Abstract
The objective of this study was to assess harvested dried inflorescences (buds) of cannabis (Cannabis sativa L., marijuana) for fungal presence and diversity. Samples from drying rooms of three licenced facilities in British Columbia were tested repeatedly during 2017–2019. A swab method was used, wherein sterile cotton swabs were gently swabbed over bud surfaces and directly streaked onto potato dextrose agar containing 140 mg L−1 streptomycin sulphate. Petri dishes were incubated at 21–24°C for 5–6 days and the fungal colonies that developed were recorded. The testing was repeated to provide >40 cumulative sampling times over a 2-year period. Representative colonies of each unique morphological type were identified to genus and species by PCR of the ITS1-5.8-ITS2 region of rDNA and sequence analysis. Among 34 different fungal species identified, the most prevalent were Penicillium (comprising 17 different species), followed by species of Cladosporium, Botrytis, Aspergillus, Fusarium, Talaromyces and Alternaria. All samples had several fungal species present and the number and composition varied at different sampling times and within different facilities. The swab method provided a qualitative assessment of viable mould contaminants on cannabis buds and reflected the diversity of mycoflora present, many of which are previously unreported. Fungi on cannabis buds may originate from spores released from diseased or decomposing plant materials, from growing substrates used in cannabis production, or as airborne contaminants in post-harvest trimming and drying rooms. Samples of dried buds exposed to electrobeam (e-beam) radiation treatment had no detectable fungal contamination when assessed using the swab method.
Résumé: Le but de cette étude était d’évaluer des inflorescences séchées (cocottes) de cannabis (Cannabis sativa L., marijuana) afin d’y déceler des agents fongiques et d’en constater la diversité. Des échantillons provenant de séchoirs de trois installations autorisées de la Colombie-Britannique ont été testés à plusieurs reprises, de 2017 à 2019, par écouvillonnage au cours duquel la surface des cocottes était légèrement frottée avec des écouvillons de coton stérile. Par la suite, la matière ainsi prélevée était directement déposée sur de la gélose dextrosée à la pomme de terre contenant 140 mg L−1 de sulphate de streptomycine. Les boîtes de Pétri ont été incubées pendant 5 à 6 jours de 21 à 24°C, et les colonies fongiques qui s’y développaient étaient notées. Les tests ont été répétés afin de fournir plus de 40 temps d’échantillonnage cumulatifs sur 2 ans. Des colonies représentatives de chaque type morphologique unique ont été identifiées au genre et à l’espèce par PCR des régions ITS1-5,8-ITS2 de l’ADNr et analyse des séquences. Parmi les 34 différentes espèces fongiques identifiées, la plus courante était Penicillium (comportant 17 espèces différentes), suivie par des espèces de Cladosporium, de Botrytis, d’Aspergillus, de Fusarium, de Talaromyces et d’Alternaria. Tous les échantillons possédaient un certain nombre d’espèces fongiques et le nombre ainsi que la composition variaient en fonction des différents temps d’échantillonnage et de l’installation d’où ils provenaient. L’écouvillonnage a fourni une évaluation qualitative des moisissures viables sur les cocottes de cannabis et a reflété la diversité des champignons qui y étaient établis, parmi lesquels de nombreux n’avaient pas encore été rapportés. Les champignons trouvés sur les cocottes de cannabis peuvent provenir de spores relâchées par du matériel végétal infecté ou en décomposition ou par les substrats de croissance utilisés pour la production du cannabis ou être des contaminants en suspension dans l’air des salles de découpe et des séchoirs. Des échantillons de cocottes séchées traités par irradiation par faisceau d’électrons n’affichaient aucune contamination fongique lorsque évalués par écouvillonnage.
Introduction
Cannabis (Cannabis sativa L., marijuana) is harvested for the inflorescences (buds) produced on pistillate (female) plants which are subsequently dried to a moisture content of around 8–10% prior to packaging and distribution to the recreational and medical cannabis sectors in Canada and elsewhere (Small Citation2017; Rodriguez & Munir Citation2019). Quality assurance of the final product requires licenced producers in Canada to meet the standards for total yeast and fungal contamination levels, as well as pesticide and mycotoxin levels, as specified in Health Canada regulations (Health Canada Citation2013). Quality assurance measurements need to be performed by certified laboratories that provide a quantitative confidential analysis for each sample submitted (European Pharmacopoeia (EP) Citation2015). Products of dried cannabis buds which exceed the limit of 1 000 total colony-forming units of yeast and mould per gram (dry weight) are rejected for sale. Prior to licenced producers performing this final product testing, however, knowledge of potential fungal contaminants on dried cannabis buds and identification of the fungal species present may provide valuable information on potential mould problems that could arise.
A few previous studies (McPartland Citation1994; McKernan et al. Citation2016b; McPartland & McKernan Citation2017) have described the fungal species that are present on dried cannabis buds using culture methods or sequencing of the ITS2 region of ribosomal DNA, respectively. A large number of yeast and bacterial species have also been detected on cannabis buds (Thompson et al. Citation2017). Diverse Penicillium and Aspergillus species were reported to be present in dispensary-derived samples from Amsterdam and Massachusetts by McKernan et al. (Citation2016b). These genera of fungal contaminants were also described to be present on cannabis grown in various parts of the world (McPartland Citation1996; McPartland & McKernan Citation2017). No similar studies describing the fungal species that could potentially be present on dried cannabis buds grown in Canada have been conducted. While total yeast and fungal contaminant levels are routinely analysed by certified laboratories prior to sale of dried cannabis, there has been no prior research on reporting the actual species that are present.
In this study, the diversity of viable fungi present on cannabis buds was assessed using a swab method which was performed on random samples of cannabis in drying rooms over a two-year period. The fungi were subcultured and identified to genus and species level using ITS1-5.8S ITS2 sequence analysis. A total of 34 fungal taxa were identified, many of which have not been previously reported to be present on cannabis buds. While some are common airborne contaminants found in indoor environments, other species have the potential for mycotoxin production if present at high population levels (Kabak & Dobson Citation2017).
Materials and methods
Sampling method
The drying rooms of three licenced production facilities, all located in the Fraser Valley of British Columbia, were included for sampling in this study. Each room was designed to meet commercial industry regulatory standards and equipped with multiple drying racks on which harvested cannabis buds were placed for 5–7 days to gradually reduce moisture content to around 10% (). All rooms were equipped with HVAC systems (Heating, Ventilation and Air-Conditioning). Presence of fungal contaminants was assessed at various times during 2017–2019 using a swab procedure. Sterile cotton swabs (Q-tip brand) were gently wiped across the surfaces of buds selected arbitrarily from different racks (). In some instances, visible fungal growth on buds was specifically targeted for sampling (). The swabs were immediately streaked in a zig-zag pattern across the surface of agar (potato dextrose agar containing 140 mg L−1 of streptomycin sulphate, PDA+S) in 100 × 15 mm Petri dishes. One dish was used for each swab and repeated for 20 replicate swab samples taken in each room from different trays at each sampling time. The samples originated from different cannabis strains (genotypes) in production at the time of sampling. With over 40 cumulative sampling times, more than 1000 Petri dishes were assessed in this study. The Petri dishes were brought to the laboratory where they were incubated under ambient conditions (temperature range of 21–24°C with 10–14 h day−1 fluorescent lighting) for 5–6 days.
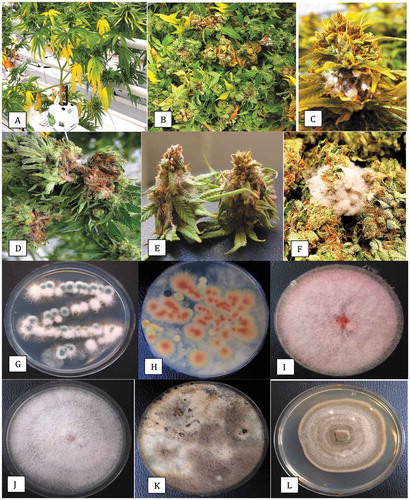
Fig. 1 Sampling of dried cannabis buds for the presence of fungi and colonies recovered on potato dextrose agar with 140 mg L−1 streptomycin sulphate (PDA+S). (a) Dried buds on rack prior to packaging. (b) Close-up of buds that were sampled. (c) Brown discolouration of buds from which Alternaria alternata and Botrytis cinerea were recovered. (d) Mould development on buds from which Penicillium spp. were recovered. (e) The swab method used to recover surface fungi. A cotton swab was gently wiped across the surface of the bud and then streaked across PDA+S. (f) Development of Aspergillus flavus (green) and Penicillium sp. (blue) from a bud streak. (g) Development of Cladosporium westeerdijkieae (brown) and some colonies of Penicillium (green). (h) Development of Botrytis cinerea (white, labelled (a)) and Penicillium citrinum (b). (i) Colonies of Aspergillus niger (a) and Penicilliumspathulatum(b). (j) Colonies of Penicillium pancosmium (a) and P. olsonii (b). (k) Colonies of Fusarium oxysporum (a), A. flavus (b) and Talaromyces pinophilus (c). Colonies of T. radicus (a), P. glabrum (b) and P. chrysogenum (c). All photographs of Petri dishes were taken after 5 days of incubation at 21–24°C

In one production facility, cannabis buds that had been treated with electrobeam radiation (4–6 kilogray, kGy) in two successive exposures to reduce overall mould contamination rates after the drying phase were sampled. These samples were vacuum-sealed and were included in the swab test to provide a comparison with untreated buds sampled at the same time. Ten replicate Petri dishes were used and the assay was repeated twice using different samples. Standard errors of the means were calculated from the data.
Mould presence in air samples
To assess the presence of fungal spores within the growing environment and in drying rooms, 9-cm diameter Petri dishes containing PDA+S were placed with the lids removed on benches in the areas of interest. The dishes were left for 60 min and then the lids were replaced and brought back to the laboratory. A minimum of 10 replicate dishes was included at each sampling location. The sampling was conducted in one indoor growing environment and in one greenhouse facility over the same time period, as well as in several drying rooms in one facility. Control dishes were placed in similar locations with the Petri dish lids left on. The sampling was conducted at various times during the study period (2017–2019). Fungal colonies that developed after 5–7 days were counted and representative ones were subcultured for identification as described below.
Identification of fungal species
Fungal colonies that grew on each Petri dish were examined and photographed and placed into morphologically distinct groups after visual examination of colony colour, size, mycelial growth and spore production. Representative colonies were transferred onto fresh PDA+S dishes by removing mycelium from the colony edge with a transfer needle or by picking up spores from the colony surface using a Q-tip and streaking that across a fresh agar plate. Once cultures were established, DNA was extracted from 10–50 mg of fungal mycelium scraped off the surface of colonies using the QIAGEN DNeasy Plant Mini Kit (cat. no. 69 104). Species-level identification was done by conducting PCR of the ITS1-5.8S-ITS2 region of ribosomal DNA with 2–50 ng µL−1 DNA. The ITS region was amplified using the universal eukaryotic primers UN-UP18S42 (5′-CGTAACAAGGTTTCCGTAGGTGAAC-3′) and UN-LO28S576B (5′-GTTTCTTTTCCTCCGCTTATTAATATG-3′) (Schroeder et al. Citation2006) to produce a DNA template for sequencing. PCR conditions were as follows: initial denaturation at 94°C for 3 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 45 s, and extension at 72°C for 2 min, and a final extension at 72°C for 7 min, followed by 4°C hold. The PCR product (~650 bp) was cut from the gel and collected using the MinElute Gel Extraction Kit (cat. no. 28 604), and 8 µL of DNA was sent to Eurofins Genomics (https://www.eurofinsgenomics.com/en/home/) for sequencing. The resulting sequences were compared with the ITS1-5.8S-ITS2 sequences from the National Centre for Biotechnology Information (NCBI) GenBank database that showed the highest sequence match (over 99%) to confirm species identity. A total of 140 representative isolates were sequenced. For each unique morphological colony type that was identified to genus and species by molecular analysis, the presence/absence was recorded on each Petri dish originating from the swabbed samples using photographs or further examination of the dishes. The cumulative number of Petri dishes containing each species was then used to obtain an overall frequency of recovery.
Effect of temperature on growth of select fungi from cannabis buds
To assess the effects of temperature on colony growth, 9-cm diameter Petri dishes containing 25 mL of PDA were inoculated with a 5 mm2 mycelial plug taken from 1-week-old cultures of B. cinerea, F. oxysporum and F. sporotrichioides recovered from cannabis buds during sampling conducted in 2019. Replicates of five Petri dishes of each of the three fungi were placed in temperature-controlled incubators maintained at 5, 10, 15, 20, 25, 30 and 35°C. The actual recorded temperatures were within +/− 1°C. The diameters of all colonies were measured after 3, 5 and 7 days of incubation from two perpendicular measurements of each colony and averaged. The experiment was conducted twice.
Pathogenicity on detached inflorescences
For pathogenicity testing, one isolate each of B. cinerea, F. oxysporum and F. sporotrichiodes, all originating from diseased cannabis bud tissues, and an isolate of F. graminearum originating from crown tissues of field-grown hemp plants showing symptoms of tissue discolouration and wilting in 2019, were used. Sequences of these isolates have been deposited in GenBank as MN853356, MN784796, MN853357 and MN853360, respectively. Detached fresh cannabis buds of strain ‘Purple God’ were placed inside a plastic container lined with moistened paper towels and an 8-mm diameter plug taken from a 10-day-old culture of each isolate grown on PDA was placed mycelial side down on the surface of the detached bud. There were two replicates for each isolate. The container was placed at ambient room temperature (21–24°C) and assessed after 5 days for extent of mycelial colonization of the tissues and photographed. The experiment was conducted three times.
Results and discussion
Sampling of cannabis buds
Representative dried cannabis buds that were sampled in this study are shown in . A close examination of some batches showed they had brown-black surface discolouration (), from which Alternaria alternata and Botrytis cinerea were isolated and identified using PCR. Other buds had visible surface mould growth, from which Penicillium spp. () were recovered. The swab method using a sterile Q-tip to transfer surface-inhabiting fungi () onto PDA dishes produced visible fungal growth after 5–6 days (). The addition of 140 mgL−1 streptomycin sulphate prevented bacterial growth.
Identification of fungal species
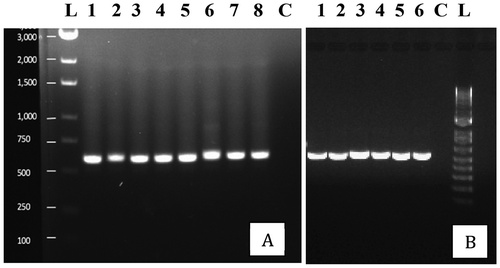
The morphologically different colony types on Petri dishes were subcultured and subsequently identified by PCR of the ITS1-5.8S-ITS2 region and comparison to BLAST. The universal ITS primers used in this study produced a band size of around 650 bp for all of the various genera and species of fungi (). The range of fungal species confirmed to be present following sequencing is presented in . The frequencies represent the presence/absence of colonies of each species observed on the PDA plates i.e. a frequency of <1% indicates that the colonies were seen on fewer than 10 dishes out of over 1000 examined. Representative colonies of the different species that were identified are shown in . These included Aspergillus flavus (), Cladosporium westeerdijkieae (), Botrytiscinerea and Penicillium citrinum (), Aspergillus niger (), Penicillium spathulatum (), Penicillium pancosmium and P. olsonii (), F. oxysporum and Talaromyces pinophilus (), and Talaromyces radicus, P. glabrum and P. chrysogenum ().
Table 1. Recovery of fungal species from cannabis inflorescences (post-harvest dried buds). The percentage of total Petri dishes which contained at least one colony of the identified species is presented. The closest GenBank sequence match to the identified species is given
Fig. 2 PCR band of ~650 bp size obtained using the universal eukaryotic primers UN-UP18 S42 and UN-LO28 S576B for fungi isolated from cannabis inflorescences. Left panel (a) – L = molecular weight ladder; 1 = Penicillium glabrum; 2 = P. manginii; 3 = Talaromyces pinophilus; 4 = P. olsonii; 5 = P. spathulatum; 6 = P. pancosmium; 7 = P. simplicissimum; 8 = P. terrigenum; C = control (no DNA). Right panel (b) – 1 = Botrytis cinerea; 2 = Alternaria alternata; 3 = Fusarium oxysporum; 4 = F. proliferatum; 5 = F. sporotrichiodes; 6 = F. graminearum; C = Control (no DNA); L = molecular weight ladder
Source of fungal contaminants
The fungi that were present on cannabis buds could have originated from a range of sources during the plant production cycle, as follows: (1) from spores released from diseased or decomposing plant materials during growth or at harvest; (2) from the growing substrates used in cannabis production, e.g. coconut coir (Punja et al. Citation2019); (3) as airborne contaminants in the post-harvest trimming and drying rooms; and (4) as internally borne endophytes that are released during the trimming process that fractures stem tissues (Punja et al. Citation2019).Flowering plants in one of the three licenced facilities included in this study showed symptoms of yellowing of the lower leaves that was determined, following isolation and identification, to be caused by Fusarium oxysporum infection of the crown tissues (). In addition, F. oxysporum was also isolated from freshly harvested and visibly diseased inflorescences in the same facility (). From diseased inflorescences with bud rot symptoms (), B. cinerea was isolated. On additional inflorescences that were harvested and incubated in a moist environment, mycelium of F. sporotrichiodes and F. proliferatum developed (). Buds in a drying room showed prolific mycelium of F. sporotrichiodes in the early stages of the drying process (). These observations confirm that diseased plants can produce inoculum that can be spread to the inflorescence and subsequently be detected as viable colonies on dried buds using the swab test ().
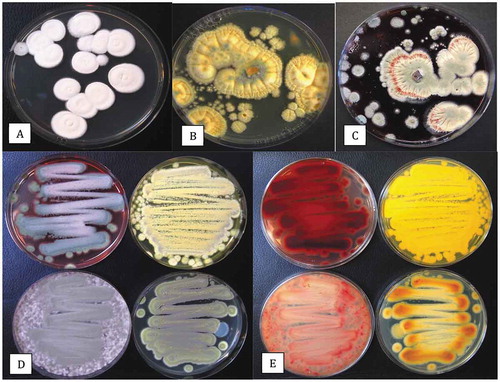
Fig. 3 Disease symptoms on greenhouse-grown cannabis plants and recovery of pathogens from diseased inflorescences. (a) Yellowing of lower leaves due to crown infection by Fusarium oxysporum. (b) Infected inflorescences (buds) from which F. oxysporum was recovered. (c) Close-up of diseased inflorescence showing mycelial growth of F. oxysporum under greenhouse conditions. (d) Symptoms of bud rot caused by Botrytis cinerea showing necrotic tissues and mycelial growth. (e) Mycelial growth of Fusarium sporotrichioides on harvested bud in the drying room. (f) Mycelial growth on freshly harvested inflorescences which were incubated under moist conditions in the laboratory for 5 days. The bud on the left has F. sporotrichioides and the one on the right has F. proliferatum. (g) Development of F. oxysporum from a bud streak. Blue-green colonies are those of P. spathulatum. (h) Development of F. proliferatum (pink-orange colonies) from a bud streak. The sparse white mycelium also growing on edges of the plate is B. cinerea. (i) Colony of F. sporotrichioides. (j) Colony of F. oxysporum. (k) Colony of B. cinerea. (l) Colony of Alternaria chlamydosporigena. Colonies shown in (i–k) were photographed after 10 days of growth on PDA at 21–24°C
Morphology and pathogenicity of fungal contaminants
Representative colonies of F. proliferatum, F. sporotrichiodes, F. oxysporum, B. cinerea and Alternaria chlamydospora that were recovered on Petri dishes in this study are shown in . All three Fusarium species are known to infect a wide range of plant species, including vegetable crops such as tomato, cucumber and pepper. In a previous study, both F. oxysporum and F. solani were shown to be capable of causing infection of cannabis inflorescences (Punja Citation2018). Inoculation of detached cannabis buds in the present study with mycelial plugs of B. cinerea, F. sporotrichiodes and F. oxysporum (from diseased buds) and an isolate of F. graminearum (originating from diseased crowns of hemp plants), all showed extensive mycelial development and caused necrosis of tissues ().The most extensive growth was observed with F. graminearum and F. sporotrichiodes. A previously unreported fungus, Alternaria chlamydosporigena, was recovered from buds with blackened tissues; this species has been reported to cause a leaf spot disease on tomato plants (Sadeghi and Mirzaei Citation2018). The pathogenicity of this species, as well as those of several other Alternaria species (), on cannabis buds remains unconfirmed. Alternaria species have been previously isolated from hemp tissues (McPartland Citation1996; Scott et al. Citation2018).
Fig. 4 Inoculation of detached inflorescences of cannabis with a mycelial plug of each of four pathogens. Buds were incubated in a moist chamber for 5 days. (a) Botrytis cinerea. (b) Fusarium oxysporum. (c) F. sporotrichioides. (d) F. graminearum. The relative growth rates of the pathogens can be seen colonizing the tissues

Effect of temperature on growth of select fungi from cannabis buds
The response of radial mycelial growth of B. cinerea, F. sporotrichiodes and F. oxysporum to different temperatures is shown in . Growth of B. cinerea was comparable at 5–25°C but completely ceased at 30°C, while F. sporotrichiodes and F. oxysporum showed greatest growth at 15–30°C and 15–35°C, respectively. Botrytis cinerea causes inflorescence (bud) rot during growth of cannabis plants (Punja et al. Citation2019) which subsequently also manifests as a post-harvest problem. It is the most widely reported pathogen affecting bud quality (Jerushalmi et al. Citation2020). In the present study, the overall frequency of recovery of B. cinerea using the swab method was 11–20%. This frequency could have been higher but was reduced by visual inspection conducted by graders after trimming, where many diseased buds are discarded prior to entering the drying process. Botrytis bud rot symptoms manifest during late summer to early autumn under greenhouse conditions, when ambient temperatures drop below 25°C. In contrast, infection by Fusarium is enhanced by temperatures in the 25–35°C range encountered during summer months(unpublished observations). Higher incidences of these pathogens causing infection of inflorescences during production generally were correlated with a higher frequency of post-harvest bud infection. The monitoring of airborne Botrytis spore levels in cannabis growing facilities and association with bud rot levels, similar to that conducted for other horticultural crops (Carisse et al. Citation2014), is worthy of investigation. Measurable levels of Botrytis spores in the air could potentially cause respiratory allergies in humans (Jürgensen & Madsen Citation2009).
Fig. 5 The effect of temperature on growth rate and colony morphology of three pathogens recovered from cannabis inflorescences. Photographs were taken after 7 days of growth at each of the temperatures, as follows. (a) Botrytis cinerea at 5, 10, 15, 20, 25, 30°C. (b) Fusarium sporotrichioides at 5, 10, 15, 20, 25, 30°C. (c) Fusarium oxysporum at 10, 15, 20, 25, 30, 35°C
Diversity of fungal species recovered
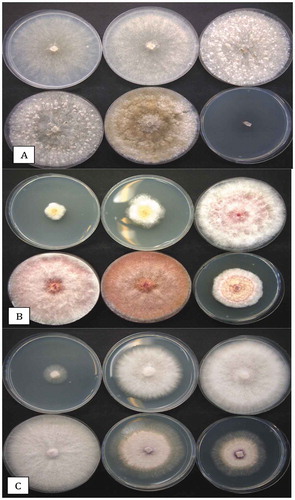
A range of other fungal species recovered from cannabis buds are shown in and include Penicillium copticola (), P. pancosmium () and Talaromyces radicus (). A comparison of the morphology of Talaromyces pinophilus, P. pancosmium, P. sclerotiorum and P. glabrum is shown in . Many of these fungi are previously unreported from cannabis buds although their frequency of recovery in many cases was less than 1%. The recovery of three Aspergillus species in this study () confirms previous reports of the presence of Aspergillus spp. on cannabis inflorescences (McPartland Citation1994; McKernan et al. Citation2016a; McPartland & McKernan Citation2017; Thompson et al. Citation2017). However, the overall incidence in the present study was low, which could be attributed, in part, to the reduced growth on PDA compared to other media with low water activity that improve growth of Aspergillus species (Tanney et al. Citation2017). In addition, the warm humid conditions under which these species are commonly found were not encountered in this study. Aspergillus spp. are also commonly reported to occur as endophytes in cannabis plants (Punja et al. Citation2019) and not detectable using the swab method.
Fig. 6 Colony morphologies of the more distinct fungi that were recovered from cannabis inflorescences. All cultures were grown on PDA at 21–24°C. (a) Penicillium copticola. (b) Penicillium pancosmium. (c) Talaromyces radicus. (d, e) Cultures made using cotton swabs to streak spores across the surface of PDA plates. Cultures were grown for 7 days. (d) Top view of colony features of Talaromyces pinophilus (upper left), P. pancosmium (upper right), P. sclerotiorum (lower left), P. glabrum (lower right). (e) Bottom view of the same cultures shown in (d) with characteristic pigment production
Comparison of the swab method with homogenized tissue samples
The swab method followed by culturing and PCR-based identification provided an indication of the colony-forming units present on the surface of cannabis buds. It is not known how the swab method compares with the commercially used procedure of plating dilutions made from homogenized tissue samples onto agar media, which is conducted by commercial cannabis quality testing labs, or how the method compares to a quantitative qPCR approach, which would also include DNA from non-viable spores (McKernan et al. Citation2016b). In samples originating from a homogenized tissue blending process, endophytic fungi naturally present inside cannabis tissues (Punja et al. Citation2019) would be released and potentially provide a different species diversity upon recovery. Many of the previously described endophytes present in cannabis pith tissues (Punja et al. Citation2019) are taxonomically different from the surface-contaminating species reported here. Further studies on the importance of these two subsets of fungal contaminants on cannabis bud quality assessments are needed.
The prevalence of Penicillium species in buds
The most prevalent group of fungi recovered in this study were Penicillium species. Many of these have been previously reported to be present within cannabis production facilities (Punja et al. Citation2019). The most frequently recovered species were P. citrinum, P. olsonii, P. simplicissimum and P. spathulatum (). Air sampling conducted in the present study confirmed that Penicillium species were present within the greenhouse production facility sampled in this study, as well as in the drying rooms (). In addition, colonies of B. cinerea and species of Alternaria, Aspergillus and Fusarium were detected on Petri dishes placed inside drying rooms. They are likely to have originated and spread from the harvested inflorescences. It was observed that the prevalence of Penicillium contamination on buds was increased with post-harvest handling and trimming processes which caused physical damage (), as reported by Punja et al. (Citation2019). The inflorescences of some cannabis strains which were more prone to physical injury (manifested as a browning of the tissues) during processing were generally more extensively colonized by Penicillium species (unpublished observations). Other factors that could influence the extent to which fungal populations may build up on dried cannabis buds include the duration of the drying period, final moisture content of the tissues, and the temperature and relative humidity conditions during the drying process.
Fig. 7 Colonies of a wide range of fungi growing on Petri dishes containing potato dextrose agar+140 mg L−1 of streptomycin sulphate. Lids of the dishes were removed and the dishes were exposed to the ambient environment in (a) a greenhouse growing facility and (b) an indoor growing facility for 60 min. Dishes were incubated for 7 days at 21–24°C after which the photographs were taken. In (a), the brown colonies are Cladosporium westeerdijkieae, the blue-green colonies are Penicillium olsonii, and the white colonies are P. spathulatum. In (b), the yellow colonies are Aspergillus ochraceus and the remainder are Penicillium spp., including P. olsonii, P. simplicissimum and P. glabrum.
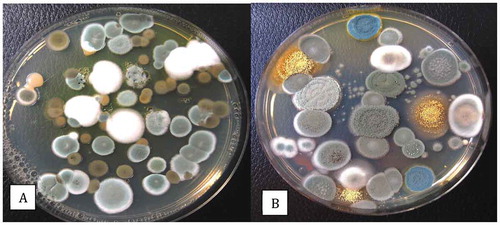
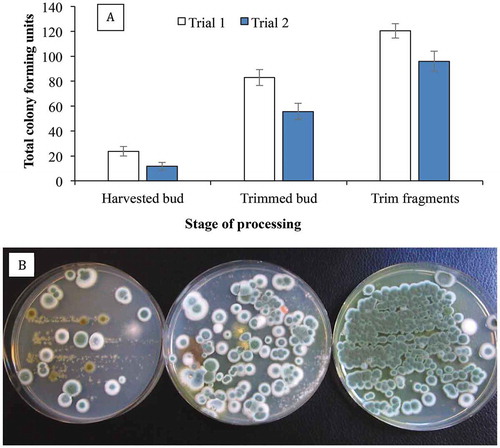
Fig. 8 Recovery of Penicillium spp. on PDA+S following swab samples taken from cannabis buds at different stages of processing. The swabs were obtained from harvested (non-dried) buds, buds after completion of mechanical trimming, and from trim fragments. The total colony-forming units are shown in (a) and the appearance of the dishes from Trial 1 is shown in (b). Data are from 10 replicate dishes in each of two trials
Penicillium species are ubiquitous and have been recovered from a range of plant species and different environments, and represent one of the most commonly identified mould species worldwide (Visagie et al. Citation2014; Aiko & Mehta Citation2016; Zheng et al. Citation2017). Over 400 taxonomic species are described (Visagie et al. Citation2014a, Citation2014b) and up to 17 species of Penicillium were previously detected in cannabis bud tissues using molecular approaches (McKernana et al. Citation2016a) as well as in the present study. Despite the complex taxonomy of species within the genus, the ITS barcode (Visagie et al. Citation2014b) provided a useful approach to identify the various species present on cannabis buds. The ITS region allows for species identification in a broad sense and generally places Penicillium strains into their respective sections as proposed by Houbraken and Samson (Citation2011). Several species secreted distinct pigments into the agar, most notably P. citrinum and P. pancosmium, which produced a yellow pigment and are members of the section Citrina and have been isolated from soil (Houbraken et al. Citation2010). Talaromyces radicus and T. pinophilus secreted a red pigment into the agar. A potential concern with the ubiquitous presence of Penicillium species on cannabis buds is the potential for mycotoxin production (Pitt Citation2002; Visagie et al. Citation2014), if sufficiently high populations proliferate on inflorescence tissues. The potential for mycotoxin production by Penicillium spp. on cannabis buds remains unexplored (Perrone & Susca Citation2017).
Effect of electrobeam radiation
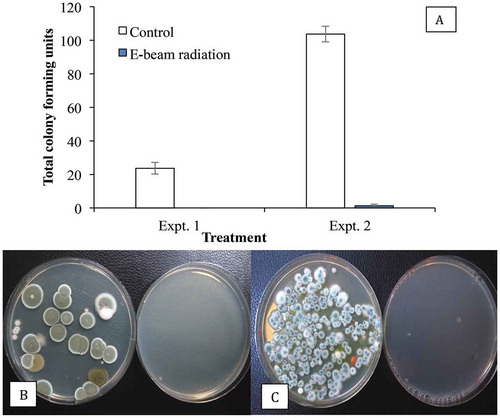
Exposure of dried buds to electrobeam radiation was observed to reduce the overall populations of Penicillium on the surface of buds in this study (). Treatments using gamma rays and electrobeam radiation have been shown to reduce mould contamination on a range of plant products (Aquino Citation2011; Calado et al. Citation2014; Jeong et al. Citation2015; Hazekamp Citation2016) and are an approved method for mould reduction for Canadian producers of cannabis (Health Canada Citation2013), except those certified for organic production. A recent study demonstrated that these forms of treatment, as well as cold plasma treatment, effectively reduced contamination of cannabis buds by B. cinerea (Jerushalmi et al. Citation2020) although the effects on Penicillium spp. were not studied. The effectiveness of electrobeam radiation on endophytic fungal populations requires further study.
Fig. 9 Recovery of Penicillium spp. on PDA+S following swab samples taken from cannabis buds after E-beam radiation compared with non-treated (control) buds. The total colony-forming units are shown in (a) and the appearance of the dishes from Expt. 1 is shown in (b) and from Expt. 2 in (c). Data are from 10 replicate dishes in each trial. In both photos, control dishes are on the left and treated dishes on the right
Conclusion
This study has demonstrated the wide range of viable colonies of fungi that can be present on cannabis buds in commercial drying rooms. While many of these fungi are present at low levels, the diversity of species found, including potentially mycotoxigenic species such as Fusarium and Penicillium species, point to the need for additional studies to determine their importance in quality assurance of commercially grown cannabis. As well, the relationships between growing practices and post-harvest handling conditions of cannabis dried products to final mould levels needs to be studied. With increasing field production of cannabis being approved in Canada, a comparison of mould contamination on these buds compared with indoor-grown product would be interesting. This study was conducted in British Columbia – further characterization of fungal contaminants of cannabis buds in other cannabis growing regions of Canada and the potential for mycotoxin production are urgently needed.
Acknowledgements
Technical assistance provided by Cameron Scott, Samantha Lung, Li Ni and Sarah Chen is gratefully acknowledged.
Additional information
Funding
References
- Aiko V, Mehta A. 2016. Prevalence of toxigenic fungi in common medicinal herbs and spices in India.3. Biotech. 5:159.
- Aquino S. 2011. Gamma radiation against toxigenic fungi in foods, medicinal and aromatic herbs. In: Mendez-Vilas A, editor. Science against microbial pathogens: communicating current research and technological advances. Formatex; p. 272–281.
- Calado T, Venancio A, Abrunhosa L. 2014. Irradiation for mold and mycotoxin control: a review. Comp Rev Food Sci Food Safety. 13(5):1049–1061. doi:10.1111/1541-4337.12095.
- Carisse O, Tremblay DM, Lefebvre A. 2014. Comparison of Botrytis cinerea airborne inoculum progress curves from raspberry, strawberry and grape plantings. Plant Pathol. 63(5):983–993. doi:10.1111/ppa.12192.
- European Pharmacopoeia (EP). 2015. Version 7.0 – section 5.1.4. Microbiological quality of non-sterile pharmaceutical preparations and substances for pharmaceutical use. Strasbourg.
- Hazekamp A. 2016. Evaluating the effects of gamma-irradiation for decontamination of medicinal cannabis. Front Pharmacol. 7:108–113. doi:10.3389/fphar.2016.00108.
- Health Canada. 2013. Technical specifications for testing dried marihuana for medical purposes. Available from https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-use-marijuana/licensed-producers/guidance-document-technical-specifications-dried-marihuana-medical-purposes.html. [Accessed 2019 Oct 30].
- Houbraken JAMP, Frisvad JC, Samson RA. 2010. Taxonomy of Penicillium citrinum and related species. Fungal Diversity. 44(1):117–133. doi:10.1007/s13225-010-0047-z.
- Houbraken JAMP, Samson RA. 2011. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud Mycol. 70:1–51. doi:10.3114/sim.2011.70.01.
- Jeong RD, Shin EJ, Chu EH, Park HJ. 2015. Effects of ionizing radiation on postharvest fungal pathogens. Plant Pathol J. 31(2):176–180. doi:10.5423/PPJ.NT.03.2015.0040.
- Jerushalmi S, Maymon M, Dombrovsky A, Freeman S. 2020. Effects of cold plasma, gamma and e-beam irradiations on reduction of fungal colony forming unit levels in medical cannabis inflorescences. J Cannabis Res. 2(1):12. doi:10.1186/s42238-020-00020-6.
- Jürgensen CW, Madsen AM. 2009. Exposure to the airborne mould Botrytis and its health effects. Ann Agric Environ Med. 16(2):183–196.
- Kabak B, Dobson AD. 2017. Mycotoxins in spices and herbs – an update. Crit Rev Food Sci Nutr. 57(1):18–34. doi:10.1080/10408398.2013.772891.
- McKernan K, Spangler J, Helbert Y, Lynch RC, Devitt-Lee A, Zhang L, Orphe W, Warner J, Foss T, Hudalla CJ, et al. 2016a. Metagenomic analysis of medicinal Cannabis samples; pathogenic bacteria, toxigenic fungi, and beneficial microbes grow in culture-based yeast and mold tests. F1000Res. Oct 7. 5:2471. doi:10.12688/f1000research.9662.1.
- McKernan K, Spangler J, Zhang L, Tadigotla V, Helbert Y, Foss T, Smith D. 2016b. Cannabis microbiome sequencing reveals several mycotoxic fungi native to dispensary grade Cannabis flowers. F1000Res. 2015, 4:1422. doi:10.12688/f1000research.7507.2.
- McPartland JM. 1994. Microbiological contaminants of marijuana. J Inter Hemp Assoc. 1:41–44. [Accessed 2019 Oct 15]. https://f1000research.com/articles/4-1422/v2.
- McPartland JM. 1996. A review of Cannabis diseases. J Inter Hemp Assoc. 3:19–23. [Accessed 2016 Mar 28]. www.internationalhempassociation.org/jiha/iha03111.html.
- McPartland JM, McKernan KJ. 2017. Contaminants of concern in Cannabis: microbes, heavy metals and pesticides. In: Chandra S, Lata L, ElSohly MA, editors. L. Cannabis sativa L. –Botany and biotechnology. Berlin: Springer-Verlag; p. 457–474.
- Perrone G, Susca A. 2017. Penicillium species and their associated mycotoxins. Meth Mol Biol. 1542:107–119.
- Pitt JI. 2002. Biology and ecology of toxigenic Penicillium species. In JW DeVries, MW Trucksess, LS Jackson, editors. Mycotoxins and food safety. Adv Exp Med Biol. 504:29–41.
- Punja ZK. 2018. Flower and foliage-infecting pathogens of marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40(4):514–527. doi:10.1080/07060661.2018.1535467.
- Punja ZK, Collyer D, Scott C, Lung S, Holmes SD. 2019. Pathogens and molds affecting production and quality of Cannabis sativa L. Front Plant Sci. 10:1120. doi:10.3389/fpls.2019.01120.
- Rodriguez G, Munir Z. 2019. Good manufacturing practices (GMP) approach to post-harvest activities for cannabis. J GXP Compliance. 23(6).
- Sadeghi B, Mirzaei S. 2018. First report of Alternaria leaf spot caused by Alternaria chlamydosporigena on tomato in Iran. Plant Dis. 102(6):1175. doi:10.1094/PDIS-09-17-1420-PDN.
- Schroeder KL, Okubara PA, Tambong JT, Lévesque CA, Paulitz TC. 2006. Identification and quantification of pathogenic Pythium spp. from soils in eastern Washington using real-time polymerase chain reaction. Phytopathology. 96(6):637–647. doi:10.1094/PHYTO-96-0637.
- Scott M, Rani M, Samsatly J, Charron JB, Jabaji S. 2018. Endophytes of industrial hemp (Cannabis sativa L.) cultivars: identification of cultural bacteria and fungi in leaves, petioles, and seeds. Can J Microbiol. 64(10):1–17. doi:10.1139/cjm-2018-0108.
- Small E. 2017. Cannabis: a complete guide. Boca Raton (FL): CRC Press.
- Tanney JB, Visagie CM, Yilmaz N, Seifert KA. 2017. Aspergillus subgenus Polypaecilum from the built environment. Stud Mycol. 88:237–267. doi:10.1016/j.simyco.2017.11.001.
- Thompson GR III, Tuscano JM, Dennis M, Singapuri A, Libertini S, Gaudino R, Torres A, Delisle JPM, Gillece JD, Schupp JM, et al. 2017. A microbiome assessment of medical marijuana. Clin Microbiol Infect. 23(4):269–270. doi:10.1016/j.cmi.2016.12.001.
- Visagie CM, Hirooka Y, Tanney JB, Whitfield E, Mwange K, Meijer M, Amend AS, Seifert KA, Samson RA. 2014a. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Stud Mycol. 78:63–139. doi:10.1016/j.simyco.2014.07.002.
- Visagie CM, Houbraken J, Frisvad JC, Hong S-B, Klaassen CHW, Perrone G, Seifert KA, Varga J, Yaguchi T, Samson RA. 2014b. Identification and nomenclature of the genus Penicillium. Stud Mycol. 78:343–371. doi:10.1016/j.simyco.2014.09.001.
- Visagie CM, Seifert KA, Houbraken J, Samson RA, Jacobs K. 2014. Diversity of Penicillium section Citrina within the fynbos biome of South Africa, including a new species from a Protea repens infructescence. Mycologia. 106(3):537–552. doi:10.3852/13-256.
- Zheng RS, Wang WL, Tan J, Xu H, Zhan RT, Chen WW. 2017. An investigation of fungal contamination on the surface of medicinal herbs in China. Chin Med. 12(1):2. doi:10.1186/s13020-016-0124-7.
