Abstract
Apple scab (Venturia inaequalis) (AS) and frogeye leaf spot or black rot (Botryosphaeria obtusa) (FLS or BR) are two diseases of apples that affect developing foliage and fruit during summer and cause significant losses in the field and during storage. In this 3-year study (2014–2016), salicylic acid (2-hydroxybenzoic acid) or its analogue (Benzothidiazole or Actigard®) were applied to field-established apple ‘Honeycrisp’ at the tight cluster stage of flower bud development and at late pink bloom as either trunk injection or foliar sprays and to ‘Cortland’ (injection only). AS and FLS leaf infections were assessed 1, 2, and 3 weeks after the second treatment while infections on fruit were assessed at harvest. Main treatment effects were significant (P = 0.05) and both salicylic acid and Actigard® showed less infections on foliage due to AS in 2014 and 2016, and only salicylic acid showed less BR infections on fruit in both years. The cultivar effects were also significant, showing consistently less AS infections on the foliage and fruit of ‘Honeycrisp’, and less FLS infections on ‘Cortland’ foliage. Injection and spray treatments of salicylic acid and Actigard® showed no differences in infections by AS and FLS or BR on foliage or fruit. The method of application × treatment interactions were only significant in 2014; both salicylic acid and Actigard®-treated ‘Cortland’ trees showed less FLS infections on foliage and less BR infections on fruit, and only salicylic acid-treated ‘Honeycrisp’ trees showed less percentage of AS and BR infected fruit. In 2015, both salicylic acid and Actigard® treatments showed less infections of FLS on foliage and lower BR-infected fruit on ‘Cortland’. In 2016, both these treatments reduced incidence of AS and FLS on ‘Cortland’ foliage, and only salicylic acid reduced AS infections on ‘Honeycrisp’ foliage. Further studies are needed to integrate salicylic acid or Actigard® treatments into an apple foliar disease management program with registered fungicides.
Résumé
La tavelure du pommier (Venturia inaequalis) (TP) et la pourriture noire/taches ocellées (Botryosphaeria obtusa) (PN/TO) sont deux maladies du pommier qui s’attaquent au feuillage et aux fruits émergents durant l’été et qui causent d’importantes pertes au champ comme en entrepôt. Durant cette étude de trois ans (2014–2016), de l’acide salicylique (acide 2-hydroxybenzoïque) ou son analogue (Benzothidiazole ou Actigard®) ont été appliqués au champ sur des pommiers ‘Honeycrisp’ établis, au stade du bouton floral vert et à celui du bouton rose avancé, en injection dans le tronc ou en pulvérisation foliaire, et sur des pommiers ‘Cortland’ (injection seulement). Les infections causées par la TP et la TO sur les feuilles ont été évaluées 1, 2 et 3 semaines après le deuxième traitement, tandis que l’infection sur les fruits a été évaluée à la récolte. Les principaux effets des traitements étaient significatifs (P=0,05) et l’acide salicylique ainsi qu’Actigard® ont permis de réduire les infections sur le feuillage causées par la TP en 2014 et 2016, et seulement l’acide salicylique a permis de réduire les infections causées par la PN sur les fruits au cours des deux mêmes années. Les effets découlant du cultivar étaient également significatifs, le feuillage et les fruits du ‘Honeycrisp’ affichant invariablement moins d’infections causées par la TP, et le feuillage du ‘Cortland’ affichant moins d’infections causées par la TO. Les injections et les pulvérisations d’acide salicylique et d’Actigard® n’ont affiché aucune différence quant aux infections causées au feuillage ou aux fruits pas la TP et la TO ou PN. La méthode d’application × les interactions des traitements se sont avérées significatives qu’en 2014. Les pommiers ‘Cortland’ traités avec l’acide salicylique et Actigard® ont affiché moins d’infections causées par la TO sur le feuillage et les fruits, et seulement les pommiers ‘Honeycrisp’ traités avec l’acide salicylique ont affiché un plus faible taux de fruits infectés par la TP et la PN. En 2015, les pommiers ‘Cortland’ traités avec l’acide salicylique et Actigard® ont affiché un plus faible taux d’infection causée par la PN tant sur le feuillage et que sur les fruits. En 2016, les deux traitements ont contribué à réduire l’incidence de la TP et de la TO sur le feuillage des pommiers ‘Cortland’, et seulement l’acide salicylique a permis de réduire l’infection causée par la TP sur le feuillage des pommiers ‘Honeycrisp’. Des études plus poussées sont nécessaires pour intégrer l’utilisation de l’acide salicylique et d’Actigard® aux fongicides homologués dans un programme de gestion des maladies foliaires.
Introduction
Apple scab caused by Venturia inaequalis (Cooke) Winter is a devastating disease of apples worldwide and is more severe in areas with prevailing cool and moist weather conditions during spring and summer months. Frogeye leaf spot or black rot on fruit (Botryosphaeria obtusa (Schwein.) Shoemaker formerly Physalospora obtusa (Schwein.) Cooke, Grevillea) is a relatively minor disease but directly affects the developing apple foliage and fruit. Botryosphaeria obtusa also causes cankers on the trunks and other woody tissues depending on the tree age. Both these polycyclic fungal diseases affect foliage early in the growing season and their infections of fruit render it unmarketable. Botryosphaeria obtusa overwinters in cankers or mummified fruit on the tree whereas V. inaequalis survives from one season to the next in infected leaves and fruit on the orchard floor. All the current preferred commercial cultivars of apples are vulnerable to foliar diseases such as apple scab, frogeye leaf spot or black rot. Breeding for disease resistance and introducing resistance genes into preferred commercial cultivars is a time-consuming process. In addition, gaining market acceptance for new cultivars can be challenging, and new pathogen races can rapidly evolve and overcome the resistance. Therefore, management of apple scab and other fungal foliar diseases has continued to rely heavily on fungicide use.
In general, 10–14 fungicide sprays are usually applied during a growing season based on the weather conditions and disease forecasts for apple disease management. This could result in the application of hundred of tonnes of active ingredients of various fungicides per year. This intensive use of fungicides can lead to deposition of chemical residues on or in fruit, contamination of groundwater and other off-target effects (Ragsdale and Sisler Citation1994; Cuthbertson and Murchie Citation2003), and resistance development in pathogens. In addition, more fungicide products have been banned or their usage restricted due to their exposure risks to farm workers. Therefore, it is essential to supplement existing disease-control strategies in order to achieve consistent and acceptable levels of foliar disease management for apples. It is also in growers’ interest that the use of some effective and safe fungicides be extended through integration with other disease management strategies.
Plants are capable of activating their innate defence response as a strategy for restricting invading microbial pathogens. This induction of resistance, which can also be triggered by chemical and biological agents, has been described as an alternative option of achieving disease control in a relatively short period of time (Lyon Citation2007; Hammerschmidt Citation2009; Vlot et al. Citation2009; Faize and Faize Citation2018). The plant defence inducers could have an effect on the primary inoculum production. Induced resistance can readily be integrated with other disease management strategies. For instance, chemical inducers can be integrated into foliar disease management programs for fruit trees. Priming of apple trees with natural products, such as salicylic acid or its analogues, prior to infection episodes, could lead to a significant reduction in the number of fungicide sprays required for foliar disease management. Disease control through induced resistance could also serve as a strategy for the fungicide resistance management (Ortega et al. Citation1998a). In recent years, interest in induced resistance research pertaining to perennial plants has increased. There have been a few published reports with positive results in reducing apple scab infection and severity by chemical inducers (Ortega et al. Citation1998b; Bini et al. Citation2008; Percival et al. Citation2009; Percival Citation2010).
High market value apple cultivar ‘Honeycrisp’ is of particular interest due to its outstanding quality and flavour. However, it is very susceptible to infection by fungal pathogens such as apple scab, black rot and bitter rot. ‘Cortland’, on the other hand, which is highly susceptible to apple scab (Aldwinckle Citation1974; Dewdney et al. Citation2003), is mostly used as a pollinator. In 2012, more than 50% of the fruit on some ‘Honeycrisp’ trees in a research plot at the Kentville Research and Development Centre, Nova Scotia, Canada was lost to black rot infections on the tree or in cold storage. In these circumstances, growers are very keen in adopting additional and alternative management strategies for fungal foliar diseases such as apple scab or frogeye or black rot on ‘Honeycrisp’ and other apple cultivars. Trunk injections provide an alternative and safe method of delivering pesticides in tree crops (Mota-Sanchez et al. Citation2009; Byrne et al. Citation2014; VanWoerkom et al. Citation2014). In tree fruit crops, trunk injections of plant protection chemicals have been reported to control foliar diseases caused by bacterial (Düker and Kubiak Citation2011; Aćimović et al. Citation2015) and fungal (Percival and Boyle Citation2005) pathogens. Trunk injections of phosphonate are widely used in South Africa as a preventive measure against avocado root rot caused by Phytophthora cinnamomi Rands (Darvas et al. Citation1984).
In a preliminary study in 2012, various inducers of resistance in host plants were evaluated for their activity in apple trees to reduce apple scab severity under field conditions. Foliar and EZ-Ject lance applications of salicylic acid were compared to a non-treated control at the one-half inch green stage of tree development in ‘Honeycrisp’. In that study, salicylic acid treatments applied as foliar sprays or EZ-Ject solid trunk injections significantly reduced the incidence of apple scab in ‘Honeycrisp’ (unpublished data). The EZ-Ject lance injections of salicylic acid or its analogues need to be further evaluated and validated for their effectiveness to reduce infections of apple scab and frogeye or black rot on foliage or fruit in a long-term field study. The objectives of this study were to evaluate the potential use of salicylic acid or its analogue Actigard® to prevent or reduce scab and frogeye or black rot infections by inducing resistance in field-established ‘Honeycrisp’ trees under field conditions. The long-term aim is to integrate these treatments into the foliar disease management programs for apples. A preliminary report of this study was published (Abbasi et al. Citation2016).
Materials and methods
Establishment of apple trees
Apple trees of cultivars ‘Honeycrisp’ and ‘Cortland’ established in a research orchard were utilized for these trials. These trees were planted in 2009 in block 79 of the Kentville Research and Development Centre (KRDC) Research Farm. The efficacy of salicylic acid and Actigard® to reduce apple scab and frogeye or black rot infections of apple foliage and fruit was evaluated in long-term field studies conducted at the over three seasons starting from 2014.
Injection and spray treatments
Salicylic acid (2-hydroxybenzoic acid 99%, Sigma Chemicals USA) and Actigard® (Acibenzolar-S-methyl 50 WG.; Syngenta, USA) were applied as trunk injections and spray treatments on ‘Honeycrisp’ and ‘Cortland’ trees. ‘Cortland’ trees only received injection treatments as there were not enough trees available for spray treatments. There were two or three (2016) replicate trees per treatment of ‘Cortland’ and three or four (2016) of ‘Honeycrisp’. Solid or powder formulations were used for trunk injections that were carried out using an EZ-Ject Lance system. The EZ-Ject lance is used to kill and remove undesirable trees with fast injections of herbicide filled shells at the base of tree, stump or brush. Same shells were used in this study but they were emptied first and then varnish-coated to avoid copper toxicity to apple trees. Shells were then filled with salicylic acid (200 mg) or Actigard® (100 mg) and injected at a depth of 5 mm into the base of each tree trunk of ‘Honeycrisp’ and ‘Cortland’ about 10–15 cm above the ground level. The control trees were injected with empty shells. Spray treatments (1 mM salicylic acid, 0.1 g/L Actigard®, or water) were prepared by first dissolving crystalline salicylic acid or Actigard® granules in ethanol and adjusting the concentration with distilled water. All spray treatments contained 0.095% ethanol. Freshly prepared solutions were applied as foliar spray using a motorized backpack sprayer (Solo) to leaf wetness on ‘Honeycrisp’. Spray and injection treatments were applied twice, once at the tight cluster stage of flower bud development and second at late pink bloom. Fungal infections for the remainder of the growing season were controlled by applying sulphur (Kumulus DF) to control secondary infections while insects were controlled using Trounce and Dipel 2X DF.
Disease severity (scab only) and incidence (scab and frogeye leaf spot) were visually assessed 1, 2, and 3 weeks after the second treatment application from 50 random leaves on each tree. Scab severity was assessed based on the percentage of infected leaf area using nearest percent estimates (Bock et al. Citation2010) and a 0 to 10 scale with some modifications, where 0 = 0, 1 = 0.75, 2 = 1.56, 3 = 3.125, 4 = 6.25, 5 = 12.5, 6 = 25, 7 = 50, 8 = 75, 9 = 87.5, 10 = 93.75 and 11 = 100% leaf area covered with infection. Incidence of scab was then calculated based on number of leaves infected out of 50 leaves assessed for disease severity. Incidence of frogeye leaf spot was also assessed from the same 50 random leaves around a tree. Disease data were presented as the mean incidence and severity based on the average of three weekly assessments. Disease incidence of scab and black rot on fruit was assessed at harvest on a whole tree basis. Incidence on fruit was calculated as the percentage infected fruit of the total number assessed.
Experimental design and statistical analysis
The experimental design was a split plot with application method (injection and spray) on the main plot and treatment (control, salicylic acid and Actigard®) on the sub plot. For each treatment, two or three (2016) replicate trees of ‘Cortland’ and three or four (2016) of ‘Honeycrisp’ were randomly assigned within the main plot. Cultivar was confounded with method of application since ‘Cortland’ received the injection application method only, and therefore, application method included cultivar as a main factor. This was repeated for 3 years and each year was analyzed separately. The analysis was a mixed model with random effects of Block, main plot and subplot; performed using Genstat for Windows 18th Edition (VSN International, Hemel Hempstead, UK. Web page: Genstat.co.uk). The fixed effects were the interactions between application method and treatment. Orthogonal contrasts were used to separate the differences between both application methods and spray types.
Results
Cultivar effects on apple scab and frogeye or black rot on foliage and fruit
The cultivar effects on incidence of scab and frogeye on foliage and scab and black rot on fruit were assessed on untreated ‘Honeycrisp’ and ‘Cortland’ trees during 2014, 2015 (‘Honeycrisp’ foliage not assessed) and 2016. The foliage of untreated control trees of ‘Honeycrisp’ (2014 and 2016) and ‘Cortland’ (2014, 2015 and 2016) was assessed for incidence of scab and frogeye whereas harvested fruit from untreated control trees of both cultivars was assessed for scab and black rot in all 3 years. The cultivar ‘Honeycrisp’ was less susceptible to scab and more susceptible to frogeye or black rot compared to ‘Cortland’ (). Mean (average of three weekly assessments) scab incidence on untreated ‘Honeycrisp’ foliage was in the range of 2‒4% in 2014 and 2016 compared to 34‒36% on untreated ‘Cortland’ foliage in all 3 years. The percentage of fruit with scab infections harvested from untreated ‘Honeycrisp’ trees was in the range of 5‒34% compared to 74‒89% from untreated ‘Cortland’ trees in all three years. Mean frogeye incidence on foliage of untreated ‘Cortland’ trees was in the rage of 13‒20% in all 3 years compared to 34‒36% on foliage of untreated ‘Honeycrisp’ trees in 2014 and 2016. The percentage of fruit with black rot infections harvested from untreated ‘Cortland’ trees was in the range of 0.5‒6% compared to 2‒17% from untreated ‘Honeycrisp’ trees in all three years.
Fig. 1 Cultivar effects on incidence of apple scab and frogeye or black rot on foliage and fruit of field-established untreated ‘Honeycrisp’ and ‘Cortland’ trees during 2014, 2015 and 2016. Mean incidence of scab and frogeye on foliage was assessed in planta three times in June at weekly intervals from 50 random leaves on each tree. The incidence of scab and black rot on fruit was determined at harvest on a whole tree basis. Means are average of three or four (2016) replicates for ‘Honeycrisp’ and two or three (2016) replicates for ‘Cortland’ and error bars represent standard error of mean.
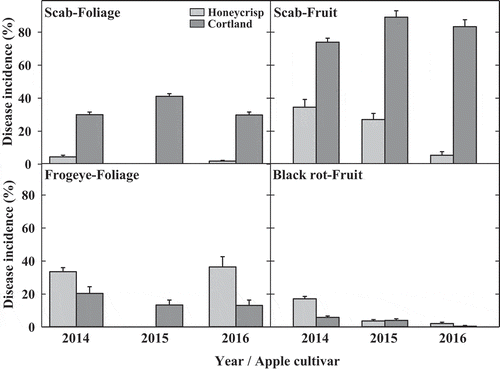
Both cultivars were part of method of application in the experimental design and analysis. Main effects of method of application or cultivars were significant for the mean incidence (P < 0.001 in 2014 and 2016) and mean severity (P < 0.001 in 2014 and 2016) of scab; and for the mean incidence of frogeye (P 0.038 in 2014 and P 0.014 in 2016) on foliage (). Scab incidence and severity on foliage were significantly lower on ‘Honeycrisp’– injected and ‘Honeycrisp’ – sprayed compared to ‘Cortland’ – injected in both years whereas the incidence of frogeye was significantly lower on ‘Cortland’ – injected compared to ‘Honeycrisp’ – injected and ‘Honeycrisp’ – sprayed (). There were no differences between ‘Honeycrisp’ – injected and ‘Honeycrisp’ – sprayed trees for scab incidence and severity and frogeye incidence on foliage (). There was also a significantly (P < 0.001) lower percentage of fruit with scab infections harvested from ‘Honeycrisp’ – injected and ‘Honeycrisp’ – sprayed trees compared to ‘Cortland’ – injected trees in all three years of the study (). Black rot-infected fruit harvested from ‘Cortland’ – injected trees was significantly (P = 0.021) lower compared to ‘Honeycrisp’– injected and ‘Honeycrisp’ – sprayed trees () when the incidence of black rot was high (2014 only). There were no differences between ‘Honeycrisp’ – injected and ‘Honeycrisp’ – sprayed trees for incidence of scab and black rot on fruit ().
Table 1. Main cultivar effects on incidence and severity of apple scab and incidence of frogeye on foliage of field-established treated and untreated ‘Honeycrisp’ and ‘Cortland’ trees a.
Table 2. Main cultivar effects on incidences of apple scab and black rot on fruit of field-established treated and untreated ‘Honeycrisp’ and ‘Cortland’ trees a.
Main effects of salicylic acid and Actigard® on disease incidence on foliage and fruit
There were significant fewer scab infections on foliage with salicylic acid and Actigard® treatments compared to the control in 2014 (P = 0.006) and 2016 (P < 0.001), but there were no treatment differences for frogeye infections (). There were no treatment differences for mean incidence of scab on fruit in any of the 3 years (). Salicylic acid treatment showed less black rot-infected fruit in 2014 when disease level was high and in 2016 when disease level was low ().
Table 3. Main effects of salicylic acid (SA) and Actigard® treatments applied to field-established ‘Honeycrisp’ and ‘Cortland’ trees on apple scab and frogeye incidence on foliage a.
Table 4. Main effects of salicylic acid (SA) and Actigard® treatments applied to field-established ‘Honeycrisp’ and ‘Cortland’ trees on incidences of apple scab and black rot on fruit a.
Effects of salicylic acid and Actigard® on disease incidence on ‘Cortland’ and ‘Honeycrisp’
The method of application × treatment interactions were significant for incidences of scab (P = 0.047) and frogeye (P = 0.036) on foliage and scab (P = 0.10) on fruit in 2014. Although interactions for 2015 and 2016 were not statistically significant, any reduction in incidence of scab and frogeye or black rot on foliage and fruit of ‘Cortland’ and ‘Honeycrisp’ trees by salicylic acid and Actigard® treatments is presented below.
In 2014 trial, ‘Cortland’ trees injected with salicylic acid or Actigard® showed no differences in the mean (average of three weekly assessments) scab incidence on foliage compared to the untreated control trees (). There were also no treatment differences in scab incidence on ‘Cortland’ fruit. ‘Cortland’ trees injected with salicylic acid and Actigard® treatments showed less frogeye infections on leaves and less (non-significant) black rot infections on ‘Cortland’ fruit compared to ‘Cortland’– injected control (). On ‘Honeycrisp’ trees, only the salicylic acid injection treatment reduced scab infections on foliage compared to the injected-control treatment, and the harvested fruit from both salicylic acid or Actigard® injection treatments showed less scab infections (). There were no treatment differences for frogeye infections on ‘Honeycrisp’ foliage, but salicylic acid injection and spray treatments resulted in lower percentage of black rot-infected ‘Honeycrisp’ fruit at harvest compared to the control treatments ().
Table 5. Effects of salicylic acid (SA) and Actigard® treatments on the incidences of apple scab and frogeye or black rot on foliage and fruit of field-established ‘Honeycrisp’ and ‘Cortland’ trees during 2014 a.
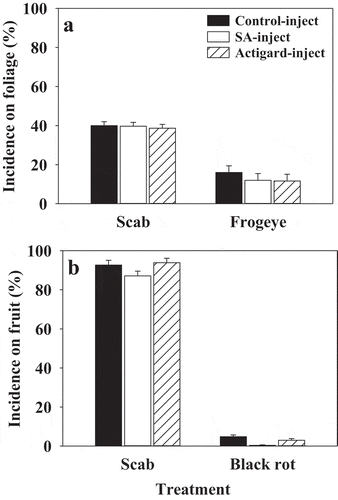
In 2015, there were no treatments differences in mean scab incidence on foliage or fruit of ‘Cortland’ trees injected with salicylic acid or Actigard® treatments (). Both salicylic acid and Actigard® injection treatments showed less (non-significant) frogeye infections on foliage of ‘Cortland’ trees and less percentage of black rot-infected fruit at harvest (). Scab incidence on ‘Honeycrisp’ foliage could not be assessed, and there were no treatment differences in the percentage of scab or black rot-infected fruit on ‘Honeycrisp’ at harvest (data not shown).
Fig. 2 Effects of salicylic acid (SA) and Actigard® injection treatments on incidence of apple scab and frogeye on foliage (a) and incidence of scab and black rot on fruit (b) of field-established ‘Cortland’ trees during 2015. Means are average of two replicates per treatment and error bars represent standard error of mean. SA (2-benzoic acid 99%; 200 mg) or Actigard® (Acibenzolar-S-methyl 50WG; 100 mg) were injected into tree trunk using an EZ-Ject Lance system. Mean incidence of scab and frogeye on foliage was assessed in planta from 50 random leaves on each tree 1, 2, and 3 weeks after the second treatment application. The incidence of scab and black rot on fruit was determined at harvest on a whole tree basis.
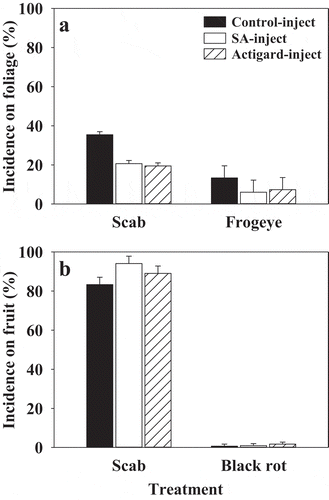
In 2016 trial, both salicylic acid and Actigard® treatments on ‘Cortland’ trees reduced mean incidence of scab and frogeye leaf spot (non-significant) on foliage but there were no treatment differences in the percentage of fruit infected with scab or black rot at harvest (). The incidence of scab was very low and the incidence of frogeye was very high on ‘Honeycrisp’ foliage and there were no treatment differences (data not shown). The percentage of infected (both scab and black rot) fruit harvested from ‘Honeycrisp’ trees was very low and there were no treatment differences (data not shown).
Fig. 3 Effects of salicylic acid (SA) and Actigard® injection treatments on incidence of scab and frogeye on foliage (a), and incidence of scab and black rot on fruit (b) of field-established ‘Cortland’ trees during 2016. Means are average of three replicates per treatment and error bars represent standard error of mean. SA (2-benzoic acid 99%; 200 mg) or Actigard® (Acibenzolar-S-methyl 50WG; 100 mg) were injected into tree trunk using an EZ-Ject Lance system. Mean incidence of scab and frogeye was assessed in planta from 50 random leaves on each tree 1, 2, and 3 weeks after the second treatment application. The incidence of scab and black rot on fruit was determined at harvest on a whole tree basis.
Discussion
Salicylic acid and its functional analogue Actigard® [(Benzothiadiazoles (BTH) or acibenzolar-S-methyl (ASM)] are known to induce plant defence responses against pathogen infections (Vlot et al. Citation2009; Faize and Faize Citation2018). This field study assesses both these products to reduce scab, frogeye or black rot infections on foliage and fruit on field-established apple trees. Although salicylic acid and Actigard® treatments showed less scab infections on foliage, this was not reflected or translated into reduced scab infections on fruit as there were no treatment differences in the percentage of scab-infected fruit in any of the 3 years. It is interesting to note that salicylic acid reduced black rot incidence on fruit when disease pressure was high. Whether the disease-reducing efficacy of induced resistance compounds such as salicylic acid is related to disease pressure levels needs to be investigated.
The main cultivar effects were consistently significant, showing less scab infections on the foliage and fruit of ‘Honeycrisp’ in all three years, and less frogeye infections on ‘Cortland’ foliage. Although both ‘Honeycrisp’ and ‘Cortland’ are considered susceptible cultivars to scab, this study indicated that there is some field tolerance in ‘Honeycrisp’ against early scab infections on foliage, and there is some tolerance in ‘Cortland’ against frogeye infections on foliage. An early study from Vermont, USA also suggested field tolerance in ‘Honeycrisp’ to apple scab (Berkett et al. Citation2013). They compared the incidence of scab on the susceptible cultivars grown under organic disease management programs (all cultivars sprayed with multiple sulphur applications) and found an overall trend of reduced scab incidence on ‘Honeycrisp’. In this study, a significantly lower incidence of scab was found on ‘Honeycrisp’ foliage compared to ‘Cortland’ in both years whereas the incidence of frogeye was significantly lower on ‘Cortland’ foliage compared to ‘Honeycrisp’. This inherent cultivar tolerance i.e. tolerance of ‘Honeycrisp’ to scab and of ‘Cortland’ to frogeye, may also have influenced the results of salicylic acid and Actigard® treatments.
The method of application × treatment interactions were only significant in 2014 for incidences of scab and frogeye on foliage and scab on fruit. Although interactions for 2015 and 2016 were not statistically significant, there was relevant biological significance among various individual components. Frogeye infections tended to be lower on ‘Cortland’ trees treated with salicylic acid or Actigard® in all 3 years. It is possible that these induced resistance compounds are more responsive to a cultivar showing field tolerance or reduced susceptibility to frogeye infections.
While both are known to induce plant defense responses against pathogen infections, salicylic acid and Actigard® show no direct toxicity to pathogens but seem to induce changes in host plant protein levels, including reactive oxygen species (ROS) and pathogenesis-related proteins and possibly others (Vlot et al. Citation2009; Faize and Faize Citation2018). Salicylic acid can confer resistance in several plants against a broad range of pathogens including in tomato against Alternaria solani (Spletzer and Enyedi Citation1999) and cherry against Monilia fructicola (Yao and Tian Citation2005). Actigard® is also effective as a host defence activator against a broad-spectrum of pathogens. For instance, foliar sprays of BTH on potted Japanese pear trees prior to inoculation with conidia of Venturia nashicola showed reduced scab severity on leaves (Ishii et al. Citation2002; Faize et al. Citation2009). The reduced scab severity in Japanese pear was correlated with enhanced expression of several PR genes and proteins (Faize et al. Citation2004) and other plant defence-related compounds, including antioxidants, polygalacturonase-inhibiting proteins (PGIP), mitogen activated protein kinase (MAPK), and leucine-rich repeat receptor-like protein kinase (LRPK) (Faize et al. Citation2007, Citation2009). In this study, liquid chromatography–mass spectrometry (LC-MS) analysis of leaf samples from ‘Cortland’ trees in 2014 and 2015 showed that both salicylic acid and Actigard® resulted in changes in several proteins involved in many metabolic and regulatory pathways (unpublished data).
In general, induced resistance compounds do not provide complete control of foliar diseases and often are comparatively less effective than standard fungicides. However, they still offer an additional management option for foliar diseases that can be easily integrated into existing disease management programs. The timing of application of induced resistance products may be an important factor to achieve a suitable level of disease control. For instance, Percival et al. (Citation2009) sprayed three commercial induced systemic resistance (ISR) products on apple and pear trees from bud break to early fruitlet formation under field conditions. They found that these applications were effective in reducing scab on both fruit tree species, though less effective than the fungicide penconazole. The study suggested that ISR agents may be used as a supplementary strategy for the management of apple or pear scab. It remains to be seen if more than two applications of salicylic acid and Actigard® during flower bud development to late pink bloom stages can provide better control of foliar diseases. In addition to the timing of application, the method used to deliver induced resistance products is another aspect that could be more effectively optimized. However, injection and spray treatments of salicylic acid and Actigard® on ‘Honeycrisp’ trees showed no differences in scab and frogeye infection on foliage or scab and or black rot infections on fruit.
Currently, foliar spraying of fungicides plays a major role in the management of apple scab, frogeye, and other fungal foliar diseases of apple. However, the intensive spraying of fungicides also increases exposure risks to workers. Worker safety during fungicide application is a serious concern for fungicide regulatory agencies and often results in the banning of a fungicide or its usage restricted. An application method with no or low exposure risks to farm workers could revive the use of these banned fungicides, which may be very important for growers. Trunk injection offers an alternative and safer method of delivering plant protection chemicals in tree crops. Trunk injection has been a preferable method of applying chemical pesticides in urban landscapes to reduce exposure risks to the public and non-target organisms. For instance, injections of imidacloprid, emamectin benzoate and other insecticides to tree xylem have been used to control Emerald ash borer (Mota-Sanchez et al. Citation2009). Similarly, trunk injections have also been used in apple trees as an alternative method of applying insecticides and fungicides to control insect pests and apple scab (VanWoerkom et al. Citation2014). The potential of trunk injection in tree fruit crops has also been described as a method for controlling foliar bacterial (Aćimović et al. Citation2015) and other fungal (Percival and Boyle Citation2005) diseases. For example, trunk injections of oxytetracycline were shown to reduce the severity of apple shoot blight caused by Erwinia amylovora (Aćimović et al. Citation2015). Similarly, microcapsule injections of penconazole, pyrifenox and carbendazim fungicides provided a significant degree of protection against apple scab and powdery mildew for two seasons in ‘Crown Gold’ and ‘English oak’ apple trees (Percival and Boyle Citation2005).
Although trunk injections show potential as an alternative delivery method of pesticides, their shortcomings should also be taken into consideration. First, the use of trunk injections may increase the production costs due to increases in labour costs. Second, there is some concern that repeated injections to a tree trunk could lead to irreparable damage to the trunk which, if not healed properly, could serve as an entry point for insects and pathogens. Third, pesticide and fungicide injection doses would need to be optimized for all test products to avoid phytotoxic effects. Finally, the disease-controlling efficacy of a product may vary depending on the uptake and translocation of the chemical from the injection point to target sites at the right time. Clifford et al. (Citation1987) found limited movement of the fungicide imazalil in apple trees with injection at bud break stage and extensive movement with post-harvest injection. However, fungicide injections in May provided a significant degree of protection against apple scab and powdery mildew for two seasons (Percival and Boyle Citation2005). Trunk injections in this study were also performed in May based on some of these previous studies and our preliminary 2012 field study.
In conclusion, salicylic acid and Actigard® treatments consistently showed less scab infections on foliage but not on fruit. There was no treatment effect on frogeye infections, but fruit harvested from salicylic acid-treated apple trees showed less black rot infections in two out of three years. The study also found significant cultivar differences for the incidence of scab and frogeye. There were less scab infections on ‘Honeycrisp’ foliage and less frogeye infections on ‘Cortland’ foliage. The method × treatment interactions were significant for incidences of scab and frogeye on foliage and scab on fruit in 2014 only. Although salicylic acid or Actigard® and other induced resistance products may not provide total and effective control of apple scab or other foliar fungal diseases of fruit trees, they can be useful in an integrated disease management program with standard conventional fungicides.
Acknowledgements
The authors wish to thank KRDC greenhouse and field crew for applying insecticides for insect control, and to summer students for providing technical help. Funding for this study was provided by AAFC Agri-Innovation Program and Organic Federation of Canada.
References
- Abbasi PA, Braun G, Bevis E, Fillmore S. 2016. Reducing initial infections of apple scab and black rot with salicylic acid and Actigard applied as trunk injections or sprays. Phytopathology. 106:S3.1 (abstr.).
- Aćimović SG, Zheng Q, McGhee GC, Sundin GW, Wise JC. 2015. Control of fire blight (Erwiniaamylovora) on apple trees with trunk injected plant resistance inducers and antibiotics and assessment of induction of pathogenesis-related protein genes. Front Plant Sci. 6:1–10.
- Aldwinckle HS. 1974. Field susceptibility of 51 apple cultivars to apple scab and apple powdery mildew. Plant Dis Rep. 58:625–629.
- Berkett LP, Bradshaw TL, Griffith MC, Kingsley-Richards SL, Darby HM, Parsons RL, Moran RE, Garcia ME. 2013. Disease and arthropod evaluation of five apple cultivars under organic management in Vermont, USA. Acta Hortic. 1001:235–248.
- Bini F, Ragaini A, Bazzi C. 2008. Resistance responses induced by the plant growth retardant prohexadione-Ca in apple against scab infections. Ann Appl Biol. 152:19–27.
- Bock CH, Gottwald TR, Parker PE, Ferrandino F, Welham S, van Den Bosch F, Parnell S. 2010. Some consequences of using the Horsfall-Barratt scale for hypothesis testing. Phytopathology. 100:1030–1041.
- Byrne FJ, Krieger RI, Doccola J, Morse JG. 2014. Seasonal timing of neonicotinoid and organophosphate trunk injections to optimize the management of avocado thrips in California avocado groves. Crop Prot. 57:20–26.
- Clifford DR, Gay CN, Gendle P, Holgate ME. 1987. Uptake and movement of the fungicide imazalil following trunk injection into apple and plum trees by a novel, rapid technique. Ann Appl Biol. 110:541–551.
- Cuthbertson AGS, Murchie AK. 2003. The impact of fungicides to control apple scab (Venturia inaequalis) on the predatory mite Anystis baccarum and its prey Aculus schlechtendali (apple rust mite) in Northern Ireland Bramley orchards. Crop Prot. 22:1125–1130.
- Darvas JM, Toerien JC, Milne DL. 1984. Control of avocado root rot by trunk injection with phosetyl-Al. Plant Dis. 68:691–693.
- Dewdney M, Charest J, Paulitz T, Carisse O. 2003. Multivariate analysis of apple cultivar susceptibility to Venturia inaequalis under greenhouse conditions. Can J Plant Pathol. 25:387–400.
- Düker A, Kubiak R. 2011. Stem injection of prohexadione carboxylic acid to protect blossoms of apple trees from fire blight infection (Erwinia amylovora). J Plant Dis Prot. 118:56–160.
- Faize L, Faize M. 2018. Functional analogues of salicylic acid and their use in crop protection. Agronomy. 8:1–20.
- Faize M, Faize L, Ishii H. 2007. Characterization of a leucine-rich repeat receptor like kinase (LRPK) gene from Japanese pear and its expression analysis upon scab infection and acibenzolar-S-methyl treatment. J Gen Plant Pathol. 73:104–112.
- Faize M, Faize L, Ishii H. 2009. Gene expression during acibenzolar-S-methyl-induced priming for potentiated responses to Venturia nashicola Japanese pear. J Phytopathol. 157:137–144.
- Faize M, Faize L, Koike N, Ishizaka M, Ishii H. 2004. Acibenzolar-S-methyl-induced resistance to Japanese pear scab is associated with potentiation of multiple defense responses. Phytopathology. 94:604–612.
- Hammerschmidt R. 2009. Systemic Acquired Resistance. Adv Bot Res. 51:174–209.
- Ishii H, Watanabe H, Tanabe K. 2002. Venturia nashicola: pathological specialization on pears and control trials with resistance inducers. Acta Hortic. 587:613–621.
- Lyon G. 2007. Agents that can elicit induced resistance. In: Walter D, Newton A, Lyon G, editors. Induced resistance for plant defence: A sustainable approach to crop protection. Oxford: Blackwell Publishing; p. 9–29.
- Mota-Sanchez D, Cregg BM, McCullough DG, Poland TM, Hollingworth RM. 2009. Distribution of trunk-injected 14C-imidacloprid in ash trees and effects on emerald ash borer (Coleoptera: buprestidae) adults. Crop Prot. 28:655–661.
- Ortega F, Steiner U, Dehne H-W. 1998a. Induced resistance: a tool for fungicide resistance management. Pestic Sci. 53:193–196.
- Ortega F, Steiner U, Dehne H-W. 1998b. Induced resistance to apple scab: microscopic studies on the infection cycle of Venturia inaequalis (Cke.) Wint. J Phytopathol. 146:399–405.
- Percival GC. 2010. Effect of systemic inducing resistance and biostimulant materials on apple scab using a detached leaf bioassay. Arboric Urban For. 36:41–46.
- Percival GC, Boyle S. 2005. Evaluation of microcapsule trunk injections for the control of apple scab and powdery mildew. Ann Appl Biol. 147:119–127.
- Percival GC, Noviss K, Haynes I. 2009. Field evaluation of systemic inducing resistance chemicals at different growth stages for the control of apple (Venturia inaequalis) and pear (Venturia pirina) scab. Crop Prot. 28:629–633.
- Ragsdale NN, Sisler HD. 1994. Social and political implications of managing plant diseases with decreased availability of fungicides in the United States. Annu Rev Phytopathol. 32:545–557.
- Spletzer ME, Enyedi AJ. 1999. Salicylic acid induces resistance to Alternaria solani in hydroponica. Phytopathology. 89:722–727.
- VanWoerkom AH, Aćimović SG, Sundin GW, Cregg BM, Mota-Sanchez D, Vandervoort C, Wise JC. 2014. Trunk injection: an alternative technique for pesticide delivery in apples. Crop Prot. 65:173–185.
- Vlot AC, Dempsey DA, Klessig DF. 2009. Salicylic acid, a multi-faceted hormone to combat disease. Annu Rev Phytopathol. 47:177–206.
- Yao HJ, Tian SP. 2005. Effects of a biocontrol agent and methyl jasmonate on postharvest diseases of peach fruit and the possible mechanisms involved. J Appl Microbiol. 98:941–950.
