Abstract
Western redcedar (WRC) trees of 48–49 years in age grown in monoculture and in admixtures with birch, Douglas-fir and pine in the interior of British Columbia were sampled for wood density and the presence and level of damage from butt decay initiated by Armillaria ostoyae in stumped and unstumped plots. Stump removal reduced butt decay incidence in WRC from 18% to 2% and damage from 3.5% of the diameter at base to 0.3% compared to when stumps remained. External lesions on tree bases caused by Armillaria root disease increased the probability of WRC stems having butt decay by 1.25 times for an average-sized lesion. The incidence of butt decay and the level of internal damage to WRC in sampled plots was not related to admixture, tree size or site index. Stump removal controlled the inoculum of Armillaria root disease, thereby reducing incidence and size of WRC basal lesions that probably act as infection courts for other fungi causing butt decay. WRC trees grown in monoculture or with birch had the highest wood densities of all planting mixes tested. Wood density in WRC was lowest at the outer edges of individual WRC trees, in larger trees, and in plots where stumps had not been removed. The lower density WRC wood is thought to occur by overtopping from fast-growing species in admixture and depletion of soil moisture affecting latewood formation. This is a first report of stump removal affecting butt decay and admixture affecting wood density in WRC.
Résumé
Les thuyas géants (TG) âgés de 48 ou 49 ans, cultivés en monoculture ou en peuplement mixte avec le bouleau, le douglas vert et le pin dans la zone intérieure de la Colombie-Britannique, ont été échantillonnés en fonction de la densité de leur bois et de l’occurrence de la carie de la souche causée par Armillaria ostoyae, ainsi que du taux de dommage qui en découle, dans des parcelles essouchées ou non. L’essouchage réduit l’incidence de la carie chez le TG de 18% à 2% et le dommage, de 3.5% du diamètre à la base à 0.3%. Des lésions externes à la base des arbres, causées par le pourridié-agaric, accroissent de 1.25 fois la probabilité que les tiges de TG soient attaquées par la carie de la souche, et ce, pour des lésions de taille moyenne. L’incidence de la carie de la souche et le taux de dommage interne causé aux TG dans les parcelles échantillonnées n’avaient aucun lien avec la mixité du peuplement, la taille des arbres ou l’indice de la station. L’essouchage a permis de restreindre la dispersion de l’inoculum du pourridié-agaric, réduisant ainsi l’incidence et la taille des lésions se développant à la base des TG et qui agissent probablement comme des zones d’infection pour d’autres champignons causant la carie de la souche. Les TG cultivés en monoculture ou avec le bouleau affichaient les plus fortes densités de bois de toutes les combinaisons d’essences testées. La densité du bois des TG était la plus faible sur les pourtours des TG poussant isolément, chez les arbres les plus gros et dans les parcelles où les souches n’avaient pas été enlevées. On croit que la plus faible densité de bois des TG dépend des essences à croissance rapide dans un peuplement mixte où celles-ci dominent la strate et accaparent l’humidité du sol, ce qui nuit à la formation du bois final. Il s’agit de la première mention relative à l’essouchage influençant la carie de la souche et à la mixité du peuplement agissant sur la densité du bois chez le TG.
Introduction
Western redcedar (Thuja plicata; WRC) is an important component of forests in western North America. The species has major ecological, economic and social importance (Antos et al., Citation2016), as well as significance to Indigenous cultures (Hebda & Mathewes, Citation1984). The current natural range of WRC, which extends from northern California to south-eastern Alaska and from the Pacific coast to west of the Rocky Mountains, comprises coastal and interior populations (Minore, Citation1990). The naturally durable heartwood of redcedar is used mostly in exterior applications such as decking, fencing, siding, shakes and shingles and utility poles. Redcedar wood products have high value, selling for two to four times more than other commercially important, North American species (Random lengths, Citation2016). Over the last decade, annual harvest levels of WRC in British Columbia have ranged from 4–5 million cubic metres. The estimated value of the species to the forest sector in British Columbia is significant; for example, it was valued at $1.2 billion in 2014 (Gregory et al., Citation2018).
The renowned natural durability of western redcedar is linked to the presence of extractives (Stirling et al., Citation2017). Extractives are secondary metabolites preferentially deposited in the heartwood conferring durability to wood products (Barton & MacDonald, Citation1971). However, western redcedar trees commonly have extensive heartwood decay in the butt of living trees, affecting up to 10% volume on the coast and up to 28% in the interior by age 250 years (Buckland, Citation1946). Decay in WRC is not well understood but likely involves an initial succession of microorganisms (e.g. bacteria, yeasts, non-decay fungi), which are thought to enter through existing basal wounds and fire scars and detoxify heartwood extractives. While more than 25 fungal species have been shown to be associated with heartwood decay in living WRC, to date only about a half dozen are considered to have notable incidence and impact on living WRC. Armillaria ostoyae has recently been proposed as potentially facilitating the entry of other decay-causing fungi in WRC via basal lesions that the fungus causes (Sturrock et al., Citation2017). Armillaria ostoyae can cause significant redcedar mortality (Koenigs, Citation1969), even though it is considered to be tolerant to this disease.
Butt decay in WRC trees is characterized by a variety of symptoms, which range from a complete absence of heartwood due to extensive decay to heartwood that appears sound. Lower density wood occurs due to colonization by fungal agents and through reduced percentage of latewood (Smith, Citation1980). Presence and extent of decay damage can be assessed through destructive sampling or coring of affected trees. More recently, the availability of microdrilling tools allows for the non-destructive evaluation of trees while providing an indirect estimate of wood density and detection of internal decay (Allikmäe et al., Citation2017).
Stumping is the machine-assisted removal of fungal inoculum in stumps from sites afflicted with root disease (Sturrock, Citation2000). Stumping has been shown to be very effective in reducing mortality of root-diseased, mostly coniferous trees in infected forest stands (Cleary et al., Citation2013). A long-term research study established in 1968 clearly demonstrated that the highest tree survival occurred where stumps had been removed prior to planting (Morrison et al., Citation2014).
This study evaluated the impact of butt decay in western redcedar using a long-term stumping experiment in the interior of British Columbia. Improved understanding of factors contributing to butt decay will lead to better planning and implementation of management practices for redcedar. The study specifically evaluated the effects of stumping treatment, stand species admixture, Armillaria root disease and tree size on redcedar butt decay incidence, damage and wood density.
Materials and methods
Site description
The Skimikin stump removal trial is located near Salmon Arm, British Columbia, Canada (BC) (50°48′32′′N, 119°25′16′′W) in the Interior Cedar-Hemlock (ICH) ecosystem (Lloyd et al., Citation1990). The trial was established in 1967. In one-half of the site, the stumps were removed with an excavator and then the soil was root raked, and in the other half stumps were left in place. Single species rows and two species mixtures in alternating rows were planted for each species combination in three 20 × 20 m plots each in both stump treatments. The four tree species planted were western redcedar (Thuja plicata Donn ex D. Don); Douglas-fir (Pseudotsuga menziesii var. glauca (Beissn.) Franco); lodgepole pine (pine) (Pinus contorta Dougl. et Loud. var. latifolia); paper birch (birch) (Betula papyrifera Marsh). There was no birch or WRC seed available when the trial was established so natural seedlings were recovered and transplanted from the surrounding area. Susceptibility to A. ostoyae in descending order by tree species is: Douglas-fir, lodgepole pine and birch. For more complete site information and layout, see Morrison et al. (Citation2014).
Tree selection and sampling
Almost five decades later in 2015, several plots had no living WRC. In the stump removal area, there was only one plot each with live WRC admixes containing Douglas-fir and pine. During 2015 and 2016 (tree age 49 years), every fifth living WRC tree (about 20/plot) was systematically selected for sampling. A total of 355 trees were sampled in 18 plots (). A Resistograph instrument model PD500 was used to drill at the base of each tree, through the centre to the other side. Each drill bit starting location was near the soil line and not started within the area of any open wound. Drills not passing through the centre of the tree were redone. The Resistograph records the relative resistance encountered by the micro-drill that is driven by an electric motor. Measurements are collected every 0.1 mm. Every 10th observation was used in the analyses since the sampling rate provides too much information per tree for statistical analysis. The presence of decay was measured from the inflection point between the vertical drop in density where decay started and zero reading line where decay persisted (i.e. the transition point). The effect of decay on density was sudden and easily recognizable, transitioning from a value greater than five to zero in about 1 mm radial distance or less. The Resistograph provides reliable estimates of wood density that correlate with X-ray densitometry by r2 > 0.8 (Rinn et al., Citation1996).
Table 1. Traits of sampled western redcedar by tree admixture and by stump treatment.
In 2016, each sampled WRC tree was measured for diameter at breast height (1.3 m) and inspected for sunken areas of bark mycelial fans in the cambium, which are indicative of aboveground A. ostoyae lesions. Some trees had open wounds but there was always enough intact bark to identify the associated fungus. All lesions were caused by A. ostoyae. All lesions visible above soil line were traced onto paper, brought back to the lab, and digitized to determine their area. The lesions had been contained within callus tissue, although a few lesions were still actively spreading ().
Fig. 1 (Colour online) Spreading A. ostoyae mycelial fans on 50-year-old WRC, arising from an older previously contained lesion below.

Site index (SI) at age 50 years was calculated in the 20 × 20 m plots based on the height of the 100 tallest trees ha−1 of each species. The software to determine this was the BC Ministry of Forests program Site Tools (BCFLNRO, Citation2018).
Statistical analyses
All analyses were completed using R version 3.4.0. Rank analyses were used where parametric model assumptions could not be assumed. Either a one-way ANOVA (Kruskal–Wallis test) or Spearman’s correlation was used. For the categorical response of incidence of butt decay in WRC trees, the mixed model Glimmer was used. The model took the form of:
where Yij is a binary variable denoting butt decay presence in tree i and plot j; a0 is the fixed intercept; area is the continuous effect of the lesion area under the bark in cm2 in tree i; treatment is the effect of removing the stumps in plot j; and plot is the random effect for plot j.
For the continuous response of relative wood density in trees, the Linear Mixed Effects (LME) model was used with a quadratic polynomial to track density for a given position inside the tree. The model took the form of:
where Yijk is the continuous response of relative wood density at per cent distance k through tree i in plot j; and pctdist and pctdist2 are the per cent distance (0–100%) and quadratic per cent distance k through the tree in tree i and plot j; mix is the categorical effect of admixture in plot j; treatment is the effect of stump removal or not on plot j; DBH0.5 is the square root of the DBH in tree i and plot j; tree is the random effect of tree i in plot j; plot is the random effect of plot location j; εijk is the residual error. To control for autocorrelation within tree, we tested several correlation structures using the Akaike information criteria (AIC), and found the exponential spatial autocorrelation (corExp) was the best fit. Correlation between observation is given by exp(-distance/ρ) where distance is the distance between the pctdist measures within tree, ρ was the range (2.63), and where distance and ρ > 0. To control for heteroscedasticity, we used an exponential variance function (varExp, −0.00391) of the per cent distance through the tree (pctdist).
Best linear unbiased predictors (BLUPs) were output from all models described above. A BLUP is an estimate of the random effect for an individual subject (plot or tree) and describes how that subject differs from the population average fixed effects. A positive BLUP means that subject has a response that is larger than the average response of all observations and the opposite is true for a negative BLUP.
Results
Incidence of decay in the butt of WRC trees
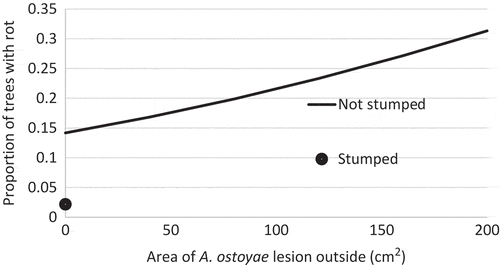
The incidence of butt decay in WRC trees was modelled (, Equation (1)) showing that the area (cm2) of A. ostoyae lesions under bark was an indicator of butt decay internally. The probability of butt decay increased by 0.005 times for each cm2 of A. ostoyae lesion (, P = 0.0003). An average lesion size of 250 cm2 (max 1000 cm2) amounts to a 1.25 times increase in butt decay incidence when there was an aboveground A. ostoyae lesion on the outside of the tree. The probability of butt decay in WRC trees also decreased where stumps were removed to 0.13 times that of locations where stumps were left in place (, P = 0.0010). This represents a reduction of butt decay in WRC from 18% on unstumped plots to 2% on stumped plots. The probability of butt decay inside WRC stems was not related to tree diameter, site index (SI), the percentage of dead and infected trees caused by A. ostoyae, or by admixture.
Table 2. Mixed model effects of area of A. ostoyae lesion and stump removal treatment on incidence of butt decay in WRC (Equation (1)).
The level of internal damage caused by decay in the stem butt
The proportion of butt decay across the diameter of the tree base was most positively correlated with the area of A. ostoyae lesions under the bark at the tree base (Spearman’s R (Sr) = 0.54, P < 2.2E-16). The next best positive correlation with decay damage was the proportion of dead and infected trees from A. ostoyae in the plots (Sr = 0.21, P < 4.3E-5). The tree diameter did not affect the proportion of decay inside the tree base (Sr = 0.05, P = 0.41), nor did SI in the plots (Sr = 0.01, P = 0.13). The tree species admixture was not related to the per cent butt decay inside the tree base (Kruskal–Wallis Chi-square (KW) = 2.1, P = 0.56). Stump removal reduced the level of decay damage inside the tree (KW = 21.1, P = 4.29E-6) from 3.5% to 0.3%. In all plots where the stumps were removed, there were no visible aboveground A. ostoyae lesions on the WRC trees, and there was almost no butt decay internally ().
Relative resistance – wood density
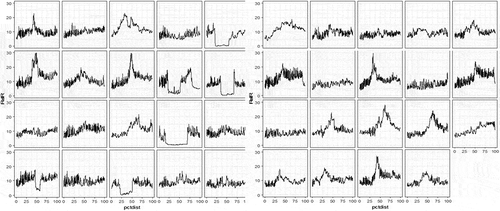
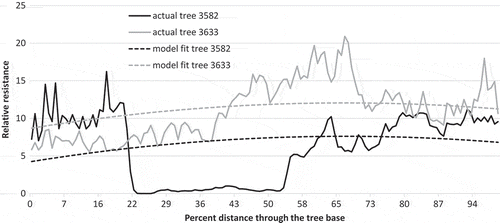
When graphs of relative resistance for each tree were examined (e.g. ), they showed little decay in the trees in plots where the stumps were removed. WRC trees with butt decay had sudden, sharp drops in internal wood density, while those without decay in the centres of tree stems had the highest wood densities. We removed all relative wood density values less than five units to eliminate observations with obvious butt decay within trees. We then tested for association between the average wood density by tree and the percentage of decay inside the base of tree stems. There was a weak positive effect of per cent decay associated with trees that had lower than average density (Sr = −0.19, P < 0.0004).
Fig. 3 Relative resistance response (RelR) indicating wood density across the diameter of WRC grown in admixture with pine. The horizontal axis is per cent distance through the tree base (pctdist) for WRC in two pine plots without stumps (right) and with stumps (left). Decay is indicated by sudden sustained drop in RelR.
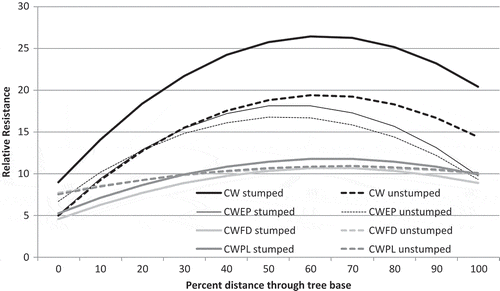
Mixed model analysis (Equation (2)) testing for tree admixture, stump treatment and tree DBH on wood density were all significant (). The largest effect was tree admixture, with monoculture WRC in both stump treatments having the highest wood densities, followed by WRC in birch, and WRC in Douglas-fir or pine having the lowest wood densities (, ). The shape of the relative wood density profile was concave down for all mixtures, except that the curvature was greatest for cedar, WRC in birch, then Douglas-fir and pine ( – B × D and C × D interaction, ). Density profiles of trees in plots without stumps also had higher curvature ( – B × E and C × E interaction, ), but this effect was lower than the effect of species mixture on curvature. Trees in plots with stumps removed had greater wood density overall by admixture, descending in order of WRC, then WRC in birch, then lowest in WRC in Douglas-fir and pine ( – D × E interaction, ). Large trees had low wood density regardless of stump treatment or admixture ( – DBH).
Table 3. Mixed-model effects of sample position within tree, tree admixture, stump treatment and tree size (Equation (2)) on wood density of WRC.
The random effects (Equation (1)) showed similar variation at the plot and tree levels ( – plot and tree intercepts). A tree level intercept partly accounted for butt decay in the tree centre ( – random effects-tree level, ), which lowered the average tree density. An attempt was made to account for additional variation by examination of the BLUPs against other variables, but no relationships were found.
Fig. 5 Relative resistance of actual data and model fit for a tree with butt rot (3582) and one without butt rot (3633) in a WRC in pine plot.
We examined the relative density variation in Resistograph readings in a 10% section of the tree base (20–30% distance through the tree base relative to the outside) where butt decay did not occur. Differences between earlywood and latewood densities, and higher values for the earlywood alone in most cases were evident between the admixtures (). The difference or change between consecutive relative resistance readings was then calculated for each tree in the 10% section. The highest variation occurred in monoculture WRC, followed by WRC in birch and then WRC in pine or Douglas-fir admixtures. The variance of the differences in descending order were: WRC (65), WRC in birch (45), WRC in WRC in pine (12) and WRC in Douglas-fir (10). The density differences between consecutive readings were significantly different among the admixtures, lower for trees with butt decay and for trees in plots with stumps remaining (KW, P < 0.006), but not related to diameter (Sr = 0.0001, P = 0.9218).
Discussion
The results from the current study located in the BC interior show that ~18% of the planted WRC trees had butt decay in plots where stumps were left in place, versus 1.7% butt decay in WRC trees where stumps were removed prior to planting. Data from BC coastal sites agree with this incidence of WRC butt decay at ~18% by age 50 years and increasing to 35% by age 100, with interior sites having 80% incidence by age 100, and both areas near 100% by age 300–400 years (Buckland Citation1946). This agrees with incidence of A. ostoyae in interior WRC being about 22% in young sites and rising to about 75% by age 100 (Morrison et al., Citation2000, Citation2001). Clearly, a large increase in butt decay incidence occurs between ages 50 and 100 years in WRC trees in interior sites, but decay incidence is delayed for a time in trees in coastal sites. Unfortunately, admixing a second tree species resistant to A. ostoyae to the WRC did not reduce butt decay incidence in WRC. This means that the resistant tree species were not able to reduce spread of the root disease or butt rot compared to when stumps were removed.
Aboveground stem lesion area and the number of dead and infected WRC trees caused by A. ostoyae in plots were positively related to the decay level inside the trees. Armillaria ostoyae lesions did not occur on trees in plots where stumps had been removed, and the percentage of dead and infected trees caused by A. ostoyae was lower in plots with stumps removed regardless of admixture (Morrison et al., Citation2014). Stumps and dead and infected trees represent a food base for pathogenic fungi, and inoculum size is known to affect lesion size (Cruickshank et al., Citation1997), which describes the inoculum potential of the fungus (Garrett, Citation1970). Therefore, lesion presence and size, and the number of dead and infected trees caused by A. ostoyae, are directly affected by stump removal. However, the possibility that stump removal may also have reduced other antagonistic fungi or increased beneficial fungi that affect decay organisms cannot be ruled out.
A number of factors could relate to the level of WRC heartwood decay damage found, including toxic compounds, nutrition, mechanical barriers, hydrophobicity (Taylor et al., Citation2002) and differences in host susceptibility to different fungi. Other studies in BC interior sites have linked increased incidence of WRC butt decay to lower elevations, which would equate to more productive sites, larger tree size (Robison, Citation2000) and more A. ostoyae. There was no effect of tree diameter on the level of WRC butt decay. This differs from other results (e.g. Robison Citation2000) and may be due to a narrower range of tree diameters occurring in planted versus naturally regenerated WRC. Tree age is related to tree diameter, and age should be considered when determining the level of decay damage (Nault, Citation1988; Robison, Citation2000). The butt rot incidence and damage in the current study site is expected to increase with age. In wood block tests using poles of several coniferous species, wood density, tree age, and pole size or site location were not related to interior decay damage level (Englerth & Scheffer, Citation1954); although, the current study found a weak association between decay level and wood density. However, more dense wood would still be stronger given the same percentage decay (Southam & Ehrlich, Citation1943) and presumably in-service longer, a trait not often recognized in WRC.
The reduction in the number of stumps that carry pathogenic fungi capable of causing WRC butt decay other than A. ostoyae, could explain some of the differences in both incidence and damage between the two stump treatments. Although there is good information on the organisms causing WRC butt decay (Sturrock et al., Citation2017), there is little information on the type of inoculum for spread of these fungi, or how they penetrate tree roots. For example, MacDonald (Citation1974) failed to isolate a decay fungus using WRC heartwood blocks buried next to a WRC tree infected with the same fungus. This suggests that WRC butt decay fungi could include root epiphytes that are pre-positioned to exploit and colonize root material, or that there is a succession of organisms needed to detoxify the wood initially. It is not clear whether spores from fungi causing butt decay can directly initiate root infections, but they could colonize stump tops and then, presumably, roots. Phellinus weirii mycelium, one of the WRC butt decay organisms, penetrated WRC roots from a source of colonized wood inoculum; further, infections entered via 1–2-year-old roots attached to the larger root and then callused over, making them difficult to detect (Sturrock & Pellow, Citation2013). Other known WRC decay fungi, such as Perenniporia subacida, a common root epiphyte, can spread at root contact with a colonized stump, and move along the root of a Chamaecyparis obtusa (Siebold and Zucc.) Endl. tree, and then penetrate the main root through smaller roots attached to it to cause butt decay (Tabata et al., Citation2002). Therefore, there is a possibility that dead trees, and especially stumps, provide a food base for these butt decay fungi, and the mycelium can penetrate at least small WRC roots. WRC root architecture is somewhat different from the other conifers it associates with: WRC has a higher number of thin absorbing roots arising from large roots near the tree base (Eis, Citation1974). An infection caught via small rootlets would take longer to cause butt decay than lesions formed directly at the tree butt by A. ostoyae or by wounding.
Armillaria ostoyae is not considered to be a major cause of WRC decay (Sturrock et al., Citation2017). However, WRC in interior BC is frequently infected by A. ostoyae appearing aboveground as cryptic, dead, vertical strips of bark. These lesions begin below ground and would be susceptible to colonization by soil fungi. WRC has some of the largest lesions caused by fungal infection, and it has the highest survival after infection as compared to other conifers it grows with (Cleary et al., Citation2012). Greater butt decay damage in interior WRC stems compared to coastal WRC stems does occur (Buckland, Citation1946), probably because A. ostoyae is known to be more aggressive there for all tree species (Morrison et al., Citation1992). Differences in WRC physiology affecting root penetration by fungi and heartwood colonization may also account for the differences between the two regions. The role that A. ostoyae plays in coastal ecosystems is uncertain, but the fungus is known to frequently infect coastal WRC (Klinka & Brisco, Citation2009). This collective information suggests that A. ostoyae, among other agents causing wounds, might be predisposing WRC to entry by other butt decay organisms that would affect both incidence and decay amount.
The current study found a negative relationship between tree diameter and wood density, and lower density at the outer edges of tree stems. This is a common pattern for WRC (Wellwood & Jurazs, Citation1968). Wood density was also greater in plots with stumps removed, presumably partly because of the lower incidence of butt decay that occurred there. After controlling for diameter, we found the highest wood density in monoculture WRC. On the study site, in 2012, WRC admixture in birch, pine and Douglas-fir plots were found to be, on average, 0.50 and 0.87 times as tall as its other species pair (Morrison et al., Citation2014). Therefore, light competition may account for some of the differences in WRC wood density between monoculture and admixture; however, this may also be related to differences in decay between the admixtures themselves.
It is possible that overtopping of WRC by pine or Douglas-fir, both fast-growing drought-tolerant species, reduced the soil moisture in plots with stumps left, and limited late season latewood formation in WRC. For example, WRC co-dominants growing with western hemlock or Douglas-fir had greater frequency of top dieback compared to WR dominants alone (60% vs. 10% respectively) (Seebacher, Citation2007). If so, then ignoring the cedar mixture type and stand structure would confound studies that only consider precipitation as a factor. Drought is recognized as a limiting factor for WRC, especially in drier sites where pine and Douglas-fir grow (Klinka & Brisco, Citation2009). In the current study, most trees with butt decay also had root disease, which suggests root damage could limit water uptake and photosynthesis. Warmer and drier summers had the greatest impact on coastal WRC growth (Laroque & Smith, Citation2005; Harrington & Gould, Citation2010), and interior climates are more extreme in temperature. The proportion of latewood is the main factor driving average wood density in most conifers (Wimmer, Citation1995). Unfortunately, there is limited information on annual ring density or latewood formation for WRC; therefore, it is difficult to understand how climate or species mixtures might affect wood density or how this may relate to drought tolerance. Drought tolerance is linked to wood density in other conifers (Martinez-Meier et al., Citation2009), and may be an additional important trait not well recognized in WRC.
Wood density can also be affected by extractives within the wood. Extractives are secondary metabolites preferentially deposited in the heartwood conferring durability to wood products (Barton & MacDonald, Citation1971). WRC has high content of these extractives compared to other conifers, with about twice that of western hemlock or Douglas-fir (Gardner, Citation1963). For example, extractives in Sitka spruce accounted for up to 14% of the wood density (Singleton et al., Citation2003). Extractive content increases from the pith towards the sapwood boundary, then declines in sapwood (Nault, Citation1988). Higher sapwood carbohydrate and lipid extract correlated with higher heartwood extractive (Taylor et al., Citation2006), suggesting that photosynthate limitation by admixture could affect extractive content.
Based on study results from one site in the southern interior of BC, this is a first report demonstrating that: (i) stump removal affects incidence and damage of butt decay in WRC; and (ii) planting of WRC admixtures affects WRC wood density. The extensive distribution of Armillaria root disease and WRC butt decay incidence and damage cautiously allow these results to apply to larger areas where WRC grows. However, further study is required to understand the connections between wood density, extractives and admixtures.
Acknowledgements
We thank past generations of Canadian Forest Service forest pathologists with the foresight to establish the Skimikin experimental site and maintain it for over 50 years. We also thank Dominique Lejour for field sampling and data processing.
Additional information
Funding
References
- Allikmäe E, Laarmann D, Korjus H. 2017. Vitality assessment of visually healthy trees in Estonia. Forests. 8:223.
- Antos JA, Filipescu CN, Negrave RW. 2016. Ecology of western redcedar (Thuja plicata): implication for management of a high-value multiple-use resource. For Ecol Manag. 375:211–222.
- Barton GM, MacDonald BF. 1971. The chemistry and utilization of western redcedar. Review. Ottawa (ON): Department of Fisheries and Forestry. Publ 1023.
- BCFLNRO 2018. Site tools. https://www2.gov.bc.ca/gov/content/industry/forestry/managing-our-forest-resources/forest-inventory/growth-and-yield-modelling/site-index-tools-sitetools.
- Buckland DC. 1946. Investigations of decay in western red cedar in British Columbia. Can J For Res. 24:158–181.
- Cleary MR, Aripova N, Morrison DJ, Thomsen IM, Sturrock RN, Vasaitis R, Gaitnieks T, Stenlid J. 2013. Stump removal to control root disease in Canada and Scandinavia: a synthesis of results from long-term trials. For Ecol Manag. 290:5–14.
- Cleary MR, van der Kamp BJ, Morrison DJ. 2012. Effects of wounding and fungal infection with Armillaria ostoyae in three confer species. II. Host response to the pathogen. For Pathol. 42:109–123.
- Cruickshank MG, Morrison DJ, Punja ZK. 1997. Incidence of Armillaria species in precommercial thinning stumps and spread of Armillaria ostoyae to adjacent Douglas-fir trees. Can J For Res. 27:481–490.
- Eis S. 1974. Root system morphology on western hemlock, western red cedar, and Douglas-fir. Can J For Res. 4:28–38.
- Englerth GH, Scheffer TC. 1954. Tests of decay resistance of four western pole species. Rep No 2006. Madison (WI): USDA Forest Service, Forest Products Laboratory.
- Gardner JAF. 1963. The chemistry and utilization of western red cedar. Vancouver (BC): Department of Forestry Canada, Forest Products Research Branch. Publication No 1023.
- Garrett SD. 1970. Pathogenic root-infecting Fungi. Cambridge (UK): University Press.
- Gregory C, McBeath A, Filipescu C. 2018. An economic assessment of the western redcedar industry in British Columbia. British Columbia: Natural Resources Canada, Canadian Forest Service, Canadian Wood Fibre Centre, Victoria; 38. Information report FI-X-017.
- Harrington CA, Gould PJ. 2010. Growth of western redcedar and yellow-cedar. In: Harrington CA, editor. A tale of two cedars: international symposium on western redcedar and yellow-cedar, May 24-28, Victoria, BC. Portland (OR): USDA Forest Service; p. 97–102. report no. PNW-GTR-828.
- Hebda RJ, Mathewes RW. 1984. Holocene history of cedar and Native Indian cultures of the North American Pacific coast. Science. 225:711–713.
- Klinka K, Brisco D. 2009. Silvics and silviculture of coastal western redcedar: a literature review. Victoria (BC): BC Ministry of Forests Range, For Sci Prog Sect. Report Series 11.
- Koenigs JW. 1969. Root rot and chlorosis of released thinned western red-cedar. J For. 67:312–315.
- Laroque CP, Smith DJ. 2005. Predicted short-term radial growth changes of trees based on past climate on Vancouver Island, British Columbia. Dendrochronologia. 22:163–168.
- Lloyd D, Angrove K, Hope G, Thompson C. 1990. A guide to site identification and interpretation for the Kamloops forest region. Victoria (BC): Research Branch, BC Ministry of Forests. Land Management Handbook 23.
- MacDonald AJ. 1974. The entry of staining fungi into the heartwood of western red cedar [B.S.F. thesis]. Vancouver (BC): University of BC.
- Martinez-Meier A, Sanchez L, Guillermina Dalla-Salda G, Gallo L, Pastorino M, Rozenberg P. 2009. Ring density record of phenotypic plasticity and adaptation to drought in Douglas-fir. For Ecol Manag. 258:860–867.
- Minore D. 1990. Silvics of North America: 1. Conifers. In: Burns RM, Honkala BH, editors. Thuja plicata Donn ex D. Don – western redcedar. Vol. 1. Washington (DC): USDA Forest Service; p. 590–600. Agriculture Handbook 654.
- Morrison DJ, Cruickshank MG, Lalumière A. 2014. Control of laminated and Armillaria root diseases by stump removal and tree species mixtures: amount and cause of mortality and impact on yield after 40 years. For Ecol Manag. 319:75–98.
- Morrison DJ, Merler H, Norris D. 1992. Detection, recognition and management of Armillaria and Phellinus root diseases in the southern interior of British Columbia. Victoria (BC): Canada-British Columbia Partnership Agreement on Forest Resource Development: FRDA II. FRDA Report 179.
- Morrison DJ, Pellow K, Norris DJ, Nemec AFL. 2000. Visible versus actual incidence of Armillaria root disease in juvenile coniferous stands in the southern interior of British Columbia. Can J For Res. 30:405–414.
- Morrison DJ, Pellow KW, Nemec AFL, Norris DJ, Semenoff P. 2001. Effects of selective cutting on the epidemiology of Armillaria root disease in the southern interior of British Columbia. Can J For Res. 31:59–70.
- Nault J. 1988. Radial distribution of thujaplicins in old growth and second growth western red cedar (Thuja plicata Donn). Wood Sci Technol. 22:73–80.
- Random lengths. 2016. 2015 yearbook: forest product market prices and statistics. Eugene (OR): Random Lengths Publications Inc.
- Rinn F, Schweingruber F-H, Schär E. 1996. Resistograph and X-ray density charts of wood comparative evaluation of drill resistance profiles and X-ray density charts of different wood species. Holzforschung. 50:303–311.
- Robison Z. 2000. The incidence of decay in western red cedar between two biogeoclimatic regions [BSc thesis]. Vancouver (BC): University of BC.
- Seebacher T. 2007. Western redcedar dieback: possible links to climate change and implication for forest management on Vancouver Island, BC [MSc thesis]. Vancouver (BC): University of BC.
- Singleton R, DeBell DS, Gartner BL. 2003. Effect of extraction on wood density of western hemlock (Tsuga heterophylla (Raf.)Sarg.). Wood Fiber Sci. 35:363–369.
- Smith JHG. 1980. Influences of spacing on radial growth and percentage latewood of Douglas-fir, western hemlock, and western redcedar. Can J For Res. 10:169–175.
- Southam CM, Ehrlich J. 1943. Decay resistance and physical characteristics of wood. J For. 41:666–673.
- Stirling R, Sturrock RN, Braybrooks A. 2017. Fungal decay of western redcedar products – a review. Int Biodeterior Biodegrad. 125:105–115.
- Sturrock RN. 2000. Management of root diseases by stumping and push-falling. Victoria (BC): Canadian Forest Service. Technology Transfer Note 16.
- Sturrock RN, Braybrooks AV, Reece PF. 2017. Decay of living western redcedar: a literature review. Victoria (BC): Natural Resources Canada, CFS, Canadian Wood Fibre Centre. Inf. Report FI-X-014.
- Sturrock RN, Pellow KW. 2013. Infection of western redcedar roots by the fungal pathogen Phellinus weirii – preliminary results. Can J Plant Pathol. 35:95.
- Tabata M, Kato T, Ohkubo M, Yasuhisa A, Shuichiro Y. 2002. Butt rot of Chamaecyparis obtusa (Sieb. et Zucc.) Endlicher trees caused by Perenniporia subacida in Shikoku district, Japan: Pathogen, distribution of damaged trees in the stand, and soil investigation. J For Res. 7:105–112.
- Taylor AM, Gartner BL, Morrell JJ. 2002. Heartwood formation and natural durability – a review. Wood Fiber Sci. 34:587–611.
- Taylor AM, Gartner BL, Morrell JJ. 2006. Western redcedar extractives: is there a role for the silviculturist? For Prod J. 56:58–63.
- Wellwood RW, Jurazs PE. 1968. Variation in sapwood thickness, specific gravity and tracheid length in western red cedar. For Prod J. 18:37–46.
- Wimmer R. 1995. Intra-annual ring characteristics and their implications for modeling softwood density. Wood Fiber Sci. 27:413–420.