Abstract
The stem and bulb nematode, Ditylenchus dipsaci (Kühn) Filipjev, is a serious threat to many important crops worldwide, including garlic. For this crop, effective detection methods are essential to discard infected seed cloves and avoid contaminated fields. This study compared the efficacy of different extraction methods for soil and garlic tissues infested with this nematode. The Baermann pan, Baermann funnel and sugar flotation were compared on four types of soil (sand, loam, silty clay and muck soil) previously inoculated with D. dipsaci. There was no significant difference between the Baermann methods which recovered an average of 57.2% of the D. dipsaci from soil. The sugar flotation only captured 20.8% of the D. dipsaci added to the soil. Slight variations were observed between soil types, especially when using the Baermann methods to extract nematodes from silty clay or loam. The two Baermann methods were also compared to a sonication technique for the extraction of D. dipsaci from garlic stems, leaves and bulbs. The Baermann methods showed greater sensitivity at low population density while the sonication allowed the recovery of more D. dipsaci at high density. Overall, this study confirmed the validity of the Baermann pan and funnel methods for the extraction of D. dipsaci from soil and garlic tissues. The sugar flotation and sonication procedures yielded significantly less D. dipsaci or had a poorer sensitivity and were not considered adapted for the diagnosis of this species from soil or garlic tissues.
Résumé
Le nématode des tiges et des bulbes, Ditylenchus dipsaci (Kühn) Filipjev, menace gravement de nombreuses cultures partout sur la planète, y compris l’ail. Pour cette culture, des méthodes de détection efficaces sont essentielles pour rejeter les gousses à planter infectées et éviter de contaminer les champs. Cette étude a comparé l’efficacité de différentes méthodes d’extraction permettant de détecter les sols et les tissus d’ail infestés par ce nématode. L’assiette et l’entonnoir de Baermann ainsi que la flottaison au sucre ont été comparés pour quatre types de sols (sable, loam, argile limoneuse et terre tourbeuse) préalablement inoculés avec D. dipsaci. Il n’y a pas eu de différence significative entre les méthodes Baermann qui ont permis de récupérer du sol, en moyenne, 57,2% des nématodes. La méthode de flottaison au sucre a permis de récupérer seulement 20,8% des nématodes ajoutés au sol. De faibles variations ont été observées entre les types de sols, particulièrement lorsque nous avons utilisé les méthodes Baermann pour extraire D. dipsaci de l’argile limoneuse ou du loam. Les deux méthodes Baermann ont également été comparées à la sonication pour extraire D. dipsaci des tiges, des feuilles et des bulbes d’ail. Les méthodes Baermann ont affiché une plus grande sensibilité pour de faibles densités de population, tandis que, pour de fortes densités, la sonication a permis de récupérer plus de nématodes. Dans l’ensemble, cette étude a confirmé le bien-fondé des deux méthodes Baermann pour ce qui est d’extraire D. dipsaci du sol et des tissus d’ail. La flottaison au sucre et la sonication ont permis de récupérer beaucoup moins de nématodes ou étaient moins sensibles, c’est pourquoi elles n’ont pas été considérées comme adaptées à la détection de cette espèce dans le sol ou les tissus d’ail.
Introduction
The stem and bulb nematode, Ditylenchus dipsaci (Kühn) Filipjev, is known to cause damage to a wide range of crops (Sturhan and Brzeski Citation1991). Garlic and onions are particularly affected and yield loss reaching 90% is reported (Abawi and Moktan Citation2010). Strict measures should be taken to avoid the introduction and contamination of the stem and bulb nematode in new fields. Good management practices start with the use of nematode-free seeds and the selection of non-infested fields by testing the soil for the presence of D. dipsaci (Abawi and Moktan Citation2010). Therefore, precise and sensitive extraction methods are needed that are optimized for extraction of D. dipsaci from soil, stem or bulb tissues, in which the nematode can be retrieved from all of these materials. Also, because onions and garlic are grown in very diverse soil types, the extraction method should not be influenced by soil texture. Unfortunately, there is no single method that is effective to extract every nematode species under all conditions (EPPO Citation2013). Some compromises are therefore usually made and the actual extraction efficacy is not always known.
Extraction methods are based on three main principles: specific density, size and shape and motility of the nematodes (Van Bezooijen Citation2006). Many methods are proposed for the extraction of plant-parasitic nematodes from soil samples, but their efficacies are influenced by many factors, such as the nematode species and soil type (Viglierchio and Schmitt Citation1983a). The Baermann funnel and Baermann pan are used for soil and plant samples. These extraction methods rely on the motility of the nematode to actively leave the soil or plant tissue (Van Bezooijen Citation2006). They are inexpensive and simple to perform, and their recovery rate is estimated to be between 50% and 80% (EPPO Citation2013). These methods are recommended by the EPPO (EPPO Citation2013) and are currently used by many diagnostic laboratories. Another method recommended for soil samples is the centrifugal flotation method. This passive technique allows the recovery of both active and inactive nematodes using the difference in specific gravity between nematodes and soil particles (Van Bezooijen Citation2006). This method is more expensive, but was shown to have a higher extraction efficacy on various species (Van Bezooijen Citation2006).
Tangchitsomkid et al. (Citation2015) proposed a new method using ultrasounds to recover nematodes from plant tissues. This method is simple and time effective, but it requires expensive materials. It has been tested with migratory parasites but not with D. dipsaci (Tangchitsomkid et al. Citation2015). All these methods are recommended for different species of nematodes, but very few reports have confirmed their utility for D. dipsaci (e.g. Viglierchio and Schmitt Citation1983b; Roberts and Matthews Citation1995; Qiao et al. Citation2013). Recently, some diagnostic labs have also questioned the efficacy of the Baermann funnel to extract D. dipsaci as this nematode is much longer in length than lesion or root-knot nematodes. Therefore, the efficacy of the Baermann pan, Baermann funnel and sugar flotation needed to be evaluated for the recovery of known numbers of the stem and bulb nematode from different types of soil. The Baermann pan and funnel methods were also compared to a sonication method for the extraction of D. dipsaci from garlic bulbs, stems and leaves.
Materials and methods
Stem and bulb nematode culture
A population of D. dipsaci originating from Québec, Canada was isolated from garlic bulbs with the Baermann pan method (Townshend Citation1963) and reared in Petri dishes on pea sprouts ‘Green Arrow’ growing on Gamborg B-5 medium with minimal organics (3.2 g/L; Sigma-Aldrich, CA) containing agar (8 g/L; Anachemia, CA) and sucrose (20 g/L; BioShop®, CA, USA) according to Poirier et al. (Citation2019). Petri dishes were incubated in the dark at 23°C for three months. Nematodes were then extracted by placing the Gamborg B-5 medium with the pea sprout in a disposable tissue (Kimwipes®, Kimberly-Clark, CA) in another Petri dish filled with tap water. Nematodes were recovered 2 h later in the water, counted under a stereomicroscope using a counting slide to adjust the concentration at 200 larvae/cc of water and used immediately to spike soil samples.
Soil samples and inoculation
Four soil types were tested: sand (97% sand, 1% silt and 2% clay), loam (42% sand, 34% silt and 24% clay), silty clay (14% sand, 39% silt and 47% clay), and muck soil (75% organic matter). They were pasteurized by heating at 70°C for 30 min to ensure the absence of live nematodes and settled to rest for at least three months before use. Volumes of 100 cc of each soil type were measured and transferred into 150 mL plastic containers. The average weight of these 100-cc samples was 119.8 g for sand, 132.8 g for loam, 99.9 g for silty clay and 60.7 g for muck soil. Before inoculation with nematodes, tap water was added to homogenize soil moisture (loam = 25 mL; clay = 20 mL; muck = 15 mL; and sand = 10 mL). Each soil sample was inoculated with 200 nematodes (mixed stages) contained in one cc of water (see above). The inoculum was poured into a hole (1-cm diameter, 2-cm depth) into each soil sample and incubated at room temperature for 2 h before soil extraction. Ten replicates of each soil type were done for each of the three soil extraction methods tested (Baermann funnel, Baermann pan and centrifugal sugar flotation). The experiment was repeated two times (fall of 2017 and 2018) with different batches of soil and D. dipsaci.
Garlic samples
Garlic plants naturally infested with the stem and bulb nematode were harvested at the experimental farm of Agriculture and Agri-Food Canada in Sainte-Clotilde, Québec. Species identification has been confirmed by visual observation of morphological characteristics and molecular methods as described in Poirier et al. (Citation2019). Approximately 1 kg of bulbs were cut in small pieces (~3 mm), mixed together and separated in three homogenous pools. In a separate experiment, stems and leaves were also cut into sections (~1 cm), mixed together and split into three homogenous parts. Nematodes were then extracted from each of these pools separately using the following methods: Baermann funnel, Baermann pan and sonication. Ten replicates were used for each method by randomly sampling 30 g of bulb or 13 g of stem and leaf materials from each pool. The experiment was repeated two times (fall of 2017 and 2018) with different batches of garlic plants.
Baermann funnel method
This method was initially developed by Baermann (Citation1917). Spiked soil (100 cc), infested bulb or stem and leave materials (30 g and 13 g, respectively) were placed on two sheets of paper towel (Bounty©, Proctor & Gamble, USA). These towels were then placed on a loose wire mesh deposited inside a glass funnel ending by a clamped rubber hose. Tap water was added halfway up the mesh to soak the plant materials or soil. After seven days at room temperature for soil samples and three days for plant tissue samples, nematodes were recovered in the water at the bottom of the tube and poured through a 25-µm sieve. Nematodes were then counted under a stereomicroscope using a counting slide.
Baermann pan method
This modification of the Baermann funnel was proposed by Oostenbrink (Citation1954) and uses a dish instead of a funnel. Spiked soil (100 cc), infested bulb (30 g) or stem and leaf (13 g) materials were placed on two sheets (to prevent rupture) of paper towel (Bounty©) previously placed on a 15-cm round mesh placed in a 16-cm round plastic plate (plant pot saucer). Preliminary tests (not shown) indicated that the number of nematodes recovered using one or two sheets of paper towel was not different. One hundred mL of tap water were added halfway up the mesh, soaking the plant materials or soil. The plates were incubated at room temperature for seven days for soil samples and three days for plant tissue samples. The nematodes were then recovered from the water at the bottom of the pan by pouring it through a 25-µm sieve. Nematodes were then counted under a stereomicroscope as described previously.
Centrifugal sugar flotation
This method was proposed by Caveness and Jensen (Citation1955). One hundred cc of spiked soil samples were suspended in 1 L of tap water and sieved through a 200-µm sieve placed on top of a 25-µm sieve. The material collected on the 25-µm sieve was then rinsed with tap water and poured into a 50-mL centrifuge tube. Tubes were centrifuged at 1800g for 4 min and the supernatant was transferred into a new tube for further rinsing and sieving. Pellets were resuspended in a sugar solution (484 g/L) (Van Bezooijen Citation2006) to a volume of 40 mL and vortexed. Then, tubes were centrifuged at 1800g for 1 min and supernatant was pooled with the previous one. Pooled supernatants were poured through a 25-µm sieve and rinsed with tap water to remove the sugar solution. Nematodes were collected from the sieve and count under a stereomicroscope as described previously.
Sonication
Plant tissue samples (30 g of chopped bulb or 13 g of stem and leaf materials) were placed into a 500-mL beaker filled to the top with tap water and placed in a Tabletop Ultrasonic Cleaners (FS220H; Fisher Scientific, USA) at 40 kHz for 40 min. The suspension was poured through a 25-µm sieve and nematodes were counted with a stereomicroscope as described previously.
Statistical analyses
Statistical analyses were performed with RStudio (R3.3.2, RStudio, Inc., USA). Normality of distribution of the data was evaluated with the Shapiro–Wilk test. As the quantitative descriptor did not follow these assumptions or the comparison was between a quantitative and a semi-quantitative descriptor, the Kruskal–Wallis test, a non-parametric one-way analysis of variance, was used (Legendre and Legendre Citation2012). A Dunn test (Dunn Citation1964) with Bonferroni correction was then used for the identification of significantly different groups. These analyses were performed with the function Kruskal_test of the package coin (Hothorn et al. Citation2008) with 100,000 permutations and the function dunn.test of the package dunn.test (Dinno Citation2017).
Results and discussion
Plant parasitic nematodes represent a serious threat to agriculture as thousands of species attack nearly all crops and are responsible for over $100 billion of losses each year (Nicol et al. Citation2011). Diagnostics are challenging due to the absence of specific symptoms, the diversity and complexity of morphological characteristics and the difficulty to extract nematodes from plant or soil samples. Official recommendations exist for the choice of extraction method for most of the species/type of sample, but surprisingly, very few actual validations have been published. In the literature, the Baermann funnel is the most frequently used method to extract D. dipsaci from garlic bulbs (e.g. Roberts and Matthews Citation1995; Qiao et al. Citation2013). This method is also recommended by the EPPO to extract D. dipsaci from seeds and plant tissues (EPPO Citation2013). On the other hand, four different methods are suggested by the EPPO to extract D. dipsaci from soil and the choice is less unanimous in published work. Many authors have used centrifugal flotation, but no valid comparisons were found. Furthermore, even if one method is better than another, the actual recovery rate is mostly unknown or vague.
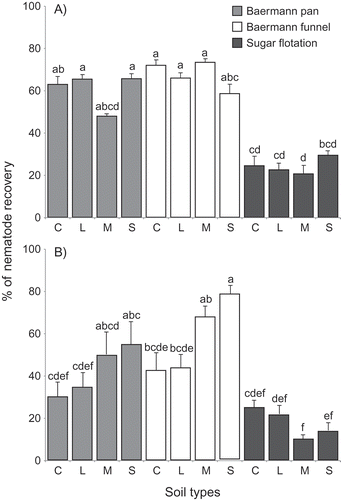
In this study, we demonstrated that the number of nematodes recovered from soil samples varied significantly between different combinations of extraction methods and soil types (p< 0.0001). The Baermann methods (pan and funnel) were clearly superior to the centrifugal sugar flotation to extract D. dipsaci from soil, yielding more than twice the number of nematodes (). In the first repetition, there was no significant difference between the Baermann pan and the Baermann funnel on any type of soil and the average recovery rate for these two methods was 63.8%. Whitehead and Hemming (Citation1965) also found similar numbers of nematodes (of other species) between clay and sand samples. However, in the second repetition, with different batches of soil and D. dipsaci, more variations were observed between soil types although significant differences were only found for sand compared to clay or loam using the Baermann funnel. The extraction efficacy remained the same with sand and muck soil, but was lower in clay and loam with higher variance between the 10 replicates. The overall recovery rate for the Baermann methods was 57.2%. This illustrates the limitation of these methods based on nematode motility in complex substrate such as soil. For the sugar flotation method, recovery was much less in both repetitions (20.8%) in comparison with the other two methods. Within each method, no significant differences among soil types were found although slight variations were observed. In sandy soil, the overall recovery rate of the added nematode inoculum was 68% for the Baermann funnel, 60.4% for the Baermann pan and only 21.5% for the sugar flotation. Miyagawa and Lear (Citation1970) reported a recovery rate of around 50% in sandy soil using the Baermann funnel. These results confirmed the superiority of the Baermann methods over sugar flotation for the extraction of D. dipsaci from soil, but also illustrates that a significant portion of the D. dipsaci present in soil is unlikely to be detected.
Fig. 1 Efficacy of three different methods for the extraction of Ditylenchus dipsaci from four soil types expressed as the percentage of recovery from artificially infested samples. The experiment was carried out in 2017 (A) and 2018 (B). Soil types are: C = silty clay; L = loam; M = muck; S = sand. Error bars represent the standard errors of 10 replicates. Letters above each bar signify statistical differences according to Dunn test.
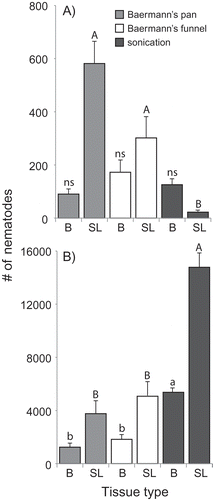
When tested with garlic bulbs or stems and leaves as the starting material, no significant difference was observed between the Baermann extraction methods at low or high population densities (). On the other hand, the sonication method yielded very contrasting results. At low density (first repetition), very few D. dipsaci were extracted from stems and leaves compared to the Baermann methods. This suggests that the new method proposed by Tangchitsomkid et al. (Citation2015), using ultrasound has only limited potential. However, at high density (second repetition), sonication recovered three times more D. dipsaci than the Baermann methods in both the bulbs or stems and leaves. Sensitivity of detection is crucial for the diagnostics and management of D. dipsaci as the presence of only a few nematodes can have consequences. Because sonication yielded very few nematodes from stems and leaves at low density, the Baermann method is preferred for the analysis of these types of materials also. Another study that compared different approaches for the extraction of D. dipsaci from seeds of the perennial plant Brachiaria ruzizensis, a common forage grass in Brazil, also identified the Baermann funnel as the best method (Jonsson Citation2015).
Fig. 2 Number of nematodes extracted from garlic bulbs or from stems and leaves using three different extraction methods. The experiment was carried out in 2017 (A) and 2018 (B). Tissue types are: B = bulbs; SL = stems and leaves. Extractions were done on 30 g of garlic bulbs or 13 g of stems and leaves cut in small pieces from a homogenous pool of naturally infested plants. Error bars represent the standard errors of 10 replicates. Letters above each bar signify statistical differences according to Dunn test.
In our study, no significant differences were found between the Baermann funnel and the Baermann pan. Because the Baermann pan is more convenient and allows the concurrent analysis of hundreds of samples, it is preferred for all large-scale experiments. However, some limitations associated with this method were identified, including its high variability between different labs, which limits its use for large-scale quantitative surveys (den Nijs And van den Berg Citation2013). There appear to be many differences (water source and towel types, number of layers, duration, etc.) in the way each laboratory conducts nematode analyses, so a standardized method should be proposed to reduce the variability in the results. For the diagnostics of D. dipsaci, this should not be problematic as any positive detection, even a very low number of nematodes, should be considered as a threat. Thus, a presence or absence result is most of the time sufficient. Several other available methods, e.g. elutriation, were not tested in this work. Some are not adapted for this species while others have shown poor results or require excessive labour time or expensive equipment. Overall, the Baermann pan was a valid option for the rapid analysis of seed cloves or soil for the presence of D. dipsaci.
Additional information
Funding
References
- Abawi GS, Moktan K. 2010. Bloat nematode problem on garlic: symptoms, distribution, and management guidelines. Cornell Univ, Geneva, NY. 14456:1–14.
- Baermann G. 1917. Ein einfache methode zur auffindung von anklyostomum (nematoden) larven in erdproben. [A simple method for the detection of nematode larvae in soil samples.]. Genees. Tijdschr Nederlandsch-Indië. 57:131–137.
- Caveness FE, Jensen HJ. 1955. Modification of the centrifugal flotation technique for the isolation and concentration of nematodes and their eggs from soil and plant tissue. Proc Helminthol Soc Wash. 22:87–89.
- den Nijs L, van den Berg W. 2013. The added value of proficiency tests: choosing the proper method for extracting Meloidogyne second-stage juveniles from soil. Nematology. 15:143–151.
- Dinno A. 2017. Package ‘dunn.test’. https://cran.r-project.org/web/packages/dunn.test/dunn.test.pdf.
- Dunn OJ. 1964. Multiple comparisons using rank sums. Technometrics. 6:241–252.
- EPPO: European and Mediterranean Plant Protection Organization. 2013. PM 7/119 (1) Nematode extraction. EPPO Bull. 43:471–495.
- Hothorn TH, Hornik K, van De Wiel MA, Zeileis A. 2008. Implementing a class of permutation tests: the coin package. J Stat Software. 28:1–23.
- Jonsson A. 2015. Methods for extracting plant pathogenic nematodes from Brachiaria seed [Master’s thesis]. Swedish University of Agricultural Sciences, Department of Ecology.
- Legendre P, Legendre L. 2012. Numerical ecology. Third English ed. Amsterdam: Elsevier Science; p. 990.
- Miyagawa ST, Lear B. 1970. Factors influencing survival of Ditylenchus dipsaci (Kühn, 1857) in soil. J Nematol. 2:139–142.
- Nicol JM, Turner SJ, Coyne DL, Den Nijs L, Hockland S, Maafi ZT. 2011. Current nematode threats to world agriculture. In: Jones JT, Gheysen G, Fenoll C, editors. Genomics and Molecular Genetics of Plant–nematode Interactions. Heidelberg: Springer; p. 21–44.
- Oostenbrink M. 1954. Een doelmatige methode voor het toetsen van aaltjesbestrijdingsmiddelen in grond met Hoplolaimus uniformis als proefdier. Mededelingen Landbouwhogeschool Gent. 19:377–408.
- Poirier S, Dauphinais N, Van Der Heyden H, Bélair G, Gravel V, Mimee B. 2019. Host range and genetic characterization of the stem and bulb nematode, Ditylenchus dipsaci, from Eastern Canada. Plant Dis. In Press. doi:10.1094/PDIS-07-18-1201-RE
- Qiao Y, Zaidi M, Badiss A, Hughes B, Celetti MJ, Yu Q. 2013. Intra-racial genetic variation of Ditylenchus dipsaci isolated from garlic in Ontario as revealed by random amplified polymorphic DNA analysis. Can J Plant Pathol. 35:346–353.
- Roberts PA, Matthews WC. 1995. Disinfection alternatives for control of Ditylenchus dipsaci in garlic seed cloves. J Nematol. 27:448–456.
- Sturhan D, Brzeski MW. 1991. Stem and bulb nematodes, Ditylenchus spp. in Manual of agricultural nematology by Marcel Dekker. New York: WR Nickle; p. 423–464.
- Tangchitsomkid N, Chanmalee T, Hodda M. 2015. Ultrasonic extraction of plant-parasitic nematodes from plant roots. Austral Plant Pathol. 44:87–96.
- Townshend J. 1963. A modification and evaluation of the apparatus for the Oostenbrink direct cottonwool filter extraction method. Nematologica. 9:106–110.
- Van Bezooijen J. 2006. Methods and techniques for nematology. Wageningen (The Netherlands): Wageningen University.
- Viglierchio DR, Schmitt RV. 1983a. On the methodology of nematode extraction from field samples: comparison of methods for soil extraction. J Nematol. 15:450–454.
- Viglierchio DR, Schmitt RV. 1983b. On the methodology of nematode extraction from field samples: baermann Funnel Modifications. J Nematol. 15:438–444.
- Whitehead AG, Hemming JR. 1965. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann Appl Biol. 55:25–38.
