figure 2 shows the stomatal density of all 10 lettuce cultivars for both the adaxial and abaxial leaf sides, and Fig. 3 provides corresponding micrographs for each leaf side and each cultivar. On the adaxial leaf side, the susceptible group exhibited a higher stomatal density (60 to 71 stomata mm−2) than the tolerant cultivars (32 to 49 stomata/mm2) (P < 0.05) (). The cultivar ROM exhibited a stomatal density similar to that of the tolerant cultivars LIG and EST (44, 49 and 49 stomata mm−2, respectively), whereas stomatal density did not vary significantly between the other intermediate cultivar TUR and the susceptible cultivars GOR, PIC and VIV (68 to 74 stomata mm−2). The tolerant cultivars HOC and BRG exhibited the lowest stomatal density (33 and 34 stomata mm−2, respectively).
Fig. 2 Stomatal density (a, b) and stomatal area (c, d) on the adaxial (a, c) and the abaxial (b, d) leaf sides of 10 lettuce cultivars (cultivars abbreviations are as in ) with different levels of resistance to bacterial leaf spot. Values are the mean (+ S.D.) of five plants (one leaf per plant). Values with the same letters are not significantly different (LSD test, P < 0.05). The white, grey and black bars indicate tolerant, intermediate and susceptible cultivars, respectively.
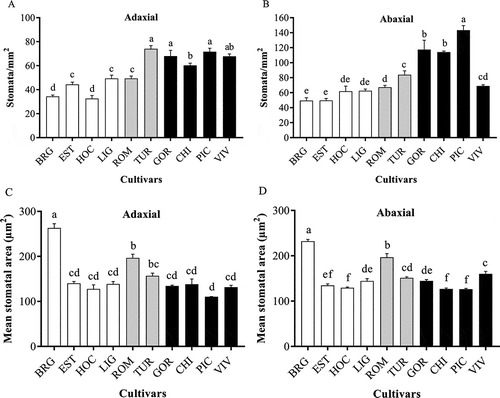
Stomatal density was higher on the abaxial leaf side than on the adaxial leaf side for all cultivars. The stomatal density on the abaxial side of the leaves varied from 69 to 143 stomata mm−2 for the susceptible cultivars and from 49 to 62 stomata mm−2 for the tolerant cultivars. All the susceptible cultivars except VIV also exhibited abaxial stomatal densities that were higher than those of the tolerant cultivars (). The intermediate cultivars ROM and TUR exhibited abaxial stomatal densities of 67 and 69 stomata mm−2, respectively. The stomatal area on lettuce leaves varied between 160 and 263 µm2stomata-1 and between 126 and 232 µm2stomata-1 on the adaxial and abaxial sides, respectively (). No relationship could be established between stomatal area and lettuce cultivar susceptibility to Xcv.
Population dynamics of XCV on lettuce cultivars
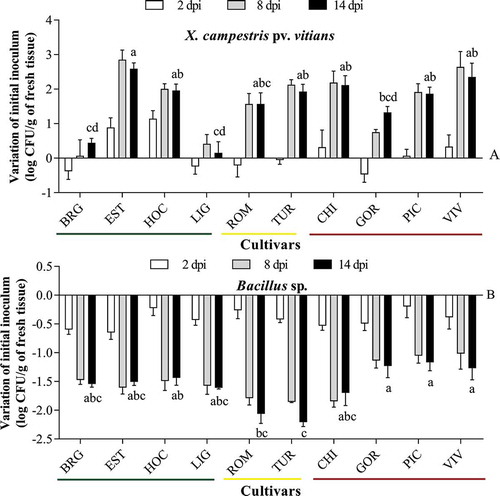
The existence of a relationship between the resistance level of the 10 lettuce cultivars and the population dynamics of Xcv and Bacillus sp. was tested over a 14-d period. For all the lettuce cultivars, the initial Xcv inoculum population had not changed considerably by 2 dpi. The population then exhibited significant growth on all cultivars except the tolerant cultivars LIG and BRG (Supplemental ). The Xcv population of LIG and BRG increased only slightly (by less than 1 logCFU g−1) from 0 to 14 dpi, whereas the Xcv population of all the other cultivars showed at least a 2-fold increase (Fig. 4a).
In contrast to Xcv, the epiphytic Bacillus sp. did not appear to multiply on lettuce leaves, given that the initial inoculum population decreased, 14 days after inoculation, by 1–2 logCFU g−1 for BRG, EST, HOC, LIG, CHI, GOR, CHI and VIV and by more than 2 logCFU g−1 for ROM and TUR ().
Fig. 3 Stomatal micrographs of 10 lettuce cultivars (20× magnification). Each pair of micrographs shows the adaxial (left) and the abaxial (right) sides of the same leaf for one cultivar. The cultivars used were as follows: ‘Batavia Reine des glaces’ (a, b), ‘Chief’ (c, d), ‘Estival’ (e, f), ‘Gorilla’ (g, h), ‘Hochelaga’ (i, j), ‘Little Gem’ (k, l), ‘Parris Island Cos’ (m, n), ‘Romora’ (o, p), ‘Turbo’ (q, r) and ‘Vista Verde’ (s, t).
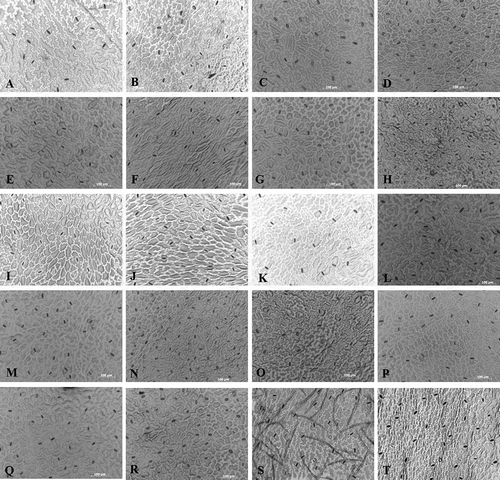
Fig. 4 Variation in the initial inoculum populations of Xanthomonas campestris pv. vitians strain B07-007 (a) and Bacillus sp. (b) on 10 lettuce cultivars (see caption of for cultivars abbreviations). Four-wk-old Lactuca sativa plants were inoculated with 108 CFU mL−1. Bacterial populations were sampled four times over a period of 14 d post inoculation (dpi). The white, grey and black bars indicate 2, 8 and 14 dpi, respectively. The green, yellow and red lines indicate tolerant, intermediate and susceptible cultivars, respectively. Multiple comparison LSD test was performed on 14 dpi values; black bar values accompanied by the same letter are not significantly different (P < 0.05).
Discussion
The cultivars used in our study showed differential levels of resistance to BLS. The most susceptible lettuce cultivars to BLS were romaine type, supporting the observations of Wang et al. (Citation2015) who demonstrated that romaine lettuce cultivars were generally more susceptible to virulent Xcv isolates. Romaine cultivars have a different morphology with better exposed leaves than other lettuce types have (), which may explain their greater susceptibility to BLS. This susceptibility may also be explained by the fact that these cultivars have seldom been used in genetic crosses and lack gene exchanges with other lettuce types, as previously proposed by Lu et al. (Citation2014). Among the 10 evaluated cultivars, LIG and BGR were the most tolerant ones. Bull et al. (Citation2007) also reported that these cultivars were among the most resistant ones under both field and greenhouse conditions. According to Hayes et al. (Citation2014), the tolerance of LIG to Xcv could be explained by the presence in its genome of resistance genes that are either allelic or closely linked to Xar1, a single dominant gene conferring a high level of resistance to Xcv. The inoculation of Xcv on LIG resulted in very small leaf spots that could effectively represent a hypersensitive reaction (Bull et al., Citation2015). Identifying other pre-existing structural and chemical defence factors that may be incorporated into a lettuce breeding programme against BLS should be considered in the future.
Although included in the tolerant cultivars group, BRG, HOC and EST have not been reported to contain the resistance gene Xar1 or other resistance genes in their genomes; however, the presence of such genes cannot be excluded, especially for BRG, which only weakly supported Xcv growth. In our study, the initial population of Xcv increased only slightly over a 14-d incubation period when Xcv was inoculated onto the leaf surfaces of the tolerant cultivars BRG and LIG. This trend toward lower pathogen populations was also described by Barton-Willis et al. (Citation1989) with respect to the growth dynamics of X. campestris pv. oryzae on leaves of rice cultivars, where the populations of bacteria were always 1 or 2 log units lower in the incompatible interaction than in the compatible interaction. Similar results were obtained by Willis & Kinscherf (Citation2009) in their study of the population dynamics of Pseudomonas syringae pv. tomato on tomato, where the mean population sizes were 4–5 orders of magnitude larger on the susceptible tomato line than on the resistant line containing the Pto gene. While BRG and LIG had relatively low Xcv populations, EST and HOC, also considered tolerant cultivars, had considerable increases in Xcv populations after inoculation. Therefore, the multiplication of the initial pathogen inoculum when the bacterium was sprayed on a given cultivar does not always seem to correlate with the extent of the resulting symptoms (Bull et al., Citation2015; Al-Saleh et al., Citation2011). Some structural aspects of lettuce leaves, such as presence of wax, cutin, lenticels, and the structure of the epidermal cell, may affect the resistance of cultivars to BLS and need to be investigated in further studies. The present work supports previous observations that the host affects the epiphytic growth of Xcv (Bull et al., Citation2015; Al-Saleh et al., Citation2011; Fayette et al., Citation2018).
The presence of Xcv on lettuce was shown to be negatively correlated with the presence of several bacterial genera, including Bacillus (Rastogi et al., Citation2012). However, unlike Xcv, the initial inoculum population of Bacillus sp. decreased independently of the cultivar tested in the present study. Species of Bacillus are frequently used in biocontrol studies of plant pathogens because of the diversity of modes of action, which include competition, antibiotic production and induction of systemic resistance (Monteiro et al., Citation2005). However, the response of lettuce cultivars to Xcv did not appear to depend on the capacity to support growth of epiphytic bacteria such as Bacillus.
Stomata represent one of the most important routes for the entry of bacterial pathogens into plants (Melotto et al., Citation2008), including Xcv (Underwood et al., Citation2007). Considering the roles played by stomata in plant infection by several plant pathogens (Montillet & Hirt, Citation2013), particularly Xanthomonas (Ryan et al., Citation2011), it is possible that lettuce cultivars with higher stomatal densities could be more susceptible to BLS. Our results suggest that stomatal density may influence the resistance of lettuce to BLS. Ramos & Volin (Citation1987) previously showed that stomatal numbers on the adaxial and abaxial leaf surfaces of tomato were correlated with the number of spot lesions produced after infection with X. campestris pv. vesicatoria. In our study, all the susceptible cultivars (VIV, CHI, GOR and PIC) had higher adaxial stomatal densities than the resistant cultivars, although the same was not always the case for the abaxial side of the leaves. Our results also show that lettuce stomatal density was generally higher on the abaxial side of the leaves than on the adaxial side. This is generally the case in most plant species (Weryszko-Chmielewska & Michałojć, Citation2009). However, no relationship could be established between stomatal area and lettuce cultivar susceptibility to Xcv. Considering that Xanthomonas cells measure 0.2–0.6 µm by 0.8–2.9 µm (Swings & Civerolo, Citation1993) and that the stomatal area on lettuce leaves varied from 110 to 263 µm2stomata-1, the stomatal area does not appear as a limiting factor to bacterial penetration. Stomatal density thus emerges as a more important parameter than stomatal area in terms of the susceptibility of cultivars to BLS. In addition to stomatal density, reducing the stomatal aperture size following bacterial infection may be important in resistance, as it has been shown that a susceptible tomato accession had a greater stomatal aperture over a longer duration after Xanthomonas perforans infection than two resistant tomato accessions (Wang et al., Citation2017).
Our study suggests that lettuce cultivars have different degrees of resistance to BLS. In particular, the tolerant cultivars BRG and LIG did not exhibit any changes in the size of the Xcv populations over a period of 14 d, whereas the populations of Bacillus sp. decreased in all cultivars without significant differences among the three susceptibility groups. Stomatal density on the adaxial side of the leaves is a relevant characteristic for predicting cultivar susceptibility to BLS. Nevertheless, there are many factors that could determine the basis of the tolerance of particular lettuce cultivars to BLS. For example, Yean et al. (Citation2009) showed that lettucenin A is a major phytoalexin produced in lettuce. Further studies on these cultivars are under way to identify factors such as mineral composition of the lettuce cultivars or lettuce proteomes correlating with lettuce tolerance to BLS.
Supplemental Material
Download PDF (27.6 KB)Acknowledgements
We thank Daniel Garneau, Sylvain Lerat, Mélanie Cadieux and Marie Ciotola for providing technical assistance and Gina Chaput and Mary Varcoe for critical review of the manuscript.
Supplemental material
Supplementary data can be accessed online here: https://doi.org/10.1080/07060661.2018.1495269
Additional information
Funding
References
- Al-Saleh MA, Ibrahim YE, Abo-Elyousr KAM, Alibrahim JS. 2011. Population dynamics of Xanthomonas campestris pv. vitians on different plant species and management of bacterial leaf spot of lettuce under greenhouse conditions. Crop Prot. 30:883–887.
- Barak JD, Koike ST, Gilbertson RL. 2001. Role of crop debris and weeds in the epidemiology of bacterial leaf spot of lettuce in California. Plant Dis. 85:169–178.
- Barton-Willis PA, Roberts PD, Guo A, Leach JE. 1989. Growth dynamics of Xanthomonas campestris pv. oryzae in leaves of rice differential cultivars. Phytopathology. 79:573–578.
- Bull CT, Gebben SJ, Goldman PH, Trent M, Hayes RJ. 2015. Host genotype and hypersensitive reaction influence population levels of Xanthomonas campestris pv. vitians in lettuce. Phytopathology. 105:316–324.
- Bull CT, Goldman PH, Hayes R, Madden LV, Koike ST, Ryder E. 2007. Genetic diversity of lettuce for resistance to bacterial leaf spot caused by Xanthomonas campestris pv. vitians. Plant Health Prog. doi:10.1094/PHP-2007-0917-02-RS
- Bull CT, Koike ST. 2005. Evaluating the efficacy of commercial products for management of bacterial leaf spot on lettuce. Plant Health Prog. doi:10.1094/PHP-2005-1121-01-RS
- Carisse O, Ouimet A, Toussaint V, Philion V. 2000. Evaluation of the effect of seed treatments, bactericides, and cultivars on bacterial leaf spot of lettuce caused by Xanthomonas campestris pv. vitians. Plant Dis. 84:295–299.
- Fayette J, Jones JB, Pernezny K, Roberts PD, Raid R. 2018. Survival of Xanthomonas campestris pv. vitians on lettuce in crop debris, irrigation water, and weeds in south Florida. Eur J Plant Pathol. 151:341–353.
- Hayes RJ, Trent MA, Truco MJ, Antonise R, Michelmore RW, Bull CT. 2014. The inheritance of resistance to bacterial leaf spot of lettuce caused by Xanthomonas campestris pv. vitians in three lettuce cultivars. Hortic Res. 1:14066.
- Huang J. 1986. Ultrastructure of bacterial penetration in plants. Annu Rev Phytopathol. 24:141–157.
- Lamprecht MR, Sabatini DM, Carpenter AE. 2007. CellProfiler™: free, versatile software for automated biological image analysis. BioTechniques. 42:71–75.
- Lu H, Hu J, Kwon SJ. 2014. Association analysis of bacterial leaf spot resistance and SNP markers derived from expressed sequence tags (ESTs) in lettuce (Lactuca sativa L.). Mol Breed. 34:997–1006.
- Lu H, Raid R. 2013. A novel screening method for evaluation of lettuce germplasm for bacterial leaf spot resistance. HortScience. 48:171–174.
- Melotto M, Underwood W, He SY. 2008. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol. 46:101–122.
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. 2006. Plant stomata function in innate immunity against bacterial invasion. Cell. 126:969–980.
- Monteiro L, Mariano RDLR, Souto-Maior AM. 2005. Antagonism of Bacillus spp. against Xanthomonas campestris pv. campestris. Braz Arch Biol Technol. 48:23–29.
- Montillet J-L, Hirt H. 2013. New checkpoints in stomatal defense. Trends Plant Sci. 18:295–297.
- Ramos LJ, Volin RB. 1987. Role of stomatal opening and frequency on infection of Lycopersicon spp. by Xanthomonas campestris pv. vesicatoria. Phytopathology. 77:1311–1317.
- Rastogi G, Sbodio A, Tech JJ, Suslow TV, Coaker GL, Leveau JHL. 2012. Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J. 6:1812–1822.
- Ryan RP, Vorhölter F-J, Potnis N, Jones JB, Van Sluys M-A, Bogdanove AJ, Dow JM. 2011. Pathogenomics of Xanthomonas: understanding bacterium–plant interactions. Nat Rev Microbiol. 9:344–355.
- Swings JG, Civerolo EL. 1993. Xanthomonas. London: Chapman and Hall.
- Toussaint V. 1999. Bacterial leaf spot, a new disease of lettuce in Quebec caused by Xanthomonas campestris pv. vitians. Phytoprotection. 80:121–125.
- Underwood W, Melotto M, He SY. 2007. Role of plant stomata in bacterial invasion. Cell Microbiol. 9:1621–1629.
- Wang Y, Lu H, Raid RN, Nuessly GS, Faroutine G. 2015. Diverse responses of lettuce cultivars and germplasm lines to infections of three isolates of Xanthomonas campestris pv. vitians. HortScience. 50:650–655.
- Wang YQ, Zhang XF, Li N, Liu X. 2017. Comparison of cellular responses to Xanthomonas perforans infection between resistant and susceptible tomato accessions. J Plant Physiol. 209:105–114.
- Weryszko-Chmielewska E, Michałojć Z. 2009. Anatomical features of leaves of sweet pepper (Capsicum annuum L.) fed with calcium using foliar nutrition. Acta Agrobot. 62:155–164.
- Willis DK, Kinscherf TG. 2009. Population dynamics of Pseudomonas syringae pv. tomato strains on tomato cultivars Rio Grande and Rio Grande‐Pto under field conditions. J Phytopathol. 157:219–227.
- Yean HC, Atong M, Chong KP. 2009. Lettucenin A and its role against Xanthomonas campestris. J Agric Sci. 1:87.
