Abstract
Fusarium spp. associated with fusarium head blight (FHB) on cereals in Ontario was monitored in this study. Nine species were recovered from 24 300 putatively infected kernels collected from 486 affected spring wheat fields from 2001 to 2017, 11 species from 13 250 kernels from 265 barley fields from 2005 to 2017, and nine species from 8800 kernels from 176 oat fields from 2008 to 2017. Fusarium avenaceum, F. equiseti, F. graminearum, F. poae and F. sporotrichioides were the most commonly isolated species, occurring in 23–66% of the infected fields and 1.3–27.0% of the infected kernels. In spring wheat, F. graminearum was predominant, occurring in 88% of fields and in 60.4% of kernels, and represented 90% of the pathogen population. In barley, F. poae and F. graminearum were equally dominant, occurring in 77% and 64% of fields, 17.6% and 17.0% of kernels, and represented 42% and 40% of the pathogen population, respectively. In oat, F. poae was predominant, occurring in 93% of fields and 23.5% of kernels, and represented 68% of the pathogen population. Growth seasons characterized as an FHB epidemic occurred in 11 of 17 years surveyed in spring wheat, 3 of 13 years surveyed in barley, and no epidemic years were observed over the 10-year period in oat. There were no significant differences in frequency of isolation of the Fusarium species, except for F. graminearum which increased by 60% in wheat and by 210% in barley in the FHB epidemic years compared with the non-epidemic years, suggesting that F. graminearum was responsible for the FHB epidemics in Ontario.
Résumé
Dans le cadre de cette étude, Fusarium spp., associé à la brûlure de l’épi causée par le fusarium (BEF) qui s’attaque aux céréales en Ontario, a fait l’objet d’un suivi. De 2001 à 2017, 9 espèces ont été récupérées sur 24 300 grains prétendument infectés, collectées dans 486 champs de blé de printemps infestés; de 2005 à 2017, 11 espèces provenant de 13 250 grains ont été collectées dans 265 champs d’orge; et, de 2008 à 2017, 9 espèces récupérées sur 8800 grains ont été collectées dans 176 champs d’avoine. Fusarium avenaceum, F. equiseti, F. graminearum, F. poae et F. sporotrichioides ont été les espèces le plus souvent isolées, ayant été trouvées dans 23 à 66% des champs infestés et sur 1.3 à 27.0% des grains infectés. Chez le blé de printemps, F. graminearum dominait, ayant été trouvé dans 88% des champs et sur 60.4% des grains, représentant 90% de la population d’agents pathogènes. Chez l’orge, l’occurrence de F. poae et de F. graminearum était similaire, ayant été trouvés dans 77% et 64% des champs, sur 17.6% et 17.0% des grains, représentant 42% et 40% de la population d’agents pathogènes, respectivement. Chez l’avoine, F. poae dominait, ayant été trouvé dans 93% des champs et sur 23.5% des grains, représentant 68% de la population d’agents pathogènes. Les saisons de croissance considérées comme ayant connu une épidémie de BEF quant au blé de printemps se sont produites durant 11 des 17 années qui ont fait l’objet du suivi; quant à l’orge, il est question de 3 années sur 13; et, pour ce qui est de l’avoine, durant 10 années, aucune épidémie n’a été observée. Il n’y avait pas de différences significatives quant à la fréquence d’isolement des espèces de Fusarium, sauf en ce qui a trait à celle de F. graminearum qui s’est accrue de 60% chez le blé et de 210% chez l’orge durant les années d’épidémie comparativement aux années où il n’y a pas eu d’épidémie, ce qui suggère que F. graminearum était responsable des épidémies de BEF en Ontario.
Introduction
Fusarium head blight (FHB) is an important disease of wheat, barley and oat worldwide (McMullen et al., Citation2012). In Canada, frequent FHB epidemics in wheat in Manitoba and eastern Canada over the past two decades have caused extensive economic losses because of reduced yield and discounted price of grains contaminated with Fusarium-damaged kernels (FDK) and their associated mycotoxins, mainly deoxynivalenol (DON) and acetylated derivatives (Gilbert & Tekauz, Citation2000; Gilbert & Haber, Citation2013). Historically, barley and oat were not considered as susceptible to FHB as wheat; however, in recent years reports of increased incidence indicate that FHB has become an economically important disease of barley in Canada (Tekauz et al., Citation2000; Tucker et al., Citation2016). Although there has not been a severe FHB outbreak observed on oat, the incidence of FHB on oats grown in the two main production regions in Canada (the Canadian prairies and the eastern provinces of Ontario and Quebec) has increased in recent years (Tekauz et al., Citation2004, Citation2008, Citation2011; Tamburic-Ilincic, Citation2010; Xue & Chen, Citation2018).
There are several Fusarium species that are capable of causing FHB. Fusarium graminearum Schwabe is the most virulent species and the major causal agent worldwide, but F. avenaceum (Fr.) Sacc., F. culmorum (Wm. G. Sm.) Sacc. and F. poae (Peck) Wollenw. are reported to prevail in some European and South American countries (Parry et al., Citation1995; Liddell, Citation2003; Barreto et al., Citation2004). In Canada, F. graminearum is the predominant species but several other species including F. acuminatum Ellis & Everh., F. avenaceum, F. equiseti (Corda) Sacc., F. poae and F. sporotrichioides Sherb. have also been frequently isolated from naturally infected FDK of cereals (Xue et al., Citation2004b, Citation2013; Bourdages et al., Citation2006; Guo et al., Citation2018). There have been no studies investigating the occurrence and frequency of infection from these Fusarium spp. with relation to the crop species and the FHB epidemics over thistime in Canada.
The objectives of this research were to examine the occurrence and prevalence of Fusarium spp. associated with head blight on spring wheat, barley and oat in Ontario in FHB epidemic and non-epidemic years during 2001–2017. Results of this study will aid in developing FHB management strategies and establishing Fusarium species priorities for screening and breeding spring wheat, barley and oat for resistance to FHB in Canada.
Materials and methods
Disease survey and sample collection
Annual surveys for FHB severities in commercial fields in Ontario were conducted in spring wheat from 2001 to 2017, barley from 2005 to 2017 and oat from 2008 to 2017. The surveys were carried out in the third and fourth weeks of July when plants were at the soft dough stage of development. The surveyed fields were chosen at random in densely cultivated regions where spring wheat, barley and oat are grown. The number of fields surveyed each year ranged from 22–40 for spring wheat, 13–35 for barley, and 10–29 for oat (). FHB was rated for incidence (% infected heads) and severity (% infected spikelets in the affected heads) based on ~200 heads at each of three random sites per field. The FHB index [(% incidence × % severity)/100] was determined for each field. Any year in which FHB was observed in ≥90% of surveyed fields with a mean FHB index value of ≥5% was considered as a FHB epidemic year. A sample consisting of 30–50 FHB-infected heads was collected from each FHB infected field during the survey period. The diseased heads were air-dried at room temperature and subsequently hand threshed.
Table 1. Prevalence and severity of Fusarium head blight (FHB) on spring wheat (2001–2017), barley (2005–2017) and oat (2008–2017) in Ontario and number of Fusarium species recovered from infected kernels collected from the surveyed fields.
Isolation and identification of Fusarium species
Approximately 2–5 weeks after the survey, a random subsample of 50 discoloured and putatively infected kernels from each sample of FHB-infected heads from each affected field was taken to determine the associated Fusarium spp. The kernels were surface-disinfected for 1.5 min with 0.5% NaOCl, rinsed three times with sterile distilled water, and drained on sterile filter paper. Five kernels were placed in each Petri dish containing modified potato dextrose agar (mPDA) (10 g dextrose per litre, which is 50% of the label rate) amended with 20 ppm streptomycin sulphate. Dishes were placed under mixed lighting consisting of fluorescent UV tubes and artificial daylight, on a 12-h light/dark cycle for 7–14 d at 22–25°C. Fusarium spp. were identified directly from mPDA or, if necessary, subcultured onto carnation leaf agar (CLA) for identification. The fungi were identified based on colony characters and by microscopic examination of macro and/or micro conidia and taxonomic keys for Fusarium spp. described by Nelson et al. (Citation1983) and illustrated morphological manuals by Samson et al. (Citation2000) and Watanabe (Citation1994).
Statistical analyses
To observe Fusarium changes across time on each of the three cereal crops, the frequency of the five common Fusarium spp. isolated from putatively infected kernels were plotted against the year of survey. The difference between FHB epidemic years and non-epidemic years in the frequency of isolation of the five Fusarium spp. was examined using contrast analysis. Since the numbers of years varied between the two paired variables, the experiment was treated as a two-independent-sample design. Analyses were performed using SAS® T Test Procedure (Version 9.3; SAS Institute Inc. Cary, NC, USA).
Results
Spring wheat
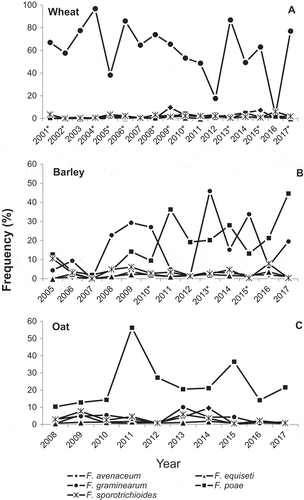
Symptoms of FHB were observed in 486 of the 492 spring wheat fields surveyed across Ontario from 2001 to 2017 (). Across the 17 years of survey, mean incidence ranged from 1.4% to 51.1%, mean severity from 1.5% to 57.8%, and mean FHB index from 0.02% to 27.0%. Eleven of the 17 years (2001–2002, 2004–2006, 2008–2010, 2013, 2015 and 2017) were considered FHB epidemic years, when FHB was observed in >90% of the surveyed fields and FHB index was greater than 5.0% on average. Fusarium spp. were isolated from 12.9% to 91.1% of the putatively infected wheat kernels and the total number of Fusarium spp. ranged from 4 to 7 species each year.
Nine Fusarium spp. were recovered from 16 489 of the 24 300 putatively infected wheat kernels tested during 2001–2017 (). Fusarium spp. was not recovered from 32.9% of these putatively infected kernels, but occasionally more than one Fusarium spp. were found in a single kernel. Fusarium graminearum was the predominant species, occurring in 88% of the infected fields and in 60.4% of the infected kernels, representing 90% of the pathogen population (). Fusarium poae, F. sporotrichioides, F. avenaceum and F. equiseti were also isolated, at frequencies ranging from 10–17% of fields and on 1–3% of infected kernels, and representing 1–4% of the pathogen population. Other Fusarium spp. including F. acuminatum, F. oxysporum Schlecht., F. culmorum and F. crookwellense Burgess, Nelson & Toussoun were least common, occurring in 1–3% of fields and 0.1–0.2% of kernels, collectively representing <1% of the pathogen population.
Table 2. Fusarium species recovered from infected kernels of wheat, barley and oat collected from surveyed fields in Ontario during 2001–2017.
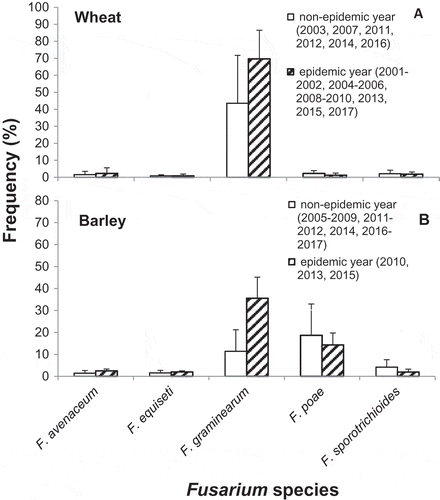
Among the five common Fusarium spp. identified, F. graminearum was the predominant species isolated in all 17 surveyed years from 2001 to 2017 (). There were no significant differences in frequency of isolation for the five most common species between the FHB epidemic and non-epidemic years, except for F. graminearum which showed a significant increase (P < 0.01) in epidemic years (). Fusarium graminearum was isolated from 45% kernels in non-epidemic years and 72% kernels in epidemic years, increasing the frequency of isolation by 60%.
Barley
Symptoms of FHB were observed in 265 of 320 barley fields surveyed across Ontario from 2005 to 2017 (). Across the 13 years of survey, mean incidence ranged from 0.8% to 41.7%, mean severity from 2.1% to 20.1%, and mean FHB index from 0.02% to 11.2%. Three of the 13 years studied (2010, 2013 and 2015) were considered FHB epidemic years, in which FHB was observed in all of the surveyed fields and FHB index was greater than 8.0%. Fusarium spp. were isolated from 2.0% to 74.1% of the putatively infected barley kernels and the total Fusarium spp. ranged from one to nine species each year.
Eleven species were isolated from 6534 of the 13 250 putatively infected barley kernels during 2005–2017 (). At least one Fusarium sp. was recovered from 42.4% of these putatively infected kernels, and the other 57.6% of kernels did not have any Fusarium spp. Fusarium poae and F. graminearum were the most predominant species, occurring in 76.8% and 64.4% of the surveyed fields, 17.6% and 17.0% of the kernels, and represented 42% and 40% of the pathogen population (), respectively. Fusarium sporotrichioides, F. avenaceum and F. equiseti were also commonly isolated and each found in 29–42% of fields and on 2–4% of kernels, and each represented 4–9% of the pathogen population. Other Fusarium spp. including F. acuminatum, F. oxysporum, F. culmorum, F. solani (Mart.) Sacc., F. verticillioides (Sacc.) Nirenberg and F. tricinctum (Corda) Sacc., were less commonly isolated, occurring in 1–5% of fields and 0.1–0.2% of kernels, collectively representing 1% of the pathogen population.
Among the five common Fusarium spp. identified, F. poae was the most frequently isolated species in 7 years of the 13 surveyed years during 2005–2017, while F. graminearum was the most frequent in the other 6 years (). There were no significant differences in frequency of isolation for these common species between the epidemic and non-epidemic years, except for F. graminearum which showed a significant increase (P < 0.01) in epidemic years (). Fusarium graminearum was isolated from 11.4% kernels in FHB non-epidemic years and 35.6% kernels in epidemic years, increasing the frequency of isolation by 210%. In contrast, F. poae was isolated from 18.6% kernels in non-epidemic years and 14.3% kernels in epidemic years, decreasing the frequency of isolation by 23.3%. This contrast in frequency of isolation of F. poae between the FHB non-epidemic and epidemic years is, however, not statistically significantly different.
Oat
In contrast to wheat and barley, the typical ‘head blighting’ symptoms on oat were not apparent, but expressed as bleached, grey or orange-pink discolouration of the individual spikelets randomly found in the surveyed fields. Because of the lack of the typical FHB symptoms on oat, the FHB incidence and severity were not visually assessed in the surveyed fields until 2010, when the evidence of FHB symptoms on oat were verified by the subsequent and high frequency of isolation of the Fusarium spp. from the surface sterilized kernels in the samples of these putatively infected spikelets or panicles collected during the disease survey in both 2008 and 2009 (). The symptoms of FHB were observed in 176 of 177 oat fields surveyed across Ontario from 2008 to 2017, with mean incidence ranging from 1.7% to 14.3%, mean severity from 3.1% to 10.3%, and mean FHB index from 0.1% to 2.7% (). Over the 10-year survey period, no FHB epidemics were observed on oat. Fusarium spp. were isolated from 17.2% to 66.5% of the putatively infected oat kernels and the total Fusarium spp. ranged from five to eight species each year.
Nine Fusarium spp. were recovered from 2945 of the 8800 putatively infected oat kernels during 2008–2017 (). Fusarium spp. was recovered from only 34.6% of these putatively infected oat kernels, with more than one Fusarium spp. found occasionally in a single kernel. Fusarium poae was the predominant species, occurring in 93% of fields surveyed, in 23.5% of the infected kernels, and represented 68% of the pathogen population (). Fusarium graminearum, F. sporotrichioides, F. avenaceum and F. equiseti were also commonly isolated and each found in 30–45% of fields and on 1–4% of kernels, and represented 3–11% of the pathogen population. Other Fusarium spp. including F. acuminatum, F. oxysporum, F. culmorum and F. solani, were less common, occurring in 1–4% of fields and 0.01–0.2% of kernels, collectively representing 1% of the pathogen population. Among the five most common Fusarium spp. identified, F. poae predominated in all of the 10 survey years (2008–2017) ().
Discussion
Representative cultures of all 12 Fusarium spp. isolated from the Ontario cereal grain in this study have been deposited in the Canadian Collection of Fungal Cultures (DAOMC). Morphological identification of Fusarium for taxonomic studies requires DNA sequencing, but for routine field surveys the known profile of species in a given geographic region defines the utility of non-molecular methods. Although F. graminearum was divided into ~20 phylogenetic species worldwide (O’Donnell et al., Citation2000), only lineage 7, which is F. graminearum in the strict sense, has been reported in Ontario. Of the other species that we commonly isolated, only F. avenaceum has been reported with species-level lineages (O’Donnell et al., Citation2012; Gräfenhan et al., Citation2013; Ceron-Bustamante et al., Citation2018). Fusarium acuminatum, F. crookwellense (also known as F. cerealis (Cooke) Sacc.), F. culmorum and F. sporotrichioides have not been separated into phylogenetic species, although some molecular variation has been documented; thus, morphological identification of these species remains reliable. There is growing evidence that the morphological concept of F. equiseti includes multiple phylogenetic lineages (O’Donnell et al., Citation2009b), and there are now three species within F. poae (Yli-Mattila et al., Citation2011), with the possibility that one of these, F. langsethiae Torp & Nirenberg, may occur in Canada among the much more common F. poae (K. Seifert, unpublished). The morphological concept of F. solani is well-documented to include a large number of phylogenetic species (O’Donnell, Citation2000), while the considerable molecular diversity within F. oxysporum has yet to be translated into named species (O’Donnell et al., Citation2009a). Neither of these species complexes occur with significance frequencies in grain.
This study investigated the severity of FHB on spring wheat, barley and oat occurring in commercial fields and the Fusarium spp. recovered from FHB-infected kernels in Ontario from 2001 to 2017. Epidemic years of FHB were recorded for 11 of the 17 years surveyed for spring wheat and 3 of the 13 years surveyed for barley. No epidemics were recorded over a 10-year survey period for oat, suggesting that the surveyed crop species differ in FHB tolerance, and the disease is more problematic on wheat than on barley and oat. On wheat, FHB resulted in significant losses in both yield and grain quality (Xue & Chen, Citation2015, Citation2018). The use of fungicides has been recommended for controlling FHB on wheat to sustain productivity and grain quality in Ontario, and we had observed that all of the wheat fields surveyed in the past five years were sprayed with fungicides to control FHB and possibly other foliar diseases. The FHB severities reported in this study and the possible losses would be much greater in the FHB epidemic years without the foliar applications of fungicides.
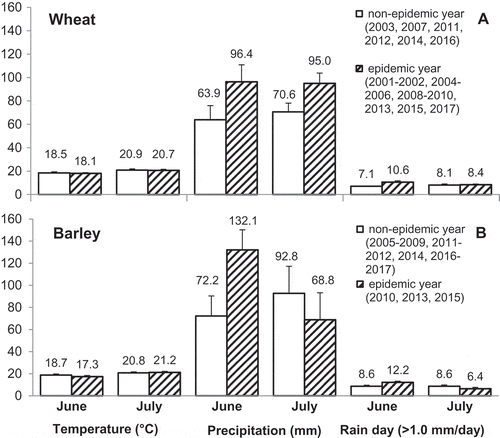
The epidemics of FHB on wheat and barley observed in the present study are probably related to the weather conditions during anthesis in June and July when FHB infection and the disease development occurred. According to records from the Environment Canada weather stations in Guelph, Peterborough, Toronto, Kemptville and Ottawa (where the majority of surveyed fields were located), average daily air temperatures were not different between the FHB epidemic and non-epidemic years (). However, the average total precipitation for June and July in wheat fields was 96.4 and 95.0 mm in the epidemic years, which was 32.5 and 24.4 mm higher than those in the non-epidemic years. Likewise, there were more days with >1.0 mm of total daily precipitation in June in the epidemic years than non-epidemic years. In barley, the differences in precipitation between the FHB epidemic and non-epidemic years were observed in June only, with 59.9 mm and 3.6 days of rain greater than non-epidemic years. These results are generally consistent with the findings of Sutton (Citation1982) and McMullen et al. (Citation1997) who demonstrated that development of FHB in the field is weather dependent and favoured by frequent rainfall and humid conditions.
Fig. 3 The difference in daily temperature, precipitation and number of rain days (>1.0 mm day−1) in June and July between Fusarium head blight (FHB) non-epidemic and FHB epidemic years in Ontario during 2001–2017. Data were collected by Environment Canada weather stations in Guelph, Peterborough, Toronto, Kemptville and Ottawa.
FHB occurs as a species mixture, where multiple species are isolated from the same field and co-infect on the same plants. Of the 12 Fusarium species recovered from the total of 46 350 putatively infected kernels over the 17 years, F. avenaceum, F. equiseti, F. graminearum, F. poae and F. sporotrichioides were the most commonly isolated species from the three crops. The results are in agreement with previous work by our group (Xue et al., Citation2004b, Citation2013; Guo et al., Citation2018). We reported that the same five most common Fusarium species were recovered from grain samples harvested from naturally infected wheat plots in Eastern Ontario during 1999–2000 and barley plots in Northeastern Ontario during 2004–2005. Notably, the current study demonstrated that F. graminearum was by far the dominant species on spring wheat (90%), F. poae on oat (68%), and F. poae (42%) and F. graminearum (40%) almost equally dominant on barley (). These results are consistent with findings from wheat in Ontario and Western Canadian provinces (Sutton, Citation1982; Gilbert & Tekauz, Citation2000; Xue et al., Citation2004b; Gilbert & Haber, Citation2013) and from barley seed from Manitoba and Quebec (Tekauz et al., Citation2000; Bourdages et al., Citation2006). The unexpected contribution of F. poae to FHB in Ontario may have practical implications on screening and breeding for FHB resistance for the specific cereal crops in Canada, which historically has focused on F. graminearum as the primary causal species of FHB in cereals and source of mycotoxin contamination (DON and acetylated derivatives) (Tekauz et al., Citation2000, Citation2008; Gilbert & Haber, Citation2013).
Fusarium graminearum was the predominant Fusarium pathogen observed on wheat in all 17 years surveyed, regardless of environmental conditions (). However for barley in non-epidemic years such as 2005, 2007, 2011 and 2012 when FHB severity and kernel infection were low, F. graminearum was a minor contributor, representing <10% of the FHB pathogen population. These findings are consistent with previous research (Xue et al., Citation2013) in which we observed that F. equiseti, F. sporotrichioides and F. poae predominated and collectively represented >80% of the pathogen population causing FHB in barley in Central and Eastern Ontario in 2004 and 2005, when the weather conditions during the two growing seasons were unfavourable for FHB epidemics. However, during years when environmental conditions became conducive to FHB epidemics, the frequency of isolation of F. graminearum from both wheat and barley increased dramatically while isolation frequency of other resident Fusarium spp. remained constant, suggesting that an increase in the F. graminearum population was responsible for disease severity during FHB epidemics in wheat and barley in Ontario.
In oat, FHB epidemics were not observed, even during 2010, 2013 and 2015 growing seasons when the environmental conditions were conducive for FHB epidemics in both wheat and barley (). However, during the 10-year period, 100% of surveyed fields were observed to have affected plants and F. poae was the dominant isolated species. This is consistent with our recent report by Guo et al. (Citation2018) who demonstrated that F. poae was the most common seed-borne Fusarium spp. affecting oat production in Canada in 2013 and 2014. Fusarium poae has also been documented as a major contributor to FHB in Argentina (González et al., Citation2008) and several European countries including France, Hungary, Ireland, Poland, Slovakia, Switzerland and the UK (Lukanowski & Sadowski, Citation2002; Ioos et al., Citation2004; Rohácik & Hudec, Citation2005; Xu et al., Citation2005; Schöneberg et al., Citation2018). Although incidence has been documented, there is a lack of information regarding when and how F. poae infects oat and other small-grain cereals. Spray inoculation at mid-anthesis on field-grown oats (3 locations, 7 oat varieties) in Switzerland resulted in no visible FHB symptoms and no significant change in yield or protein content of harvested oats, but all genotypes at all locations were infected with F. poae at rates ranging from 2–35% of oat grains (Martin et al., Citation2018). Previous studies by Xue et al. (Citation2004a, Citation2006) indicated that F. poae was only moderately pathogenic on wheat and weakly pathogenic on barley in causing FHB. This raises a question as to whether F. poae grows within the plants as a pathogen or more like an opportunistic biotroph and disseminated as an endophyte.
In North America, native grasses represent a natural endophytic niche of Fusarium species, and these species are repeatedly isolated as an endophyte from the same host over time and geographic space (Lofgren et al., Citation2018). For example, both F. avenaceum and F. graminearum are reported as symptomless colonizers of wild grass species and have been recovered from non-symptomatic tissues including symptomless, non-inoculated flowers and from seed lots of native grass species (Inch & Gilbert, Citation2003; Turkington et al., Citation2011; Lofgren et al., Citation2018). Fusarium graminearum persists in agricultural fields by forming perithecia that overwinter on debris on the surface of soil. At the time of flowering, the perithecia forcefully eject ascospores into the air to be carried upwards towards the wheat flowers, which then cause infection and initiate the disease. However, for other Fusarium species such as F. poae, no sexual state has been observed in nature and there is no equivalent mechanism for infection of flowers by fungal propagules overwintering on debris, as asexual spores would require passive dissemination from the soil level to the flowers. In native grass species, where the presence of fungal hyphae is associated with asymptomatic tissue, vertical transmission of the fungus through seeds may occur (Lofgren et al., Citation2018). Dissemination of F. poae through vertical transmission might explain the observed high frequency rate of isolation of F. poae in oat kernels even during non-epidemic years.
Each Fusarium spp. in the FHB complex produces a unique array of secondary metabolites, many of which are considered mycotoxins. Reduced yields due to FHB are compounded by the production and deposition of mycotoxins in infected kernels. Of immediate concern is the production of DON and acetylated derivatives by F. graminearum because these toxins are nationally and internationally regulated mycotoxins with strict acceptable tolerances in grains for human consumption and finished animal feed. Emerging mycotoxins on the other hand are currently neither routinely determined, nor presently legislatively regulated. However, they are of concern as they are currently being re-evaluated in the EU because of increasing evidence of their incidence in food and feed (Kovalsky et al., Citation2016). Many of these emerging mycotoxins such as beauvericin, enniatins and additional type A & B trichothecenes (i.e. diacetoxyscirpenol and fusarenone-X) are produced by many of the Fusarium species isolated.
The predominance of F. poae in this survey is of particular concern as it has been reported to produce all of the aforementioned emerging mycotoxins in culture (Thrane et al., Citation2004; Stenglein et al., Citation2014; Vanheule et al., Citation2017). All trichothecenes of the scirpentriol subgroup have been associated with F. poae (as well as F. sporotrichioides and F. equisiti) (Schollenberger et al., Citation2007) and certain isolates of F. poae originating in the upper midwest region of the USA were reported to produce these trichothecenes in barley (Sala et al., Citation1999). Because FHB occurs as a mixture of species, currently regulated mycotoxins such as DON can co-occur with other emerging mycotoxins. Kovalsky et al. (Citation2016) found that from 300 samples of finished feed obtained from Central European countries, for kernels exceeding acceptable threshold levels of DON and zearalenone, 100% of samples also contained enniatins and 85% contained beauvericin (reported to be produced by F. avenaceum and F. poae but not by F. graminearum). In this case increasing concentrations of these emerging mycotoxins correlated with increasing concentrations of DON. To date, little to no information exists regarding what effect mycotoxin combinations (such as enniatin or beauvericin with DON) will have upon biological systems. There is a need for further investigation to define the importance of F. poae as a contaminant of oat and barley grains in Canada and the possible presence of excessive levels of multiple mycotoxins in harvested grains in a FHB epidemic year. Research is underway at Ottawa Research and Development Centre, Agriculture and Agri-Food Canada to assess the mycotoxin potential for Canadian strains of F. poae and production of its corresponding mycotoxins in oat.
As is the case with wheat and barley, breeding for improved resistance to FHB in oat has been considered as the best management option and initiated in oat breeding programmes across Canada in recent years (Yan et al., Citation2010; Mitchell Fetch et al., Citation2016). Further studies investigating the pathological mechanisms of F. poae and its contribution to the overall FHB development and mycotoxin levels in oat relative to other aetiological Fusarium species would provide valuable information required for effective FHB resistance screening and breeding efforts in Canada.
Acknowledgements
This research was funded in part through AAFC’s Growing Forward-II Policy Framework Program, AAFC and CFCRA Canadian Field Crop Genetics Improvement Cluster Project No. J-000305.
References
- Barreto D, Carmona M, Ferrazini M, Zanelli M, Perez BA. 2004. Occurrence and pathogenicity of Fusarium poae in barley in Argentina. Cereal Res Commun. 32:53–60.
- Bourdages JV, Marchand S, Belzile FJ, Rioux S. 2006. Diversity and prevalence of Fusarium species from Quebec barley fields. Can J Plant Pathol. 28:419–425.
- Ceron-Bustamante M, Ward TJ, Kelly A, Vaughan MM, McCormick SP, Cowger C, Leyva-Mir SG, Villaseñor-Mir HE, Ayala-Escobar V, Nava-Díaz C. 2018. Regional differences in the composition of Fusarium head blight pathogens and mycotoxins associated with wheat in Mexico. Int J Food Microbiol. 273:11–19.
- Gilbert J, Haber SM. 2013. Overview of some recent research developments in Fusarium head blight of wheat. Can J Plant Pathol. 35:149–174.
- Gilbert J, Tekauz A. 2000. Review: recent developments in research on Fusarium head blight of wheat in Canada. Can J Plant Pathol. 22:1–8.
- González HHL, Moltó GA, Pacin A, Resnik SL, Zelaya MJ, Masana M, Martínez EJ. 2008. Trichothecenes and mycoflora in wheat harvested in nine locations in Buenos Aires province, Argentina. Mycopathologia. 165:105–114.
- Gräfenhan T, Patrick SK, Roscoe M, Trelka R, Gaba D, Chan JM, McKendry T, Clear RM, Tittlemier SA. 2013. Fusarium damage in cereal grains from Western Canada. 1. Phylogenetic analysis of moniliformin-producing Fusarium species and their natural occurrence in mycotoxin-contaminated wheat, oats, and rye. J Agric Food Chem. 61:5425–5437.
- Guo W, Chen Y, Al-Rewashdy Y, Foran N, Ma BL, Yan W, Frégeau-Reid J, Liu J, Ren C, Pageau D, et al. 2018. Effect of nitrogen fertilization on seed-borne Fusarium species in oat. Can J Plant Sci. 98:38–46.
- Inch S, Gilbert J. 2003. The incidence of Fusarium species recovered from inflorescences of wild grasses in southern Manitoba. Can J Plant Pathol. 25:379–383.
- Ioos R, Belhadj A, Menez M. 2004. Occurrence and distribution of Microdochium nivale and Fusarium species isolated from barley, durum and soft wheat grains in France from 2000 to 2002. Mycopathologia. 158:351–362.
- Kovalsky P, Kos G, Nährer K, Schwab C, Jenkins T, Schatzmayr G, Sulyok M, Krska R. 2016. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize — an extensive survey. Toxins. 8:363.
- Liddell CM. 2003. Systematics of Fusarium species and allies associated with fusarium head blight. In: Leonard KJ, Bushnell WR, editors. Fusarium head blight of Wheat and Barley. St. Paul (Minnesota, USA): APS Press; p. 35–43.
- Lofgren LA, LeBlanc NR, Certano AK, Nachtigall J, LaBine KM, Riddle J, Broz K, Dong Y, Bethan B, Kafer CW, et al. 2018. Fusarium graminearum: pathogen or endophyte of North American grasses? New Phytol. 217:1203–1212.
- Lukanowski A, Sadowski C. 2002. Occurrence of Fusarium on grain and heads of winter wheat cultivated in organic, integrated, conventional systems and monoculture. J Appl Genet. 43:69–74.
- Martin C, Schöneberg T, Vogelgsang S, Mendes Ferreira C, Morisoli R, Bertossa M, Bucheli TD, Mauch-Mani B, Mascher F. 2018. Responses of oat grains to Fusarium poae and F. langsethiae infections and mycotoxin contaminations. Toxins. 10:E47. doi:10.3390/toxins10010047
- McMullen M, Bergstrom G, De Wolf E, Dill-Macky R, Hershman DE, Shaner GE, Van Sanford D. 2012. A unified effort to fight an enemy of wheat and barley: fusarium head blight. Plant Dis. 96:1712–1728.
- McMullen M, Jones R, Gallenberg D. 1997. Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis. 81:1340–1348.
- Mitchell Fetch J, Tekauz A, Wang X, Kumar S, Burt A. 2016. Fusarium head blight of oat -progress in dealing with a sly foe. Proc. Canadian Workshop on Fusarium Head Blight, Ottawa, ON.
- Nelson PE, Toussoun TA, Marasas WFO. 1983. Fusarium species. An illustrated manual for identification. University Park (PA): The Pennsylvania State Univ. Press.
- O’Donnell K. 2000. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia. 92:919–938.
- O’Donnell K, Gueidan C, Sink S, Johnston PR, Crous PW, Glenn A, Riley R, Zitomer C, Colyer P, Waalwijk C, et al. 2009a. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet Biol. 46:936–948.
- O’Donnell K, Humber RA, Geiser DM, Kang S, Park B, Robert VARG, Crous PW, Johnston PR, Aoki T, Rooney AP, et al. 2012. Phylogenetic diversity of insecticolous fusaria inferred from multilocus DNA sequence data and their molecular identification via FUSARIUM-ID and Fusarium MLST. Mycologia. 104:427–445.
- O’Donnell K, Kistler HC, Tacke BK, Casper HH. 2000. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc Natl Acad Sci USA. 97:7905–7910.
- O’Donnell K, Sutton DA, Rinaldi MG, Gueidan C, Crous PW, Geiser DM. 2009b. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United States. J Clin Microbiol. 47:3851–3861.
- Parry DW, Jenkinson P, McLeod L. 1995. Fusarium ear blight (scab) in small grain cereals – a review. Plant Pathol. 44:207–238.
- Rohácik T, Hudec K. 2005. Influence of agroenvironmental factors on Fusarium infestation and population structure in wheat kernels. Ann Agr Env Med. 12:39–45.
- Sala B, Steffenson BJ, Casper HH, Tacke B, Prom LK, Fetch T, Schwarz PB. 1999. Fusarium species pathogenic to barley and their associated mycotoxins. Plant Dis. 83:667–674.
- Samson RA, Hoekstra ES, Frisvad JC, Filtenborg O. 2000. Introduction to food- and airborne fungi. Utrecht (The Netherlands): Centraalbureau voor Schimmelcultures; p. 389.
- Schollenberger M, Drochner W, Müller HM. 2007. Fusarium toxins of the scirpentriol subgroup: a review. Mycopathologia. 164:101–118.
- Schöneberg T, Jenny E, Wettstein FE, Bucheli TD, Mascher F, Bertossa M, Musa T, Seifert K, Gräfenhan T, Keller B, et al. 2018. Occurrence of Fusarium species and mycotoxins in Swiss oats—impact of cropping factors. Eur J Agron. 92:123–132.
- Stenglein SA, Dinolfo MI, Barros G, Bongiorno F, Chulze SN, Moreno MV. 2014. Fusarium poae pathogenicity and mycotoxin accumulation on selected wheat and barley cultivars at a single location in Argentina. Plant Dis. 98:1733–1738.
- Sutton JC. 1982. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can J Plant Pathol. 4:195–209.
- Tamburic-Ilincic L. 2010. Fusarium species and mycotoxins associated with oat in southwestern Ontario, Canada. Can J Plant Sci. 90:211–216.
- Tekauz A, Gilbert J, Stulzer M, Beyene M, Kleiber F, Ghazvini H, Kaethler R, Hajipour Z. 2011. Monitoring Fusarium head blight of oat in Manitoba in 2010. Can Plant Dis Surv. 91:84–85.
- Tekauz A, McCallum B, Ames N, Mitchell Fetch J. 2004. Fusarium head blight of oat - current status in western Canada. Can J Plant Pathol. 26:473–479.
- Tekauz A, McCallum BD, Gilbert J. 2000. Fusarium head blight of barley in western Canada: a review. Can J Plant Pathol. 22:9–16.
- Tekauz A, Mitchell Fetch J, Rossnagel BG, Savard ME. 2008. Progress in assessing the impact of Fusarium head blight on oat in western Canada and screening of Avena germplasm for resistance. Cereal Res Commun. 36(Suppl. B):49–56.
- Thrane U, Adler A, Clasen P-E, Galvano F, Langseth W, Lew H, Logrieco A, Nielsen KF, Ritieni A. 2004. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Int J Food Microbiol. 95:257–266.
- Tucker J, Badea A, Hiebert C, Legge B, McCartney C, Fernando D. 2016. Evaluation of genomic selection as a breeding method for developing FHB resistance and reducing DON accumulation in two-row barley. Proc. 8th Canadian Workshop on Fusarium Head Blight, November 20-22, 2016, Ottawa, ON; p. 72.
- Turkington TK, Clear RM, Demeke T, Lange R, Xi K, Kumar K. 2011. Isolation of Fusarium graminearum from cereal, grass and corn residues from Alberta, 2001–2003. Can J Plant Pathol. 33:179–186.
- Vanheule A, De Boevre M, Moretti A, Scauflaire J, Munaut F, De Saeger S, Bekaert B, Haesaert G, Waalwijk C, van der Lee T, et al. 2017. Genetic divergence and chemotype diversity in the Fusarium head blight pathogen Fusarium poae. Toxins. 9:255.
- Watanabe T. 1994. Pictorial atlas of soil and seed fungi – morphologies of cultured fungi and key to species. Tokyo (Japan): Lewis Publishers; p. 411.
- Xu XM, Parry DW, Nicholson P. 2005. Predominance and association of pathogenic fungi causing Fusarium ear blighting wheat in four European countries. Eur J Plant Pathol. 112:143–154.
- Xue AG, Armstrong KC, Voldeng HD, Fedak G, Babcock C. 2004a. Comparative aggressiveness of isolates of Fusarium species causing head blight on wheat in Canada. Can J Plant Pathol. 26:81–88.
- Xue AG, Chen Y. 2015. Diseases of oat in central and eastern Ontario in 2014. Can Plant Dis Surv. 95:106–107.
- Xue AG, Chen Y. 2018. Diseases of oat in central and eastern Ontario in 2017. Can Plant Dis Surv. 98:115–116.
- Xue AG, Frégeau-Reid J, Rowsell J, Babcock C, Hoekstra GJ, Sparry E. 2004b. Effect of harvesting time on incidence of seed-borne Fusarium spp. in spring wheat in eastern Ontario. Can J Plant Sci. 84:757–763.
- Xue AG, Ho KM, Butler G, Vigier BJ, Babcock C. 2006. Pathogenecity of Fusarium species causing head blight in barley. Phytoprotection. 87:55–61.
- Xue AG, Rowsell J, Ho KM, Chen Y, Chi DT, Manceur A, Ren CZ, Zhang SZ. 2013. Effect of harvesting time on grain contamination with Fusarium spp. and deoxynivalenol in barley in northern and eastern Ontario. Phytoprotection. 93:1–7.
- Yan W, Frégeau-Reid J, Rioux S, Pageau D, Xue AG, Martin R, Fedak G, de Haan B, Lajeunesse J, Savard M. 2010. Response of oat cultivars to Fusarium head blight in eastern Canada. Crop Sci. 50:134–142.
- Yli-Mattila T, Ward TJ, O’Donnell K, Proctor RH, Burkin AA, Kononenko GP, Gavrilova OP, Aoki T, McCormick SP, Gagkaeva TY. 2011. Fusarium sibiricum sp. nov, a novel type Atrichothecene-producing Fusarium from northern Asia closely related to F. sporotrichioides and F. langsethiae. Int J Food Microbiol. 147:58–68.