Abstract
Common bean (Phaseolus vulgaris L.) can be affected by several viral diseases, including golden mosaic caused by the Bean golden mosaic virus (BGMV). When infected by BGMV, bean plants undergo major physiological changes, leading to a reduction or complete loss of productivity. This study aimed to evaluate and compare seven bean cultivars and lineages, as well as the legume calopo (Calopogonium mucunoides Desv.), for the severity of golden mosaic symptoms and BGMV titre following inoculation. The study was conducted in the greenhouse under controlled environmental conditions. Plants were inoculated with viruliferous whiteflies [Bemisia tabaci (Gennadius)], and evaluated for severity of disease symptoms at 7, 10, 15, 20, and 25 days after inoculation (DAI). The virus titre was measured by quantitative PCR (qPCR) analysis with primers specific for the virus coat protein gene. All bean genotypes were symptomatic for BGMV infection, but calopo was not. There were differences among the tested cultivars and lineages for the expression of symptoms. Differences in BGMV titre also were observed among the cultivars at 10 and 25 DAI. The bean cultivar ‘Tangará’ had a greater viral titre but exhibited lower symptom severity than the other cultivars. Calapo was tolerant to BGMV.
Résumé
Le haricot ordinaire (Phaseolus vulgaris L.) peut être sujet à plusieurs maladies virales, dont la mosaïque dorée causée par le virus de la mosaïque dorée du haricot (BGMV). Lorsqu’ils sont infectés par le BGMV, les plants subissent des transformations physiologiques majeures engendrant une réduction ou une perte totale de productivité. Cette étude visait à évaluer et à comparer sept cultivars et lignées de haricot, ainsi que la légumineuse fourragère calopo (Calopogonium mucunoides Desv.), quant à la gravité des symptômes de la mosaïque dorée et au titre du BGVM à la suite de l’inoculation. L’étude a été menée en serre dans des conditions contrôlées. Les plants ont été inoculés par l’intermédiaire de thrips virulifères (Bemisia tabaci [Gennadius]) et évalués pour la gravité des symptômes à 7, 10, 15, 20 et 25 jours après inoculation (JAI). Le titre du virus a été mesuré par PCR quantitative avec des amorces spécifiques du gène de la protéine de coque du virus. Tous les génotypes de haricots, sauf le calopo, ont affiché les symptômes de l’infection au BGVM. Il y avait des différences entre les cultivars et les lignées testés quant à l’expression des symptômes. À 10 et à 25 JAI, des différences quant au titre du BGVM ont également été observées chez les cultivars. Le titre viral du cultivar de haricot ‘Tangará’ était plus élevé, mais ce dernier affichait une gravité de symptômes plus faible que les autres cultivars. Le calapo était résistant au BGVM.
Introduction
The common bean (Phaseolus vulgaris L.) is a leguminous plant belonging to the Fabaceae family (Singh et al. Citation1991). It is of high economic importance in several countries, including Brazil, India, China and Mexico. Brazil is the largest producer and consumer of common beans in the world, with an annual production of 3.39 million tons (Conab Citation2018). Moreover, the state of Paraná is the main producer of beans in Brazil, yielding approximately 300 thousand tons annually (Conab Citation2018).
Several factors, such as viral diseases, limit the production of common bean. Among them, the golden mosaic disease caused by Bean golden mosaic virus (BGMV) is considered the most economically and agriculturally important disease for the crop in tropical and subtropical regions (Aragão et al. Citation2013). The disease not only affects both grain yield and quality, but also increases production costs due to frequent application of costly insecticides to control its insect vector, the whitefly [Bemisia tabaci (Gennadius)] (Costa Citation1987; Galvez & Morales Citation1989; Coutinho et al. Citation2016).
Bemisia tabaci is a species complex. Presently, more than 41 biotypes (over 35 species) have been described worldwide. In Brazil, the whitefly species include Middle East-Asia Minor 1 (MEAM 1; biotype B), Mediterranean (MED; biotype Q), and the native species New World (NW; biotype A) and New World 2 (NW2) (Marubayashi et al. Citation2013; Barbosa et al. Citation2014). In the state of Paraná, the most prevalent species is MEAM 1 (biotype B) (Walz Citation2017), while the species MED (biotype Q) is detected occasionally (Moraes et al. Citation2018).
BGMV is transmitted by the whitefly in a persistent-circulative nonpropagative manner, where the virus is acquired by the vector during feeding on phloem sap. The virus then circulates in the body of the insect, reaches its salivary glands, and subsequently is injected into a healthy plant during feeding (Rezende & Kitajima Citation2018).
BGMV belongs to the genus Begomovirus, family Geminiviridae. The virus capsid is characterized by ‘twinned particles’ with icosahedral morphology and a genome composed of two circular single-stranded DNA molecules (DNA-A and DNA-B) (Lazarowitz Citation1992), each containing a unique nucleotide sequence with a size of ~2.6 kb (Lazarowitz Citation1992). DNA-A contains genes encoding the coat protein and proteins involved in replication, while DNA-B contains genes related to viral movement and symptom induction (Timmermans et al. Citation1994; Brown Citation1997).
The symptoms of the disease caused by BGMV are broadly grouped into two types: wrinkling (or severe deformation) and leaf mosaic. Plants with wrinkling undergo a drastic size reduction, and may develop excessive lateral sprouts, resulting in witches’ brooms (Bianchini et al. Citation2005). In contrast, plants with leaf mosaic symptoms have a less pronounced reduction in development (Bianchini et al. Citation2005).
Within the cell, the virus affects the chloroplast morphology, especially the lamellar system (Lemos et al. Citation2003). Symptoms may appear in the phloem and the cells adjacent to the parenchyma (Lemos et al. Citation2003). The virus causes an enlargement of the nucleolus, which condenses in fibril granular regions, forming ring-shaped structures of different sizes and numbers within the nucleus (Lemos et al. Citation2003). Finally, when viral particles appear in the nucleus, the solubility of solutes in the plant decreases, affecting the productivity of the bean plant (Faria & Maxwell Citation1999).
There are no completely effective control measures for golden mosaic disease (Faria & Zimmermann Citation1988; Faria Citation1994; Faria & Maxwell Citation1999; Bianchini et al. Citation2005). The elimination of weeds within the planting site that could serve as hosts for the insect vector and as reservoirs for the virus is recommended (Yokoyama Citation1995; Bianchini et al. Citation2005; Rocha et al. Citation2013), as is planting of the crop when disease pressure is low. The use of common bean cultivars with resistance or tolerance to BGMV is also suggested (Bianchini Citation1999), and represents the best approach for controlling golden mosaic disease. While highly resistant bean lines have been developed, the transfer of this trait to new cultivars is difficult due to variation and recombination of the virus.
The objective of this study was to evaluate the severity of golden mosaic disease by quantifying the BGMV titre in a variety of bean cultivars and lineages and in the legume calopo (Calopogonium mucunoides Desv.).
Materials and methods
Host plants
The experiment was carried out in a completely randomized design using eight plant genotypes: five bean cultivars (‘Eldorado’, ‘Juriti’, ‘Tangará’, ‘Pérola’, and ‘Carioca’), two bean lineages (Lineage A and Lineage B), and the legume calopo. For each genotype, four plants were inoculated with the virus and four were used as a control (virus-free plant). The experiment was carried out under greenhouse conditions in Londrina, Paraná (−23.22; −51.10; 585 m above sea level). Disease development in the plants was assessed at 7, 10, 15, 20, and 25 days after inoculation (DAI) (Bianchini Citation1998).
Inoculation of plants with BGMV
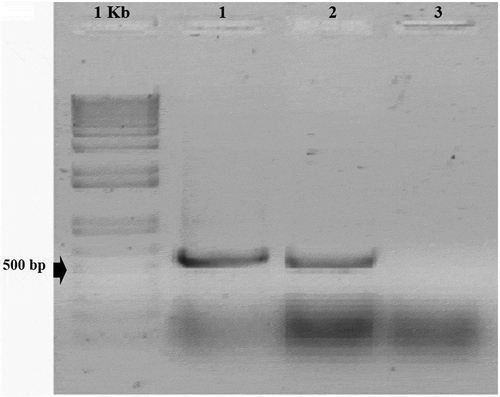
The BGMV isolate was obtained from a bean plant, cultivar ‘Carioca’, with typical symptoms of golden mosaic disease. The BGMV infection was confirmed by conventional PCR analysis with the primers AV494/AC1048 specific for the DNA-A coat protein gene of the virus () (Wyatt & Brown Citation1996).
Fig. 1 Agarose gel electrophoresis of PCR products for detection of Bean golden mosaic virus (BGMV). Primers specific for the DNA-A coat protein gene of the virus were used in the PCR analysis and amplified a 550 bp product. The gel was stained with SYBR Gold nucleic acid gel stain. Lane labelling is as follows: 1Kb – molecular weight marker; 1 – amplification from a bean plant of cultivar ‘Carioca’ showing mosaic and wrinkling symptoms; 2 – positive control; 3 – negative control (no DNA included in reaction mixture)
Virus inoculation was carried out using whiteflies (B. tabaci) raised in a cage under controlled greenhouse conditions (Walz Citation2017). The adult whiteflies were kept in aphid-proof cages and fed bean plants symptomatic for bean golden mosaic disease for 48 h. The whiteflies were then transferred to cages containing plastic gerbox-type boxes with plant seedlings and allowed to feed for 24 h. Thereafter, the seedlings were sprayed with a systemic insecticide to kill the whiteflies and transplanted to pots containing a clay-based potting mix.
Evaluation of disease severity
The plants were evaluated for symptoms of bean golden mosaic. The severity of mosaic and wrinkling (including foliar curling, pod distortions, and plant stunting) symptoms were recorded. Disease severity was scored on a 0 to 5 scale adapted from a descriptive procedure developed specifically for golden mosaic disease (Bianchini Citation1999). For mosaic symptoms, the categories were: 0 = absence of symptoms; 1 = initial symptoms (lightening or chlorosis of young leaf veins, or mild chlorosis in older leaves without apparent damage to the plant); 2 = obvious symptoms with yellow-chlorotic spots scattered on the leaf, and the affected leaf area is much smaller than the green area; 3 = bright yellow speckling distributed throughout the leaf interspersing the green area that is greater or equal to the affected leaf area; 4 = intense yellow splashing distributed throughout the leaf with coalescence of yellow spots, but with interspersed green areas smaller than the affected area; and 5 = intense yellow discolouration throughout the leaf, separate yellow spots can no longer be distinguished and the green areas are restricted to the larger veins, or absent.
For symptoms of wrinkling, the disease severity categories were: 0 = absence of symptoms; 1 = slight reduction in the development of younger leaves without apparent damage or initial symptoms in apical trifoliate; 2 = noticeable deformation (or curvature) of the leaflets in the upper third of the plant with an apparent reduction (~20%) in plant size, and shortening of the apical guide; 3 = accentuated curling in more than half of the leaves with 40%–50% reduction in leaf size, and a reduction in plant size by one third; 4 = severe curling or wrinkling of most leaves (60% or more) with a diameter of 1–3 cm, a reduction in plant size or volume by half, axillary shoots with deformed and small leaves with an appearance of overgrowth; and 5 = 80%–100% of leaves are malformed and small (diameter 2.5 cm or less), plant size or volume reduced to about a quarter of normal size, axillary sprouting and pods are unlikely to form.
Total DNA extraction
Total DNA was extracted from the plants at 7, 10, 15, 21, and 25 DAI. Top leaves were identified, collected individually, snap-frozen in liquid nitrogen, and stored at −80°C. The cetyl trimethylammonium bromide (CTAB) method was used for DNA extraction (Murray & Thompson Citation1980), with some changes. Snap-frozen young petioles and central veins (500 mg) were macerated in 3 mL of CTAB extraction buffer [CTAB, Tris HCl 1 M (pH8.0), EDTA 0.5 M (pH8.0), Polyvinylpyrrolidone 10 000 and NaCl] with 0.2% β-mercaptoethanol. The extract was transferred to a 2 mL microtube and incubated at 65°C for 30 min. After incubation, the sample was centrifuged at 10 000 × g for 5 min, 1 mL of the supernatant was transferred to a new microtube, and 1 mL of 24:1 chloroform:isoamyl was added. The sample was vortexed and centrifuged at 16 000 × g for 10 min. Then, 0.9 mL of the aqueous phase was recovered and the cleaning process was repeated with 24:1 chloroform:isoamyl. The aqueous phase (0.9 mL) was mixed with 540 µL of isopropanol, incubated at −20°C for 30 min, and centrifuged for 20 min at 16 000 x g. After centrifugation, the DNA precipitated by forming a pellet which was washed twice with 1 mL of 70% ethanol and centrifuged for 10 min at 14 000 x g. The pellet was dried and resuspended in 100 µL of milli-Q water.
Detection and quantification of BGMV by quantitative-PCR (q-PCR)
The primers 2371 (5ʹ-TTCCGTCTCCCATGCCAATA-3ʹ) and 2271 (5ʹ-CTAGCAGATCTTCCGTCGACTTG-3ʹ), which amplify 150 bp of the coat protein gene in the DNA-A viral genome, were used for the quantification of the BGMV in plants by quantitative-PCR (q-PCR) analysis. The final volume of the reaction mixture was 12.5 μL, containing 1X Power SYBR Green PCR Master Mix® (Applied Biosystems, Foster City, CA, EUA) (6.25 μL); 0.4 μM of each primer (2371 and 2271); 5 ng of DNA obtained from the plants; and 4.25 μL of Milli-Q water. The amplification was conducted in a StepOnePlus™ Real-Time PCR System with the following settings: 95°C for 10 min, 40 cycles at 95°C for 15 s, and 60°C for 1 min. The viral titre was quantified using a serial dilution of a positive control sample as the standard. Each sample was analysed in triplicate.
Statistical analysis
The results of the experiment were subjected to analysis of variance, followed by the Scott Knott averages test at 5% probability level, using Sisvar v. 5.6 (Ferreira Citation2015).
Results
All bean cultivars and lineages in this study were symptomatic for BGMV infection, presenting mosaic () and wrinkling () symptoms (). In contrast, the calopo plants were asymptomatic. The most severe mosaic symptoms were observed at 20 and 25 DAI () and wrinkling at 25 DAI ().
Table 1. Severity of mosaic symptoms at 7, 10, 15, 20, and 25 days after inoculation of different cultivars and lineages of common bean (Phaseolus vulgaris) and Calopogonium mucunoides with Bean golden mosaic virus.
Table 2. Severity of wrinkling symptoms at 7, 10, 15, 20, and 25 days after inoculation of different cultivars and lineages of common bean (Phaseolus vulgaris) and Calopogonium mucunoides with Bean golden mosaic virus.
Fig. 2 Bean cultivar ‘Carioca’, infected with the Bean golden mosaic virus (BGMV) showing symptoms of: (a) Mosaic and (b) Wrinkling

At 10 DAI, ‘Juriti’ and ‘Eldorado’ had developed more severe mosaic symptoms than the other cultivars, with ‘Juriti’ presenting the highest average score (). However, at 15 DAI, the highest severity was observed for Lineages A and B, and ‘Eldorado’ and ‘Juriti’ (). At 25 DAI, more severe symptoms were observed in Lineage A and the cultivars ‘Tangará’ and ‘Carioca’ (). For the Lineages A and B, the most severe symptoms were at 15 DAI, while for ‘Pérola’, ‘Carioca’ and ‘Tangará’, the highest severity was found at 20 and 25 DAI (). There were no differences in symptom severity between ‘Eldorado’ and ‘Juriti’ throughout the experiment ().
Differences in wrinkling intensity were also observed among the bean cultivars and lineages (). Calopo remained asymptomatic during the duration of the experiment, while ‘Tangará’ remained asymptomatic until 10 DAI. The other bean cultivars and lineages showed wrinkling by 7 DAI, and differences in symptom severity were observed after 10 DAI. Wrinkling symptoms were most severe in Lineages A and B, and in cultivars ‘Carioca’ and ‘Juriti’ at 15 DAI (). At 20 DAI, ‘Pérola’ was most symptomatic, followed by Lineage A and cultivar ‘Carioca’. Finally, Lineage A and ‘Pérola’, ‘Carioca’, and ‘Juriti’ showed more symptoms at 25 DAI ().
The most severe symptoms in Lineage A were observed at 15, 20, and 25 DAI, whereas in the cultivar ‘Pérola’, the greatest symptom severity was observed at 20 and 25 DAI (). The greatest symptom severity level for Lineage B was at 10 DAI, and at 10, 15, and 25 DAI for ‘Juriti’ (). In contrast, ‘Eldorado’, ‘Carioca’, and ‘Tangará’ did not show changes in symptom severity throughout the experiment ().
Quantification of the virus titre carried out by q-PCR indicated differences among the genotypes at 20 and 25 DAI (). The highest BGMV titre was observed in ‘Tangará’, with cycle threshold (Ct) values of 6.71, and 9.33, at 10, and 25 DAI, respectively (). There were also differences in the relationship between bean cultivar (or lineage) and days after inoculation (). At 10 DAI, ‘Tangará’ presented the highest viral titre, followed by Lineage A, ‘Pérola’, ‘Carioca’, and ‘Juriti’, which presented Ct values of 11.69, 12.61, 12.22, and 12.17, respectively. However, at 25 DAI, all cultivars and lineages had a lower viral titre than ‘Tangará’ ().
Table 3. Quantification of the Bean golden mosaic virus by RT q-PCR in different cultivars and lineages of common bean (Phaseolus vulgaris) and Calopogonium mucunoides.
Discussion
The most prevalent symptom of bean golden mosaic disease is the intense yellow mosaic throughout the leaf blade, although dwarfism, internode shortening, loss of apical dominance, and budding of axillary buds can also occur (Furlan Citation2004). In the present study, different symptom severity levels were observed among the cultivars. This large variability in disease expression may reflect the genetic variability of the bean BGMV isolates (Costa Citation1987; Bianchini et al. Citation1989, Citation2004).
Both recombination and pseudo-recombination result in a high rate of virus evolution and can play a role in disease expression (Pita et al. Citation2001; Lima et al. Citation2017). Viral recombination characterized by the exchange of DNA or RNA fragments between distinct virus genomes (Roossinck Citation1997) is frequently observed among begomoviruses of the same strain or between viruses of different species. Similarly, pseudo-recombination corresponds to a pseudo-recombinant formation through the exchange of whole genomic components between members of the same or different species (García-Arenal et al. Citation2003; Andrade et al. Citation2006).
The symptoms varied between bean cultivars and lineages, as well as with the time since inoculation. Lemos et al. (Citation2003) also observed that bean genotypes reacted differently to BGMV infection. Symptom variation, specifically the intensity of mosaic foliar yellowing, leaf curling, pod distortion, and severity of plant stunting with abnormal axillary sprouting (typical of witches’ broom), may be related to the changes in genomic regions of the begomoviruses associated with replication, virus movement, and the processes that control the level of virus accumulation in the infected plants (Lazarowitz Citation1992; Timmermans et al. Citation1994; Brown Citation1997). The phenotypes exhibited by plants when subjected to the virus result from interactions between plant and viral genes. The adaptation and specificity to certain plant species may involve viral proteins or regulatory elements present in the DNA-A and DNA-B of the begomovirus (Hou et al. Citation1998).
The bean cultivars and lineages varied with respect to virus multiplication in the host. The cultivar ‘Tangará’ had the highest BGMV titre at different DAIs compared with the other cultivars and lineages. Despite this high virus titre, this cultivar showed mild to moderate symptoms of disease, suggesting that ‘Tangará’ may have the ability to delay the manifestation of symptoms of the pathogen in a highly dynamic and coordinated manner (Abreu et al. Citation2016).
The response of host genotypes to BGMV can be important information for the development of management strategies for bean golden mosaic, such as the selection of cultivars that show less severe disease symptoms. The prevalence of golden mosaic epidemics reflects the lack of highly disease-resistant sources in bean germplasm banks (Inoue-Nagata et al. Citation2016). Therefore, the introduction of cultivars with improved resistance to golden mosaic is necessary in order to reduce crop productivity losses (Fé-Montenegro et al. Citation2016).
‘Tangará’ may be a good source of resistance to bean golden mosaic, because despite high viral titre, it displayed no symptoms of wrinkling and mosaic symptoms were not observed until 20 DAI. Similarly, calopo, despite having a high viral titre, showed no symptoms of the disease, consistent with previous studies reporting calopo as tolerant to BGMV. This legume is widely used in agriculture as a green manure, and therefore, if it was not resistant to the virus, it could be a source of virus inoculum (Teodoro et al. Citation2011; Silva et al. Citation2012).
This study contributes to information that could be useful for the development of new bean cultivars with greater resistance to golden mosaic. However, the transfer of resistance to a new cultivar may be a difficult process, due to variability in the virus and the type of resistance involved. It will be necessary to identify specific resistance mechanisms to the main strains of the virus and the relationship between BGMV and different common bean genotypes.
Acknowledgements
We would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES).
References
- Abreu PMV, Vaz AB, Fernandes PMB. 2016. Enfoque biotecnológico para o controle de vírus de plantas [Biotechnological approach to plant virus control]. In: Resende RR, editor. Biotecnologia aplicada à agro & indústria: fundamentos e aplicações [Biotechnology applied to agro & industry: fundamentals and applications]. São Paulo (SP): Blucher; p. 709–734. Portuguese.
- Andrade EC, Manhani GC, Alfenas PF, Calegario RF, Fontes EPB, Zerbini FM. 2006. Tomato yellow spot virus, a tomato-infecting begomovirus from Brazil with a closer relationship to viroses from Sida sp., forms pseudorecombinants with begomoviruses from tomato but not from Sida. J Gen Virol. 87(12):3687–3696. doi:10.1099/vir.0.82279-0.
- Aragão FJL, Nogueira EOPL, Tinoco MLP, Faria JC. 2013. Molecular characterization of the first commercial transgenic common bean immune to the Bean golden mosaic virus. J Biotechnol. 166(1–2):42–50. doi:10.1016/j.jbiotec.2013.04.009.
- Barbosa L, Marubayashi J, De Marchi B, Yuki V, Pavan M, Moriones E, Navas-Castillo J, Krause-Sakate R. 2014. Indigenous American species of the Bemisia tabaci complex are still widespread in the Americas. Pest Manag Sci. 70(10):1440–1445. doi:10.1002/ps.3731.
- Bianchini A 1998. Mosaico dourado do feijoeiro: crescimento do hospedeiro, progresso da doença e produção [Bean golden mosaic: host growth, disease progress and production] [PhD thesis]. São Paulo (SP): Universidade de São Paulo. Portuguese.
- Bianchini A. 1999. Resistance to Bean golden mosaic virus in bean genotypes. Plant Dis. 83(7):615–620. doi:10.1094/PDIS.1999.83.7.615.
- Bianchini A, Maringoni AC, Carneiro SMTPG. 2005. Doenças do Feijoeiro (Phaseolus vulgaris) [Bean (Phaseolus vulgaris) diseases]. In: Kimati H, Amorim L, Bergamin Filho A, Camargo LEA, Rezende JAM, editors. Manual de Fitopatologia: doenças das Plantas Cultivadas [Plant pathology manual: cultivated plant diseases]. São Paulo (SP): Agronômica Ceres; p. 33–349. Portuguese.
- Bianchini A, Menezes JR, Maringoni AC. 1989. Doenças e seu controle [Diseases and their control]. In: IAPAR, editor. O Feijão no Paraná [Beans in Paraná]. Londrina (PR): IAPAR; p. 189–216. Portuguese.
- Bianchini A, Motomura KF, Leite RP. 2004. Geminivirus variability affecting bean and other species in Paraná state. Fitopatol Bras. 29:253.
- Brown JK. 1997. The biology and molecular epidemiology of the Geminiviridae subgroup III. In: Stacey GE, Keen NT, editors. Plant-microbe interactions. New York (NY): ITP; p. 125–195.
- Conab. 2018. Acompanhamento da safra brasileira de grãos, v. 6 - safra 2018/19, n. 2 - Segundo levantamento [Follow up of the Brazilian grain crop, v. 6-2018/19 crop, n. 2 - Second survey]. Brasília (DF): Conab. [ accessed 2019 Jan 20]. Available from; https://www.conab.gov.br/info-agro/safras/graos Portuguese.
- Costa AS. 1987. Fitoviroses do feijoeiro no Brasil [Bean phytoviruses in Brazil]. In: Bulisani EA, editor. Feijão: Fatores de Produção e Qualidade [Beans: production and quality factors]. Campinas (SP): Fundação Cargil; p. 173–256. Portuguese.
- Coutinho G, Carneiro LMS, Juliatti FC. 2016. Resistance of bean genotypes to Bean golden mosaic virus (VMDF). Biosci J. 32(1):73–80. doi:10.14393/BJ-v32n1a2016-29436.
- Faria JC. 1994. Mosaico dourado [Golden Mosaic]. In: Sartorato A, Rava CA, editors. Principais doenças do feijoeiro comum e seu controle [Major common bean diseases and their control]. Goiânia (GO): EMBRAPA-CNPAF; p. 262–284. Portuguese.
- Faria JC, Maxwell DP. 1999. Variability in Geminivirus isolated with Phaseolus spp. in Brazil. Phytopathology. 89(3):262–268. doi:10.1094/PHYTO.1999.89.3.262.
- Faria JC, Zimmermann MJO. 1988. Control of bean golden mosaic virus in common bean (Phaseolus vulgaris L.) by varietal resistance and insecticides. Fitopatol Bras. 13:32–35.
- Fé-Montenegro CFF, Piedra AL, Travieso RMC, Pérez JH. 2016. Agronomic response of newly-introduced common bean (Phaseolus vulgaris L.) cultivars in Cuba. CulTrop. 37:102–107.
- Ferreira DF 2015. Programa computacional Sisvar, versão 5.6 [Sisvar computer program, version 5.6]. UFLA. Lavras (MG): UFLA. Portuguese.
- Furlan SH. 2004. Doenças bióticas e abióticas do feijoeiro. Guia de identificação e controle de doenças do feijoeiro [Bean biotic and abiotic diseases. Bean disease identification and control guide]. Campinas (SP): APTA - Instituto Biológico. Portuguese.
- Galvez GE, Morales FJ. 1989. Whitefly-transmitted viruses. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production in the tropics. Cali (CO): International Center for Tropical Agriculture; p. 379–390.
- García-Arenal F, Fraile A, Malpica JM. 2003. Variation and evolution of plant virus populations. Int Microbiol. 6(4):225–232. doi:10.1007/s10123-003-0142-z.
- Hou Y, Paplomatas EJ, Gilbertson RL. 1998. Host adaptation and replication properties of two bipartite geminiviruses and their pseudorecombinants. Mol Plant-Microbe Int. 11(3):208–217. doi:10.1094/MPMI.1998.11.3.208.
- Inoue-Nagata AK, Lima MF, Gilbertson RL. 2016. A review of geminivirus (begomovirus) diseases in vegetables and other crops in Brazil: current status and approaches for management. Hort Bras. 34(1):008–018. doi:10.1590/S0102-053620160000100002.
- Lazarowitz SG. 1992. Geminiviruses: genome structure and gene function. Crit Rev Plant Sci. 11(4):327–349. doi:10.1080/07352689209382350.
- Lemos LB, Fornasieri Filho D, Silva TRB, Soratto RP. 2003. Common bean genotypes behavior to gold mosaic virus. Pesq Agro Bras. 38(5):575–581. doi:10.1590/S0100-204X2003000500004.
- Lima ATM, Silva JCF, Silva FN, Castillo-Urquiza GP, Silva FF, Seah YM, Mizubuti ESG, Duffy S, Zerbini FM. 2017. The diversification of begomovirus populations is predominantly driven by mutational dynamics. Virus Evol. 3(1):1–7. doi:10.1093/ve/vex005.
- Marubayashi J, Yuki V, Rocha K, Mituti T, Pelegrinotti F, Ferreira F, Moura MF, Navas-Castilho J, Moriones E, Pavan MA, et al. 2013. At least two indigenous species of the Bemisia tabaci complex are present in Brazil. J Appl Entomol. 137(1–2):113–121. doi:10.1111/j.1439-0418.2012.01714.x
- Moraes LA, Muller C, Bueno RCOF, Santos A, Bello VH, Marchi BR, Watanabe LFM, Marubayashi JM, Santos BR, Yuki VA, et al. 2018. Distribution and phylogenetics of whitefies and their endosymbiont relationships after the mediterranean species invasion in Brazil. Sci Rep. 8(1):14589. doi:10.1038/s41598-018-32913-1
- Murray MG, Thompson WF. 1980. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8(19):4321–4325. doi:10.1093/nar/8.19.4321
- Pita JS, Fondong VN, Sangaré A, Otim-Nape GW, Oqwal S, Fauquet CM. 2001. Recombination, pseudorecombination and synergism of geminiviruses are determinant keys to the epidemic of severe cassava mosaic disease in Uganda. J Gen Virol. 82(3):655–665. doi:10.1099/0022-1317-82-3-655
- Rezende JAM, Kitajima EW. 2018. Vírus e Viróides [Viruses and viroids]. In: Amorim L, Rezende JAM, Bergamin Filho A, editors. Manual de Fitopatologia: Princípios e Conceitos [Plant pathology manual: principles and concepts]. Ouro Fino (MG): Agronômica Ceres; p. 161–190. Portuguese.
- Rocha CS, Castillo-Urquiza GP, Lima AT, Silva FN, Xavier CA, Hora-Junior BT, Beserra-Junior JE, Malta AW, Martin DP, Varsani A, et al. 2013. Brazilian begomovirus populations are highly recombinant, rapidly evolving, and segregated based on geographical location. J Virol. 87(10):5784–5799. doi:10.1128/JVI.00155-13
- Roossinck MJ. 1997. Mechanisms of plant virus evolution. An Rev Phytopathol. 35(1):191–209. doi:10.1146/annurev.phyto.35.1.191.
- Silva SJC, Castillo-Urquiza GP, Hora-Júnior BT, Assunção IP, Lima GSA, Pio-Ribeiro G, Mizubutia ESG, Zerbini FM. 2012. Species diversity, phylogeny and genetic variability of begomovirus populations infecting leguminous weeds in northeastern Brazil. Plant Pathol. 61(3):457–467. doi:10.1111/j.1365-3059.2011.02543.x.
- Singh SP, Gepts P, Debouch DG. 1991. Races of common bean (Phaseolus vulgaris, Fabaceae). Econ Bot. 45(3):379–396. doi:10.1007/BF02887079.
- Teodoro RB, Oliveira FL, Silva DMN, Fávero C, Quaresma MAL. 2011. Leguminosas herbáceas perenes para utilização como coberturas permanentes de solo na Caatinga Mineira [Perennial herbaceous legumes used as permanent cover cropping in the Caatinga Mineira]. R Cien Agro. 42(2):292–300. Portuguese. doi:10.1590/S1806-66902011000200006.
- Timmermans MCP, Prem-Das O, Messing J. 1994. Geminiviruses and their uses as extrachromossomal replicons. Annu Rev Plant Physiol Plant Mol Biol. 45(1):79–112. doi:10.1146/annurev.pp.45.060194.000455.
- Walz DM 2017. Caracterização de biótipos e detecção de Begomovirus em mosca-branca (Bemisia tabaci) no estado do Paraná [Biotype characterization and detection of begomovirus in whitefly (Bemisia tabaci) in Paraná state] [master’s thesis]. Londrina (PR): Instituto Agronômico do Paraná. Portuguese.
- Wyatt SD, Brown JK. 1996. Detection of subgroup III geminivirus isolates in leaf extracts by degenerate primers and polymerase chain reaction. Phytopathology. 86(12):1288–1293. doi:10.1094/Phyto-86-1288.
- Yokoyama M. 1995. Whitefly on common bean: biological aspects and control. Cor Agri. 1:8–9.
