Abstract
Diseases caused by Stemphylium vesicarium are an increasing problem in the major asparagus and onion production regions of eastern and central Canada. Replicated and repeated controlled environment and field trials were conducted in Ontario to assess the disease reaction of asparagus and onion cultivars to determine if isolates from one crop can cross-infect other hosts, to assess the variation of disease symptoms among cultivars of onion and of asparagus, and to assess the ability of the pathogen to survive in a non-host species. All of the varieties of asparagus and onion assessed were susceptible to infection by S. vesicarium. There were small differences in susceptibility among varieties and in aggressiveness among isolates. These differences were not strongly associated with host crop. Onion cultivars that developed higher numbers of lesions often had lower levels of leaf dieback and vice versa. This indicated that resistance to infection was independent of susceptibility to development of dieback symptoms. In asparagus, infection on spears was not consistently correlated with severity on ferns. All isolates from asparagus and onion produced typical symptoms on wounded, but not unwounded, pear fruit. Finally, the pathogen was shown to colonize and sporulate on necrotic leaves of autumn rye, which is often used as a cover crop in asparagus fields. Stemphylium vesicarium can cross-infect asparagus and onion.
Résumé
Les maladies causées par Stemphylium vesicarium constituent un problème croissant dans les principales régions productrices d’asperges et d’oignons du Canada. Des essais en environnement contrôlé et sur le terrain ont été menés en Ontario afin d’évaluer la réaction des cultivars d’asperges et d’oignons à la maladie pour déterminer si les isolats d’une culture peuvent infecter d’autres hôtes, la variation des symptômes de la maladie entre les cultivars d’oignons et d’asperges, et la capacité de l’agent pathogène à survivre chez une espèce non hôte. Toutes les variétés d’asperges et d’oignons évaluées étaient sensibles à l’infection. Il y avait de petites différences au niveau de la sensibilité entre les variétés et de l’agressivité entre les isolats. Ces différences n’étaient pas fortement associées à la culture hôte. Les cultivars d’oignons qui ont développé un plus grand nombre de lésions présentaient souvent des niveaux inférieurs de dépérissement des feuilles et vice versa. Cela indique que la résistance à l’infection était indépendante de la susceptibilité à l’apparition de symptômes de dépérissement. Chez les asperges, l’infection sur les tiges n’était pas toujours corrélée à la sévérité des symptômes du feuillage. Tous les isolats provenant des cultures d’asperges et d’oignons ont provoqué des symptômes typiques chez les fruits de poirier blessés, mais pas chez ceux de poiriers non blessés. En outre, l’agent pathogène colonisait et sporulait sur les feuilles nécrosées du seigle d’automne, souvent utilisé comme culture de couverture dans les champs d’asperges. Stemphylium vesicarium peut provoquer une infection croisée entre l’asperge et l’oignon.
Introduction
Stemphylium vesicarium (Wallr.) E.G. Simmons (teleomorph: Pleospora herbarum [Pers.] Rabenh, syn. P. allii [Rabenh.] Ces. & De Not.) is the most important foliar pathogen of asparagus and onion in Ontario. Symptoms on these hosts start out as small elliptical lesions on the infected tissue. The lesions coalesce, resulting in elongated lesions in asparagus and leaf dieback in onion (Rao & Pavgi, Citation1975; Lacy, Citation1982). The pathogen was identified on asparagus for the first time in Ontario in the 1990s but was not reported on onion until 2008 (Paibomesai et al., Citation2012), although it had been present on asparagus in Michigan since the 1980s (Lacy, Citation1982). The long interval between reports on asparagus and onion is not surprising since the production of these crops is geographically separated: asparagus in south-western Ontario and onion in the Holland Marsh.
Stemphylium vesicarium has a diverse host range that includes cultivated crops such as asparagus (Lacy, Citation1982), Allium spp. (Rao & Pavgi, Citation1973, Citation1975; Miller et al., Citation1978; Basallote et al., Citation1993), parsley (Koike et al., Citation2013) and pear (Vilardell, Citation1988). The disease is known as Stemphylium leaf spot or purple spot on asparagus, Stemphylium leaf blight on onion, and brown spot on pear. Other Stemphylium spp. have also been identified as pathogens in asparagus (Suzui, Citation1973; Falloon et al., Citation1987; Leuprecht, Citation1990) and garlic (Zheng et al., Citation2009). Brown spot of pear has not been identified in North America, but is widely distributed in Europe (Llorente & Montesinos, Citation2002).
Despite similar symptomatology on onion and asparagus, it is unclear whether S. vesicarium cross-infects between these host crops. In one report, seedlings of asparagus were inoculated with isolates of Stemphylium spp. (1 × 105 conidia mL−1, sprayed until runoff) from asparagus and onion, but only isolates from asparagus produced symptoms on asparagus (Falloon et al., Citation1987). In another report, symptoms developed on onion leaves inoculated with isolates of S. vesicarium from both onion and asparagus (6.3 × 103 conidia mL−1 and 8.8 × 104 conidia mL−1, respectively, sprayed until runoff) (Shishkoff & Lorbeer, Citation1989). In pear, only 1 of 52 isolates from onion or asparagus (inoculated with a 10-µL drop of 2.5 × 104 conidia mL−1 or 1 × 105 conidia mL−1 for each leaf or fruit, respectively) produced symptoms on detached pear leaves, and no isolates produced symptoms on pear fruit (Köhl et al., Citation2009). In addition, S. vesicarium was recently identified as a pathogen of parsley, and the isolates collected from parsley caused symptoms on other Apiaceae crops (inoculated with 1.5 × 105 conidia mL−1, sprayed until runoff) and artificially wounded pears, but not Allium spp. and unwounded pears (Koike et al., Citation2013).
Asparagus is a perennial vegetable crop. Aerial shoots (spears) grow up from the below-ground crown each spring and are harvested before the branches and modified leaves (cladophylls) unfold. For several weeks after the spears are harvested, the shoots are allowed to grow and fully develop into ferns. In asparagus, S. vesicarium infects the spears in spring, reducing the quality of the spears and resulting in premature defoliation, which can greatly reduce harvests in subsequent years (Hausbeck et al., Citation1999). The primary inoculum is airborne ascospores from pseudothecia produced on crop residue from the previous year (Falloon, Citation1982; Evans & Stephens, Citation1984; Falloon et al., Citation1987; Hausbeck et al., Citation1999). The aetiology of S. vesicarium on onion is not as well understood.
None of the asparagus and onion cultivars commonly grown in Ontario are known to be resistant to S. vesicarium and fungicides do not provide consistent disease reduction (Tayviah, Citation2017). Since 2000, asparagus growers have switched from producing the ‘Jersey’ cultivars to ‘Guelph Millennium’, a cultivar with high yield and superior winter hardiness (Landry & Wolyn, Citation2011). However, ‘Guelph Millennium’ appears to be as susceptible as the other common lines.
Generally, Stemphylium spp. are weak pathogens that colonize senesced plant tissue (Hudson, Citation1971). Wounding, however, even by erosion of the wax layer by wind-blown soil, can increase disease incidence in both asparagus (Lacy, Citation1982; Johnson & Lunden, Citation1986) and onion (Shishkoff & Lorbeer, Citation1989). In pear orchards, S. vesicarium has been isolated from the dead leaves of weeds and grass species (Rossi et al., Citation2005, Citation2008; Köhl et al., Citation2013), and isolates of S. vesicarium collected from seven orchard grass species were pathogenic on pear leaves (Rossi et al., Citation2005). Asparagus growers plant autumn rye (Secale cereale) in the autumn and kill it with herbicide in spring to provide a dense plant cover to minimize wind erosion during harvest (Brainard, Citation2012). Although rye is considered a non-host, S. vesicarium may survive on the dead plant tissue as a saprophyte.
Little is known about the prevalence of S. vesicarium in Ontario, despite its impact on asparagus and onion production and the lack of effective control measures. The objectives of this research were to assess the pathogenicity and host specificity of S. vesicarium isolates on onion, asparagus, pear and rye, and to assess the disease reaction of commonly grown asparagus and onion cultivars to S. vesicarium in Ontario.
Materials and methods
Isolate collection and maintenance
From 2012 to 2016, 38 fields across five counties in Ontario (ON) and one county in Nova Scotia (NS) were scouted for symptoms caused by S. vesicarium in asparagus and onion. Symptomatic onion leaves and asparagus ferns were collected, surface sterilized, and the pathogen isolated onto potato dextrose agar (PDA). The resulting fungal colonies were purified using hyphal tip culture and single-spore isolation. In addition, five isolates of S. vesicarium collected from commercial onion fields in Kings County, NS, were included (provided by P.D. Hildebrand, N.S. Dept. of Agriculture). The isolates were identified based on morphological characteristics, where conidia are olive-brown, oval to ovoid, and are borne on conidiophores that are pale to brown with dark edges and bands. The conidia have 1–5 transverse septa and are constricted at 1–3 transverse septa (Simmons, Citation1969). These identifications were confirmed using PCR as described below. The isolates were maintained in long-term storage on half strength PDA and water agar slants at 5 ± 1°C. For each experiment, isolates were transferred onto PDA from long-term storage and incubated for 7 days at 20 ± 2°C. The identifier for each isolate indicated the province where it was collected (O – Ontario and N – Nova Scotia) and the host plant (A – asparagus or O – onion) ().
Table 1. Isolates of Stemphylium vesicarium, collected from asparagus and onion fields in Canada, used to assess pathogenicity on onion, asparagus, pear and rye. Checkmarks indicate which isolates were used in DNA sequencing and for each host assessment.
DNA extraction, PCR amplification and identification
DNA of eight S. vesicarium isolates (OA20, OO27, OO31, NO35, NO36, OA46, OA48 and NA51) was extracted from the mycelia as follows. The isolates were cultured on PDA for 3 days at room temperature (20°C), then plugs with actively growing hyphal tips were transferred into a flask containing 100 mL potato dextrose broth (Difco, Becton Dickinson and Co., Sparks, MO). The flasks were incubated at room temperature on an orbital shaker (ThermoFisher Inc., Marietta, OH) at 90 rpm, and mycelial mats were collected 14 days later by filtering through several layers of sterile cheesecloth. The mats were transferred to sterile microcentrifuge tubes and flash frozen in liquid nitrogen. DNA was extracted using a DNeasy Plant Kit (Qiagen, Toronto, ON) according to the manufacturer’s protocol and stored at −80°C.
Species-specific primers were selected based on previous literature to amplify regions of the internal transcribed spacer (ITS), glyceraldehyde-3-phosphate dehydrogenase (GPD), translational elongation factor EF-1 alpha (TEf-A), and cytochrome b (cytB) genes of S. vesicarium, based on GenBank accessions. The primers used for each of the regions, respectively, were ITS1/ITS4 (White et al., Citation1990), gpd1/gpd2 (Berbee et al., Citation1999), EF446f/EF1473r (Inderbitzin et al., Citation2005) and KES1999/KES2000 (Graf et al., Citation2016). The ITS, GPD and TEf-A sequences were able to separate S. vesicarium (syn. P. herbarum) into a clade apart from other closely related Stemphylium species, but were unable to differentiate Pleospora alfalfae, P. sedicola and P. tomatonis from S. vesicarium (Inderbitzin et al., Citation2009). However, a recent study has suggested that these species be synonymized (Woudenberg et al., Citation2017). The cytB sequence described by Graf et al. (Citation2016) was included in this experiment to differentiate S. vesicarium from S. botryosum, since the two pathogens have similar host ranges and distributions.
The PCR reaction was performed in a total volume of 50 μL containing 1× PCR buffer (50 mM Tris-HCl, pH 8.5); 2.0 mM MgSO4; 0.2 mM dNTP; 0.2 μM of each primer separately; 0.04 U Tag DNA polymerase (Biobasic, Scarborough, ON); and 4 μL DNA template. Amplifications were performed in a Mastercycler pro384 thermal cycler (Eppendorf Canada, Mississauga, ON). The PCR program consisted of an initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 59°C for 30 s, and extension at 72°C for 60 s, followed by a final extension at 72°C for 7 min. Amplicons were resolved by horizontal gel electrophoresis in 1.0% agarose gels in 0.5× (Tris-borate-EDTA, TBE) buffer at 100 V cm−1 for 75 min. Gels were pre-stained with ethidium bromide (0.5 μg mL−1), then digitally visualized and photographed on a Gel Doc XR Imaging System (BioRad, Hercules, CA).
The PCR products showing a single band of the expected size were purified by removing the excess dNTPs, primers and reagents by using RapidTip ‘clean-up’ pipette tips (Diffinity Genomics, West Henrietta, NY) and the Mag-Bind SeqDTR kit (Omega Bio-Tek, Norcorss, GA). Sanger sequencing was completed using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) and 3500 Series Data Collection Software 2 (Applied Biosystems, Foster City, CA). The resulting sequences were compared to previously reported S. vesicarium gene sequences in the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) using BLASTN 2.8.0+ (Zhang et al., Citation2000; Morgulis et al., Citation2008). The forward and reverse sequences were assembled into contigs using the sequence assembly software CAP3 (http://doua.prabi.fr/software/cap3) using default settings.
Inoculum preparation for pathogenicity bioassays
Ten isolates were selected from long-term storage for pathogenicity bioassays. Subsets of the isolates were evaluated for pathogenicity on asparagus spears (OA46, OA48, OA56, NA51, NA52, OO27, OO55 and NO36), onion leaves (OA46, OO27, OO54, OO55 and NO35), pear fruit (OA48, OA20, OA56, OO31, NO35 and NO36), and autumn rye leaves (OA46, OA48 and OO55).
Agar plugs were transferred from stock plates onto V8 agar (160 mL unfiltered V8 juice, 16 g agar, 30 mM CaCO3 and 840 mL deionized water) and incubated for 7 days under constant fluorescent lights at 20 ± 2°C. Ten drops of sterile water were placed around the outer edge of the culture, and a sterile glass rod was used to gently press the mycelia against the media. The cultures were then placed under UV light at 20 ± 2°C for 12 h, followed by darkness for 12 h, to induce production of conidia. Inoculum was prepared by flooding sporulating cultures with sterile water and dislodging the conidia with a sterile glass rod or microscope slide. The conidial suspension was filtered through sterile cheesecloth to remove mycelia fragments and a drop of Tween 20 surfactant (J.T Baker Inc., Philipsburg, NJ) was added to every 10 mL of conidial suspension. The concentration of conidia was estimated using a hemocytometer and adjusted by dilution as required.
Asparagus spear bioassays
Two similar bioassays were conducted to determine if isolates of S. vesicarium collected from onion and asparagus were pathogenic to wounded spears of commercial asparagus cultivars and breeding lines from the asparagus breeding programme at the University of Guelph. Both assays included the varieties ‘Guelph Millennium’, ‘Guelph Equinox’ and UG010. The first assay also included ‘Guelph Eclipse’ and UG009, while a second also included ‘Jersey Giant’, ‘Tiessen’ and UG023.
In the first assay, spears were collected from trials at the University of Guelph Simcoe Research Station. Spears for the second assay were collected from cultivar trials at Simcoe or Plattsville, ON (described below). Spears were stored for a maximum of 2 weeks at 2 ± 1°C. One day prior to inoculation, spears were returned to room temperature, surface-disinfected in 0.5% sodium hypochlorite for 10 min, and rinsed with sterile water.
Five isolates from asparagus (OA46, OA48, OA56, NA51 and NA52) and three from onion (OO27, OO55 and NO36) were selected for the study. Each isolate was inoculated onto each asparagus variety. Prior to inoculation, each spear was wounded at five locations on the spear using a sterile needle to a depth of 1–2 mm, except for the non-wounded, non-inoculated control. Sterile water was used as the negative control. The first wound was located 2.5 cm from the spear tip and wounds were spaced every 2.5 cm down the length of the spear. A 10-µL drop of conidial suspension (1 x 105 conidia mL−1) was pipetted onto each wound site. Spears were placed onto plastic racks in sterile humidity chambers, and sterile water was added to each chamber to maintain high humidity. The chambers were sealed and maintained under constant fluorescent lights at 20 ± 2°C.
Lesion development on the spears was evaluated on a 0 to 5 scale, where 0 = no lesion, 1 = 1 mm lesion length, 2 = 2 mm lesion, 3 = 3 mm, 4 = 3.1–7 mm and 5 = 7.1–10 mm lesion length. The area under the lesion growth curve was calculated (Shaner & Finney, Citation1977) based on disease scores to estimate the cumulative disease severity throughout the assessment period. However, this assessment showed a similar pattern of response to DSI, so only the DSI is presented. Both assays were repeated. At the conclusion of each repetition, 10% of the symptomatic spears were collected to re-isolate and verify the identity of the pathogen based on morphology in culture, as described previously.
Onion bioassay
A bioassay was conducted to determine if isolates of S. vesicarium collected from onion and asparagus were pathogenic on onion leaves in a controlled environment. Twelve onion cultivars were inoculated with five isolates of S. vesicarium, one from asparagus (OA46) and four from onion (OO27, OO54, OO55 and NO35), plus a water-only control. Each experimental unit consisted of two onion plants. The study was arranged in a completely randomized design with four replicates.
Seed of 12 onion cultivars were planted (one per cell) into 12-cell plug trays filled with soil-less media (Growers-mix, ABS Greenworld Inc., Mount Elgin, ON), and placed in a greenhouse at the Muck Crops Research Station. At 30 days after planting (DAP), the leaves were trimmed according to standard commercial practice to produce short, dense transplants with strong root growth. The seedlings were transplanted at 44 DAP into 1.5-litre pots filled with soil-less media (Sunshine ProMix BX, Sun Gro Horticulture Canada Ltd, Agawam, MA).
At the four-leaf stage (74 DAP), each onion leaf was rubbed gently with cheesecloth to remove the cuticular wax, to simulate the weathering that occurs in the field. A conidial suspension (2 × 106 conidia mL−1) was sprayed until run-off on each plant using an Optimus® hand atomizer (Home Depot, Canada). Sterile water was applied to the non-treated control. After inoculation, the plants were placed in moist chambers for 72 h to maintain high humidity and enhance conidial germination. The plants were misted every 12 h to maintain the humidity and leaf wetness. The temperature and relative humidity were recorded using an Enviro-Meter™ (Fisher Scientific, TX). The plants were removed and kept under controlled environment conditions (60–70% RH, 18–23°C and 16 h photoperiod) and observed at regular intervals for symptom development. Isolations from the margins of lesions on diseased leaves were used to confirm pathogen identity, as described above.
The number of lesions per leaf and leaf dieback (% of leaf length per leaf with symptoms) were assessed 42–45 days post inoculation. The number of lesions per plant was assessed by counting lesions on the second and third youngest fully developed leaves 9–14 days post inoculation. On the same leaves, leaf dieback was assessed by measuring total leaf length and length of necrotic section and calculating the mean proportion of the leaf length with visible symptoms.
Pear fruit bioassay
A bioassay was conducted to assess the pathogenicity of isolates of S. vesicarium from asparagus and onion on pear cultivar ‘Hargold’. Pear fruits were harvested from a commercial orchard located in Ontario that had not received a fungicide application in season. The fruit were stored for a maximum of 2 weeks at 2 ± 1°C, until required for the assay. One day prior to inoculation, the fruit were returned to room temperature, surface-disinfected with 0.5% sodium hypochlorite for 10 min, and rinsed with sterile water.
It proved difficult to produce consistent spore suspensions from all of the isolates of S. vesicarium. As a result, availability of inoculum influenced the choice of the isolates assessed and the conidial concentration applied in each repetition. The lowest concentration produced was 2.25 × 105 conidia mL−1. For those isolates that produced > 2.25 × 105 conidia mL−1, the fruit were inoculated with the suspension concentration that was produced prior to dilution as well as the adjusted standardized concentration of 2.25 × 105 conidia mL. All isolates, and each concentration, were replicated five times, and an experimental unit consisted of one pear fruit. A sterile needle was used to wound pears once to a depth of 1–2 mm. A 10-µL drop of the conidial suspension was pipetted onto each wound site. Control treatments included a non-wounded control, a non-inoculated control and a water-only control for both wounded and non-wounded fruit. Fruits were placed onto plastic racks in sterile humidity chambers with water on the bottom of the chamber to maintain high humidity. Chambers were sealed and maintained at room temperature (20 ± 2°C).
Brown spot severity was evaluated by measuring the lesion diameter every day for 12 days. The area under the lesion progress curve was calculated (Shaner & Finney, Citation1977) based on lesion diameter to describe the cumulative severity throughout the assessment period. However, this assessment showed a similar pattern of response to lesion diameter, so only the lesion diameter is presented. Upon conclusion of the experiment, 10% of the symptomatic fruit were collected to isolate and verify the identity of the pathogen. The study was repeated three times.
Rye bioassay
An assay was conducted to test the pathogenicity of S. vesicarium on autumn rye ‘cultivar’. The study was arranged in a randomized complete block design with four replicates (pots). Five rye seeds were planted into each tall, narrow plastic pot (19-cm-tall SC7 Ray Leach Cone-tainers, Stuewe and Sons, Inc. Corvallis, OR) filled with soil-less media (Promix PGX, Premier Tech Horticulture, Rivière-du-Loup, QC). Seedlings were maintained in a growth chamber at 22/18°C day/night cycle with 16-h photoperiod. One month after seeding, plants were inoculated with conidial suspensions of isolates OA46, OA48 and OO55, plus a sterile-water control and a no water control. Each isolate and control were replicated four times. Immediately after inoculation, plants were bagged to maintain humidity and returned to the growth chamber. At 14 days after inoculation, the plants were visually examined for symptoms of disease, e.g. lesions, yellowing. Each seedling was divided into green and brown tissue and these were cut into 2-cm-long pieces. The leaf pieces were surface sterilized in 0.5% sodium hypochlorite for 10 min, rinsed with sterile water, and placed in a flow hood to dry. Five pieces each of green and brown tissue per pot were placed onto PDA medium, incubated under constant fluorescent light at 20°C ± 2°C for 7 d, and examined for cultures of S. vesicarium based on culture morphology and pigmentation. The experiment was repeated twice.
Asparagus field trials
Field trials were established in 2011 at the University of Guelph Simcoe Research Station in Norfolk County, ON, on a silt loam soil (23% sand, 73% silt, 4% clay, pH 7.4 and 1.7% OM) and Syngenta Canada Inc. Honeywood Research Farm, Plattsville, ON, on a silt loam soil (34% sand, 58% silt, 8% clay, pH 6.5 and 2.5% OM). In mid-March, seeds were planted in 288-cell plug trays and the plugs were transplanted 4 weeks later into 50-cell trays filled with Sunshine mix #5 (Sun Gro Horticulture, Agawam, MA). Seedlings of ‘Guelph Millennium’, ‘Jersey Giant’, ‘Guelph Equinox’, ‘Tiessen’, UG010 and UG023 were then grown in a greenhouse for 10 weeks, placed outside for 7 days to acclimate and transplanted by hand at each site. Each plot consisted of one 6-m-long row, with 1.5 m between rows, 0.3 m between plants within a row, and 2 m between blocks. The plots were arranged in a randomized complete block design with five replicates.
In 2011–2014, the trials were fertilized with 50 kg N ha−1 in June, July and August. In 2015 and 2016, the trials were fertilized with 100 kg N ha−1 and 100 kg K ha−1 immediately following the final harvest. Weeds and insects were managed according to standard commercial practice and integrated pest management recommendations.
From 2014 to 2016, spears were harvested three times a week for 6 weeks. Spears harvested when no rain event had occurred were collected and held at 4°C to be used in other studies (described previously). After most rain events, 10 spears per plot were collected and assessed for purple spot incidence and severity. Spears were assessed four (2014), eight (2015) and 13 (2016) times per year at the Plattsville site and three (2014), four (2015) and 12 (2016) times at the Simcoe site. Each spear was rated on a scale of 0 to 4, where 0 = no lesions, 1 = 1–20 lesions, 2 = 21–50 lesions, 3 = 51–90 lesions and 4 > 90 lesions (Falloon et al., Citation1987). A disease severity index (DSI) was calculated as described previously.
After harvest was complete in 2015 and 2016, ferns were assessed weekly for the presence of Stemphylium leaf spot on the branches and cladophylls by rating 10 branches per plot on a scale of 0 to 5, where 0 = no lesions, 1 = 1–20 lesions, 2 = 21–50 lesions, 3 = 51–90 lesions, 4 = 90–200 lesions and 5 > 200 lesions (modified from Falloon et al., Citation1987). A disease severity index (DSI) and area under the disease progress curve (AUDPC) were calculated as described previously (Kobriger & Hagedorn, Citation1983). The AUDPC was standardized (sAUDPC) by dividing AUDPC by the number of days the plants were assessed (Simko & Piepho, Citation2012). This assessment showed a similar pattern of response to DSI, so only the DSI is presented. Both assays were repeated.
Onion field trials
Field trials to assess the disease reaction of selected onion cultivars to Stemphylium leaf blight were established at the Muck Crops Research Station (MCRS) of the University of Guelph in 2015 and 2016 at sites where Stemphylium leaf blight had occurred in previous years. In 2015, plugs (three seedlings per plug) of 13 onion cultivars were transplanted by hand at the end of May into organic soil (62% OM, pH = 7.2) at the Jane Street research site of MCRS. In 2016, lines were direct seeded in early May into organic soil (71% OM, pH = 5.7) at the MCRS at a rate of 35 seeds m−1 with a custom-built precision seeder. In 2016, ‘Braddock’ replaced ‘Madras’ because seed of ‘Madras’ was not available, and ‘Pontiac’ was not assessed due to poor germination. Each plot consisted of four 5.0-m-long rows on a 1.6-m-wide bed. The trials were arranged in a randomized complete block design with four replicates. Weeds and insects were managed according to standard recommendations and integrated pest management, supplemented with hand weeding as required.
The study was rated weekly for symptoms of Stemphylium leaf blight. The number of lesions, disease incidence (% of plants) and leaf dieback (% length affected) were assessed on the two middle rows of each bed. Lesions on the first and second fully developed true leaves of eight transplant plugs per plot were counted in 2015 and on 20 plants per plot in 2016. In 2015, disease incidence was assessed based on the total number of plants per plot with symptoms of Stemphylium leaf blight. In 2016, incidence was assessed for only 100 plants per plot. Leaf dieback was assessed by estimating the percentage of leaf length with chlorosis or necrosis. Leaf dieback was assessed on eight transplant plugs per plot in 2015, and on 20 plants per plot in 2016.
Data analysis
Statistical analyses were conducted using SAS v.9.4 (SAS Institute Inc., Cary, NC). Mixed model analysis of variance was used to assess the disease and yield data (PROC GLIMMIX). The normality of each data set was assessed using PROC UNIVARIATE.
In the controlled environment asparagus spear and onion cultivar assays, variance was portioned into random (block) and fixed (isolate, cultivar, and cultivar × isolate) effects. The repeated experiments were not pooled due to significant interactions (P > 0.05) between isolate and repeated experiment for both species. In the pear fruit and rye assay, variance was portioned into random (block) and fixed (isolate) effects within each repetition. The non-inoculated controls were removed from the ANOVA.
Field data could not be pooled across years because of significant year × treatment interactions (P > 0.05). Data in the asparagus field trial data was reported for each location separately. Variance for the onion and asparagus field trials was partitioned into random effects (block) and fixed effects (year, variety, and year × variety). In the onion field trial, variance was partitioned into random effects (block) and fixed effects (year, cultivar, and year × cultivar).
Means were separated using Tukey’s HSD (P ≤ 0.05). Pearson’s rank correlation (PROC CORR at P < 0.05) was used for correlations between the mean number of lesions per leaf and the mean percentage leaf dieback in the onion growth room study, and for correlations between two locations in the asparagus field trials.
Results
Isolate phenotyping and genotyping
Isolates were obtained from four commercial onion fields in Ontario and five fields in Nova Scotia. Isolates were also obtained from 16 commercial asparagus fields in Ontario and two fields in Nova Scotia. When characteristic symptoms were observed on onion and asparagus, isolates of S. vesicarium were obtained from plants with symptoms. The conidia were oblong in shape with size averaging between 19–23 × 22–46 µm and with 4–7 transverse septa and 1–2 complete longitudinal septa. Conidia of representative isolates are shown in .
Fig. 1 (Colour online) Conidia of the five isolates of Stemphylium vesicarium assessed in a growth room study of pathogenicity and aggressiveness on onion.
Molecular analysis and DNA sequencing of eight isolates confirmed that each isolate was S. vesicarium, based on species-specific primers (GenBank accession numbers MH628098–MH628128). The alignment of retrieved sequences from NCBI database from the ITS, GPD, TEf-A and cytB regions of the fungal isolates OA20, OO27, OO31, NO36, OA46, OA48 and NA51 exhibited 98–100% homology over 83–99% query cover with S. vesicarium (GenBank accession numbers MG065799.1, MG020760.1 and KF993418.1 for the ITS region, and DQ000654.1, JF331624.1 and KJ934233.1 for the GPD, TEf-A and cytB regions, respectively). When aligned using ClustalW (https://www.genome.jp/tools-bin/clustalw), the pairwise alignment scores of the created contigs of the 8 isolates were 96.6–100% for each gene sequence. The ITS, TEf-A and cytB regions of NO35 also exhibited 99–100% homology with 83–98% query cover, although the GPD region of NO35 was not completely sequenced due to experimental error. The ITS, TEf-A and GPD regions (GenBank accession numbers AY329212.1, AY324708.1 and AY317016.1, respectively) aligned with 99–100% homology over 73–88% query cover to EGS37-067, which is an ex-type strain deposited by Simmons (Citation1967) and sequenced by Inderbitzin et al. (Citation2005). Thus, representative isolates from both provinces, Nova Scotia and Ontario, and from both asparagus and onions were all confirmed as S. vesicarium.
Asparagus spear bioassays
In both bioassays, all of the asparagus varieties were susceptible to S. vesicarium, and no symptoms were observed on the wounded and non-wounded control spears (data not shown). All isolates were pathogenic on asparagus spears, regardless of the host or location the isolate was collected from, but the isolates differed in aggressiveness (). Lesions developed at the wound site and grew lengthwise along the spear. The pathogen was re-isolated from selected lesions and was confirmed to be S. vesicarium based on morphology.
Table 2. Differences in final disease severity index (DSI) on detached spears of asparagus inoculated with eight isolates of Stemphylium vesicarium from asparagus and onion. The experiment was repeated twice.
There was no isolate × cultivar × repetition interaction, but there was an interaction between repetition and isolate in both the first (P = 0.0046) and second (P < 0.0001) assay, so data could not be combined. In the first bioassay DSI was lower for NO36, an isolate from onion, than for the other isolates (). Similarly, in the second bioassay DSI was lower for OO27, another isolate from onion, than for the asparagus isolate OA46. The data were not combined between repetitions for cultivar and ‘Guelph Eclipse’ had a lower final DSI than UG010 in both repeated experiments ().
Table 3. Differences in disease severity index (DSI) on asparagus spears of several cultivars and lines hybrids inoculated with Stemphylium vesicarium. The experiment was repeated twice.
Onion bioassay
Inoculation with each of the isolates of S. vesicarium from onion and asparagus produced symptoms on onion leaves. The first lesions were observed at 9–14 days after inoculation. Mock-inoculated plants did not develop symptoms.
There was no treatment × repetition interaction, but the data were not combined for analysis to prevent a significant cultivar × isolate interaction. There were significant differences between isolates and cultivars for the number of lesions per leaf and leaf dieback (P < 0.001). Isolates OO27 and OA46 produced the highest number of lesions, and NO35 and OO54 produced the lowest. Isolate OO27 produced the highest dieback and isolates NO35 and OA46 the lowest (). The only difference in the number of lesions per leaf was that ‘Pontiac’ had a lower number of lesions compared with ‘Patterson’ in one experiment. There were also differences in leaf dieback on the cultivars; ‘Pontiac’ had the lowest dieback and ‘Highlander’ had the highest (). The mean number of lesions per leaf was not correlated with the per cent leaf dieback for each cultivar.
Table 4. Lesions per leaf and final leaf dieback (%) on seedlings of 12 onion cultivars1 inoculated with Stemphylium vesicarium by isolate. The experiment was repeated twice.
Table 5. Lesions per leaf and final leaf dieback (%) of 12 onion cultivars inoculated with Stemphylium vesicarium (isolates OA46, OO27, OO54, OO55 and NO35). The experiment was repeated twice.
Pear fruit bioassay
Symptoms were observed as early as 2 days after inoculation. No symptoms developed on non-wounded pear fruit (data not shown), but all isolates were able to cause symptoms on wounded pear fruit. Generally, higher concentrations of conidia of S. vesicarium produced larger lesions than lower concentrations of S. vesicarium. Specifically, isolate OO31 did not produce lesions at 2.25 × 105 conidia mL−1 but did produce disease symptoms at 4.5 × 105 conidia mL−1. In the second repetition, when the concentration of conidia was diluted to 2.25 × 105 conidia mL−1, NO35 produced larger lesions (20 mm diameter) than OA48, OA56, NO36 and OA20 (7.4–18 mm diameter).
Rye bioassay
Symptoms were not observed on inoculated rye plants or controls, and S. vesicarium was not recovered from green leaf tissue. However, S. vesicarium was re-isolated from necrotic, brown tissue. The per cent of inoculated plants producing cultures of S. vesicarium varied by isolate and was 13%, 63% and 75% for isolates OA48, OA46 and OO55, respectively.
Asparagus field trials
All six cultivars developed characteristic symptoms on spears, branches and cladophylls, although there was no clear pattern of disease response among cultivars over sites and years. There was a cultivar × location interaction (P < 0.0001) for purple spot incidence and severity on spears; ‘Guelph Millennium’ had the lowest incidence and severity at Simcoe and the highest incidence at Plattsville (). Similarly, there was a year × location × variety interaction for final DSI on ferns, where differences among varieties was identified at one site in only one of two years. In 2016 at the Simcoe site, ‘Jersey Giant’, ‘Tiessen’ and UG010 had a lower DSI than ‘Guelph Millennium’, ‘Guelph Equinox’ and UG023 (). Severity (DSI) on spears and ferns were negatively correlated (r = −0.44, P = 0.0004) at Simcoe and positively correlated (r = 0.44, P = 0.0002) at Plattsville.
Table 6. Incidence (%) and disease severity index (DSI) on spears of six asparagus hybrids at two field locations in Ontario in 2014, 2015 and 2016.
Table 7. Incidence (%) and standardized area under the disease severity index (DSI) on ferns of six asparagus hybrids at two field locations in Ontario in 2015 and 2016.
Asparagus rust (Puccinia asparagi DC) developed on ferns at the Simcoe site but not at Plattsville, and all six cultivars were susceptible. There was no interaction in final DSI between year and cultivar so the data were pooled across the two years at the Simcoe site. ‘Guelph Equinox’ had a lower DSI than ‘Tiessen’. The DSI of rust and Stemphylium leaf spot were negatively correlated (r = −0.82, P < 0.0001).
Onion field trials
In both years, all of the onion cultivars were susceptible to Stemphylium leaf blight and differences among cultivars were generally small. The number of lesions per leaf and proportion of leaf dieback were higher in 2015 than in 2016 (). The mean number of lesions per leaf was not correlated with the overall leaf dieback per cultivar, and there was no correlation between leaf dieback and the days to maturity for each cultivar (data not shown).
Fig. 2 Reaction of onion cultivars to Stemphylium leaf blight at the MCRS, Holland Marsh, ON in 2015 and 2016: (a) leaf lesions and (b) leaf dieback. Bars topped with the same lower case letter (2015) or upper case letter (2016) do not differ based on Tukey’s HSD at P ≤ 0.05.
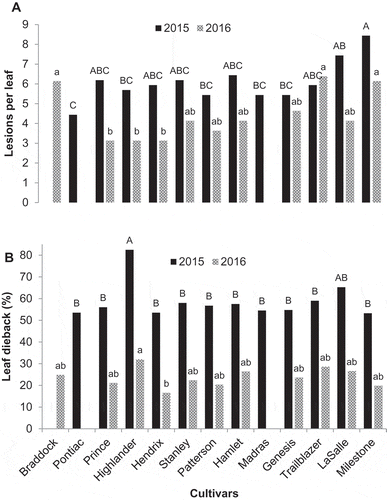
In 2015, ‘Pontiac’ had the lowest number of lesions, but this was only different from ‘LaSalle’ and ‘Milestone’, whereas ‘Highlander’ had higher leaf dieback (83%) than all of the other cultivars except ‘LaSalle’ (65%). In 2016, ‘Hendrix’, ‘Highlander’ and ‘Prince’ had fewer lesions than ‘Milestone’ and ‘Trailblazer’, furthermore ‘Highlander’ again had numerically higher leaf dieback (32%) than the other cultivars but this was only significantly different from ‘Hendrix’ (17%) ().
There was no correlation between growth room and field results for numbers of lesions per leaf. There was a positive correlation (r = 0.68, P = 0.02) between leaf dieback in the growth room and the field trial 2015, but not in 2016. However, ‘Milestone’ was most susceptible to initial infection (lesions) and ‘Highlander’ was most susceptible to leaf dieback in both the growth room and field studies.
Discussion
In the current study, all of the cultivars and lines of asparagus and onion assessed were susceptible to S. vesicarium in both controlled environment and field trials. Small differences in susceptibility were identified, but no cultivars demonstrated economically important levels of resistance. There was no correlation between lesion counts and leaf dieback in onion. Onion cultivars that developed more lesions often had low levels of leaf dieback, and vice versa. This indicated that susceptibility to infection differed from susceptibility to leaf dieback. Similarly, infection of spears was not consistently correlated with severity on ferns in asparagus.
The role of toxins produced by S. vesicarium in the disease reaction of asparagus and onion cultivars is not known; however, the pathogenicity of Stemphylium isolates has been attributed to host-specific toxins in pear cultivars (Montesinos et al., Citation1995; Singh et al., Citation1999; Pattori et al., Citation2006). The chemistry of these toxins has not been characterized. Differences in reaction to initial infection and subsequent disease development in asparagus and onion cultivars may relate to differential response to similar host-specific toxins, although further research is needed to confirm this. Investigation into these toxins could assist plant breeders in cultivar selection for resistance in both asparagus and onion.
Isolates of S. vesicarium collected from asparagus infected onion and vice versa. Overall, isolates differed in lesion production on asparagus spears and onion leaves, and in leaf dieback of onion. Although not always significant, isolates from onion were generally less aggressive on asparagus spears than isolates from asparagus. Unfortunately, only one isolate from asparagus was included in the onion bioassay. It resulted in high numbers of lesions but low levels of leaf dieback, which suggests that it may lack the host-specific toxins produced by isolates that are highly aggressive on onion. Further studies using more isolates would be needed to confirm aggressiveness of the pathogen.
In the current study, wounding was required to initiate infection by S. vesicarium and symptom development on both asparagus spears and pear fruit. The pathogen is known to infect asparagus spears through wounds and open stomata (Lacy, Citation1982; Falloon et al., Citation1984, Citation1987; Johnson & Lunden, Citation1986; Sutherland et al., Citation1989). Isolates collected from pear, however, were able to infect both pear fruit and leaves without wounding (Llorente et al., Citation2006; Köhl et al., Citation2009). In a study where pear fruit and leaves were not wounded prior to inoculation, only 1 of 52 isolates from onion and asparagus were pathogenic on pear leaves and none were pathogenic on pear fruit (Köhl et al., Citation2009). Taken together, this indicates that isolates adapted to other hosts were only able to cause symptoms on wounded pears, whereas isolates adapted to pear did not require wounding to produce symptoms.
The reaction of the asparagus lines and cultivars to purple spot differed among the controlled environment studies and both field sites. Similar to previous studies, in the detached spear assay, infection occurred only at the site of wounding (Lacy, Citation1982; Johnson & Lunden, Citation1986; Falloon et al., Citation1987). In attached spear studies, like the field trials, the spears can be infected through either natural openings or wounding (Falloon et al., Citation1987). If a mechanism of resistance was physical, such as stomatal opening and closing, wounding of the spears would artificially circumvent the mechanism. Further studies could investigate the reaction of asparagus in attached spear bioassays to establish which cultivars are resistant in the absence of artificial wounding. In addition, the asparagus rust outbreak at Simcoe influenced the results of the Stemphylium leaf spot severity at this site. These results demonstrate the difficulty of screening cultivars for resistance when multiple pathogens are present, and also demonstrate the importance of conducting trials across multiple sites for plant breeding research.
In the current study, S. vesicarium was shown to colonize and sporulate on necrotic leaves of rye, which is normally considered to be a non-host. Inoculation with S. vesicarium did not produce visible symptoms on autumn rye, but the pathogen was later re-isolated from dead, surface-sterilized rye leaves. This indicated that S. vesicarium was able to survive as a saprophyte on dead autumn rye tissue, which may provide a reservoir of overwintering inoculum for the pathogen. Isolates from non-host tissue obtained from pear orchards were able to re-infect pear fruit (Rossi et al., Citation2005; Köhl et al., Citation2009, Citation2013). It is unknown how S. vesicarium overwinters in onion fields in Ontario, since these fields are tilled, which minimizes the potential biomass of infected residue on the soil surface. In Japan, S. vesicarium has been observed overwintering as pseudothecia produced on leaves with or without symptoms (Misawa & Yasuoka, Citation2012). The potential for S. vesicarium to survive on living or senesced weed species and cover crops should be assessed to further understand the aetiology of Stemphylium leaf blight in onion.
Stemphylium vesicarium now occurs in all of the major asparagus and onion production regions of Ontario and Nova Scotia. The pathogen is endemic in asparagus in Ontario, but has only recently been recognized as a constraint to onion production in Ontario or Nova Scotia. Similarly, S. vesicarium is present in asparagus production in Michigan (Lacy, Citation1982; Meyer et al., Citation2000) but has yet to be reported on onion, despite its occurrence on onion in New York State for decades (Miller et al., Citation1978; Shishkoff & Lorbeer, Citation1989). The cause(s) of the recent increase in severity observed in Ontario and Nova Scotia are unknown, but may be the result of a change in virulence of the pathogen, the introduction of more susceptible cultivars, or changes in production practices, such as herbicide use.
Future research is required to investigate the host-specific toxins produced in onion and asparagus, mechanisms of resistance in asparagus, and the importance of non-host crops on the aetiology of S. vesicarium in both asparagus and onion.
Acknowledgements
The authors thank P.D. Hildebrand, E. Roddy and A. Shi for providing isolates of S. vesicarium or infected plant tissue, and Dr A. Abdelmagid, Dr F. Al-Daoud, S. Boersma, A. Fang Shi, K. Goldenhar, M. Gras, K. Knip, C. Mackay, A. Van Den Nieuwelaar, J. Van Den Nieuwelaar and H. Van Lith for technical assistance.
Additional information
Funding
References
- Basallote MJ, Prados AM, Pérez DA, Melero-Vara JM. 1993. First report in Spain of two leaf spots of garlic caused by Stemphylium vesicarium. Plant Dis. 77:52.
- Berbee ML, Pirseyedi M, Hubbard S. 1999. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 91:964–977.
- Brainard D. 2012. Irrigation and rye living-mulch effects on Michigan asparagus. Acta Hort. 950:53–58.
- Evans TA, Stephens CT. 1984. First report in Michigan of the teleomorph of Stemphylium vesicarium, the causal agent of purple spot of asparagus. Plant Dis. 68:1099.
- Falloon PG. 1982. The need for asparagus breeding in New Zealand. NZ J Exp Agric. 10:101–109.
- Falloon PG, Falloon LM, Grogan RG. 1984. Purple spot and Stemphylium leaf spot of asparagus. California Agric. 38:21.
- Falloon PG, Falloon LM, Grogan RG. 1987. Etiology and epidemiology of Stemphylium leaf spot and purple spot of asparagus in California. Phytopathology. 77:407–413.
- Graf S, Bohlen-Janssen H, Miessner S, Wichura A, Stammler G. 2016. Differentiation of Stemphylium vesicarium from Stemphylium botryosum as causal agent of the purple spot disease on asparagus in Germany. Eur J Plant Pathol. 144:411–418.
- Hausbeck MK, Hartwell J, Byrne JM. 1999. Epidemiology of Stemphylium leaf spot and purple spot in no-till asparagus. Acta Hortic. 479:205–210.
- Hudson HJ. 1971. The development of the saprophytic fungal flora as leaves senesce and fall. In: Preece TF and Dickinson CH, editors. Ecology of Leaf Surface Micro-Organisms. London: Academic Press; p. 447–455
- Inderbitzin P, Harkness J, Turgeon BG, Berbee ML. 2005. Lateral transfer of mating system in Stemphylium. Proc Natl Acad Sci USA. 102:11390–11395.
- Inderbitzin P, Mehta YR, Berbee ML. 2009. Pleospora species with Stemphylium anamorphs: a four locus phylogeny resolves new lineages yet does not distinguish among species in the Pleospora herbarum clade. Mycologia. 101:329–339.
- Johnson DA, Lunden JD. 1986. Effects of wounding and wetting duration on infection of asparagus by Stemphylium vesicarium. Plant Dis. 70:419–420.
- Kobriger KM, Hagedorn DJ. 1983. Determination of bean root rot potential in vegetable production fields of Wisconsin’s central sands. Plant Dis. 67:177–178.
- Köhl J, de Jong PF, Kastelein P, Groenenboom-de Haas BH, Anberge RHN, Balkhoven H, Wubben JP. 2013. Dynamics of pear-pathogenic Stemphylium vesicarium in necrotic plant residues in Dutch pear orchards. Eur J Plant Pathol. 137:609–619.
- Köhl JB, Groenenboom-de Haas B, Goossen-van de Geijn H, Speksnijder A, Kastelein P, de Hoog S, van den Ende BG. 2009. Pathogenicity of Stemphylium vesicarium from different hosts causing brown spot in pear. Eur J Plant Pathol. 124:151–162.
- Koike ST, O’Neill N, Wolf J, Van Berkum P, Daugovish O. 2013. Stemphylium leaf spot of parsley in California caused by Stemphylium vesicarium. Plant Dis. 97:315–322.
- Lacy ML. 1982. Purple spot: a new disease of young asparagus spears caused by Stemphylium vesicarium. Plant Dis. 66:1198–1200.
- Landry EJ, Wolyn DJ. 2011. Cold acclimation attributes of two asparagus cultivars with varying patterns of fern senescence. J Amer Soc Hort Sci. 136:177–189.
- Leuprecht B. 1990. Stemphylium botryosum Wallr. on asparagus. Gesunde Pflanzen. 42:187–191.
- Llorente I, Montesinos E. 2002. Effect of relative humidity and interrupted wetness periods on brown spot severity of pear caused by Stemphylium vesicarium. Phytopathology. 92:99–104.
- Llorente I, Vilardell A, Montesinos E. 2006. Infection potential of Pleospora allii and evaluation of methods for reduction of the overwintering inoculum of brown spot of pear. Plant Dis. 90:1511–1516.
- Meyer MP, Hausbeck MK, Podolsky R. 2000. Optimal fungicide management of purple spot of asparagus and impact on yield. Plant Disease. 84:525–530.
- Miller ME, Taber RA, Amador JM. 1978. Stemphylium blight of onion in South Texas. Plant Dis Rept. 62:851–853.
- Misawa T, Yasuoka S. 2012. The life cycle of Stemphylium vesicarium, the causal agent of Welsh onion leaf blight. J Gen Plant Pathol. 78:18–29.
- Montesinos E, Moragrega C, Llorente I, Vilardell P. 1995. Susceptibility of selected pear cultivars to infection by Stemphylium vesicarium and the influence of leaf and fruit age. Plant Dis. 79:471–473.
- Morgulis A, Coulouris G, Raytselis Y, Madden TL, Agartala R, Schaffer AA. 2008. Database indexing for production MegaBLAST searches. Bioinformatics. 24:1757–1764.
- Paibomesai M, Celetti M, Tesfaendrias M. 2012. Update on Stemphylium leaf blight in onions in Ontario. Hort Matters Ontario Ministry of Agriculture, Food and Rural Affairs. 12(19):11–12. http://www.omafra.gov.on.ca/english/crops/hort/news/news_hortmatt.html
- Pattori E, Rossi V, Bugiani R, Giosue S 2006. Virulence of Stemphylium vesicarium isolates from pear and other host species. International Organisation for for Biological Control/wprs Bull. 29:195–205.
- Rao NNR, Pavgi MS. 1973. Pleospora allii on onions from Varanasi. Curr Sci. 42:734.
- Rao NNR, Pavgi MS. 1975. Stemphylium leaf blight of onion. Mycopathologia. 56:113–118.
- Rossi V, Pattori E, Bugiani R. 2008. Sources and seasonal dynamics of inoculum for brown spot disease of pear. Eur J Plant Pathol. 121:147–159.
- Rossi V, Pattori E, Giosue S, Bugiani R. 2005. Growth and sporulation of Stemphylium vesicarium, the causal agent of brown spot of pear, on herb plants of orchard lawns. Eur J Plant Pathol. 111:361–370.
- Shaner G, Finney RE. 1977. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology. 67:1051–1056.
- Shishkoff N, Lorbeer JW. 1989. Etiology of Stemphylium leaf blight of onion. Phytopathology. 79:301–304.
- Simko I, Piepho HP. 2012. The area under the disease progress stairs: calculation, advantage, and application. Phytopathology. 102:381–389.
- Simmons EG. 1967. Typification of Alternaria, Stemphylium, and Ulocladium. Mycologia. 59:67–92.
- Simmons EG. 1969. Perfect stages of Stemphylium. Mycologia. 61:1–26.
- Singh P, Bugiani R, Cavanni P, Nakajima H, Kodama M, Otani H, Kohmoto K. 1999. Purification and biological characterization of host-specific SV-toxins from Stemphylium vesicarium causing brown spot of European pear. Phytopathology. 89:947–953.
- Sutherland PW, Hallett IC, Parkes SL, Templeton MD. 1989. Structural studies of asparagus spear infection by Stemphylium. Can J Bot. 68:1311–1319.
- Suzui T. 1973. Stemphylium leaf spot (Stemphylium botryosum Wallr.) on asparagus plants. Ann Phytopathol Soc Jpn. 39:364–366.
- Tayviah CS. 2017. Epidemiology and management of stemphylium leaf blight on onion (Allium cepa L.) in The Holland Marsh, Ontario [M. Sc. dissertation]. Guelph (ON): University of Guelph.
- Vilardell P. 1988. Stemphylium vesicarium en plantacione de peral. Fruitic Prof. 18:51–55.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Orlando (FL): Academic Press; p. 315–322.
- Woudenberg JHC, Hanse VLGCM, Groenewald JZ, Crous PW. 2017. Stemphylium revisited. Stud Mycol. 87:77–103.
- Zhang Z, Schwartz S, Wagner L, Miller W. 2000. A greedy algorithm for aligning DNA sequences. J Comput Biol. 7:203–214.
- Zheng L, Lv R, Hsiang T, Huang J. 2009. Host range and phytotoxicity of Stemphylium solani, causing leaf blight of garlic (Allium sativum) in China. Eur J Plant Pathol. 124:21–30.
