 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Root-knot nematodes are responsible for substantial losses in rice production. These parasites are difficult to control, particularly in flooded fields, which require carefully designed strategies to avoid contamination of water bodies. This study investigated whether biotic and abiotic elicitors can induce resistance to the root-knot nematode Meloidogyne graminicola in rice plants. An initial screening test was performed to determine the elicitors with the greatest potential to control nematodes. Then, a second experiment was conducted under net house conditions to assess the effects of a mannanoligosaccharide-based fertilizer (MOS), acibenzolar-S-methyl (ASM), and silicate clay on nematode reproduction, penetration, and activation of plant defence-related enzymes. Elicitor treatment reduced nematode reproduction by 57.4 to 75.6% at 60 days after inoculation (DAI). Two factors that may have contributed to this result include reduced nematode penetration and delayed development, as observed in the screening test. All elicitors increased phenylalanine ammonia-lyase activity at 10 DAI. MOS and silicate clay also increased catalase, peroxidase, and glucanase activity at 8 DAI. Elicitor treatment activates defence responses in rice plants and may be an environmentally friendly strategy for controlling M. graminicola in an integrated management system.
Résumé
Les nématodes cécidogènes sont la cause de lourdes pertes subies par les producteurs de riz. Ces parasites sont difficiles à gérer, particulièrement dans les champs inondés, ce qui requiert des stratégies savamment planifiées pour éviter de contaminer les plans d’eau. Cette étude a cherché à savoir si des éliciteurs biotiques et abiotiques pouvaient induire la résistance au nématode cécidogène Meloidogyne graminicola chez le riz. Un test initial de criblage a été effectué pour déterminer les éliciteurs les plus susceptibles de maîtriser les nématodes. Puis, une deuxième expérience a été menée sous filet afin d’évaluer les effets d’un fertilisant à base de mannanoligosaccharides (MOS), de l’acibenzolar-S-méthyl (ASM) et de l’argile foisonnante sur la reproduction et la pénétration des nématodes ainsi que sur leur activation des enzymes associées à la défense des plantes. Le traitement aux éliciteurs a réduit la reproduction des néma\\todes de 57,4 à 75,6 %, 60 jours après inoculation (JAI). Deux facteurs qui peuvent avoir contribué à ce résultat incluent la pénétration réduite des nématodes et leur développement tardif, comme cela a été observé lors du test de criblage. Tous les éliciteurs ont accru l’activité de la phénylalanine ammoniac-lyase 10 JAI. Les MOS et l’argile foisonnante ont également accru l’activité de la catalase, de la peroxydase et de la glucanase, et ce, 8 JAI. Le traitement aux éliciteurs active les réactions de défense chez les plants de riz et peut être une stratégie respectueuse de l’environnement pour maîtriser M. graminicola dans le cadre d’un système intégré de gestion.
Introduction
Rice (Oryza sativa L.), one of the major staple cereals, feeds almost half of the world’s population (Prasad et al. Citation2017). Several factors affect rice production and yield, such as water scarcity, poor management practices, high labour costs, and plant diseases.
Over 35 genera of nematodes parasitize rice. The main pathogen of irrigated rice is the root-knot nematode Meloidogyne graminicola Golden & Birchfield (Dutta et al. Citation2012; Kyndt et al. Citation2014), responsible for yield losses of 20 to 80% (Kyndt et al. Citation2014; Zhan et al. Citation2018). Second-stage juveniles (J2), also called infective juveniles, penetrate root tips and migrate into the cortex until reaching the vascular cylinder (Dutta et al. Citation2012; Kyndt et al. Citation2014), where they induce the formation of feeding sites. These complex structures are composed of large feeding cells with increased number of nuclei and ribosomes and dense, granular cytoplasm. Cell hyperplasia and hypertrophy present macroscopically as hook-like galls (Kyndt et al. Citation2013).
Nematode control in rice is a challenge aggravated by the lack of resistant genotypes. Most commercial cultivars of O. sativa are susceptible to nematodes (Cabasan et al. Citation2018). Chemical control methods, although effective, are often not economically viable because of the low market value of rice (Le et al. Citation2009) and carry the risk of contaminating groundwater. Biological control is environmentally friendly but difficult to apply in flooded systems (Le et al. Citation2009; Seenivasan et al. Citation2012). For increased efficacy, these management techniques should be combined with other methods.
Resistance induction through biotic or abiotic elicitors may be a promising method to manage Meloidogyne in rice. Bacillus and Trichoderma are prominent examples of biotic elicitors. These beneficial microorganisms were shown to activate induced systemic resistance (ISR) through the jasmonic acid and ethylene pathways (Molinari & Baser Citation2010). Abiotic elicitors are in general chemical compounds that induce systemic acquired resistance (SAR) by activating the salicylic acid route (Araújo et al. Citation2016; Puerari et al. Citation2019). Important examples are silicates and acibenzolar-S-methyl (ASM), both of which were shown to enhance plant defences against nematodes through a cascade of hypersensitivity reactions, production of reactive oxygen species, lignin deposition, and upregulation of defence proteins (Molinari & Baser Citation2010; Zhu et al. Citation2013; Molinari et al. Citation2014; Puerari et al. Citation2019). The results are impaired nematode penetration, movement, and multiplication (Passardi et al. Citation2005; Wuyts et al. Citation2007; Ji et al. Citation2015).
Several molecules have shown great potential as elicitors of plant defence against nematodes in rice, such as dl-β-amino-n-butyric acid (BABA), ASM, and silicon (Ji et al. Citation2015; Mattei et al. Citation2017; Zhan et al. Citation2018). However, the development of nematode management strategies necessitates novel, more effective elicitors and a deeper understanding of their effects on plant responses to pathogens. This study aimed to identify compounds with the ability to control M. graminicola in rice and investigate their effects on plant defence-related enzyme activity.
Material and methods
General experimental procedures
All experiments were conducted in a net house at geographical coordinates 23°24′17.2″S 51°56′27.1″W, 551 m elevation, in Maringá, Paraná, Brazil. Rice (O. sativa cv. IRGA 424) seeds were sown in pots containing 700 mL of a 1:1 (v/v) mixture of Dark Red Latosol (classified according to EMBRAPA, Citation2018), and sand, previously autoclaved at 120°C for 2 h. The inoculum used in the experiments consisted of a single-species population of M. graminicola maintained on rice (O. sativa cv. IRGA 424). Nematodes were extracted from roots by the method developed by Hussey & Barker (Citation1973) and adapted by Boneti & Ferraz (Citation1981). Suspensions of eggs and second-stage juveniles (J2) were adjusted to the appropriate concentrations using a nematode counting chamber under a light microscope. Inoculations were performed at 15 days after germination by pipetting 1 mL of nematode suspension into two holes, each 3-cm deep and 1 cm from the base of the plant, and covering them with soil. Elicitors were applied at the doses recommended by the manufacturers. Treatments were applied via foliar spraying immediately after inoculation and every 15 days thereafter. All leaves of the plant were sprayed until complete wetting was achieved. Inoculated and uninoculated controls were sprayed with water.
Selection of the most effective elicitors of plant defence
The first experiment was conducted in a completely randomized design (CRD) to determine the most effective elicitors of M. graminicola resistance in rice. Ten treatments were used (), with six replications per treatment. The experiment was performed between February and April 2016 (average daily temperatures of 27.5°C) and repeated between April and June 2016 (average daily temperatures of 24.1°C). In this experiment, the rice plants were inoculated with 1500 eggs and J2 of M. graminicola.
Table 1. Treatments used in the study, resistance elicitors and applied doses
At 60 days after inoculation (DAI), plants were harvested and separated into roots and shoots. Shoots were incubated in dry paper bags in a ventilated oven at 60°C for 72 h and weighed using an analytical balance to obtain the shoot dry weight. Roots were washed under running water, placed on paper towels to remove excess moisture, and weighed to obtain the root fresh weight. Nematode extraction from roots was performed according to Hussey & Barker (Citation1973) and Boneti & Ferraz (Citation1981), & extracted nematodes were counted. The total number of nematodes was divided by the root weight to obtain the nematode population density (number of nematodes per gram of root), and the nematode reproduction factor was calculated by dividing the final population density by the initial population density.
This preliminary investigation revealed that two elicitors were the most effective in reducing M. graminicola population density in roots: a mannanoligosaccharide-based fertilizer (MOS), silicate clay, and ASM was also selected because it is officially registered as a resistance inducer by the Brazilian Ministry of Agriculture (MAPA). Thus, these compounds were selected for further experiments.
Validation of selected elicitors
A second experiment was conducted in a CRD, from March to May 2017, to confirm the effectiveness of the selected elicitors. The average daily temperature during this period was 24.7°C. Four treatments (ASM, MOS, silicate clay, and water) with six replications were used. Plants were inoculated with 1500 eggs and J2 of M. graminicola, as described in the ‘General experimental procedures’ section. At 30 and 60 DAI, plants were evaluated for nematode count, nematode population density, reproduction factor, shoot dry weight, and root fresh weight.
In vivo penetration assay
An in vivo experiment was performed in a CRD, from October to December 2017, to determine the effects of the selected elicitors on nematode penetration. The average daily temperature was 25.1°C. Four treatments (ASM, MOS, silicate clay, and water) and five replications were used. Fifteen-day-old rice seedlings were inoculated with 500 eggs and J2 of M. graminicola and treated by foliar spraying every 15-day-old. Nematode penetration in roots was determined at 6, 12, 18, 24, and 30 DAI by the acid fuchsin method (Byrd et al. Citation1983). Nematodes were counted and classified into J2, third-stage (J3), and fourth-stage (J4) juveniles and females (Kumari et al. Citation2016). The total nematode count of roots was determined at 30 DAI.
Plant defence-related enzyme activity
A fourth experiment was conducted in a CRD, between April and May 2018, to assess the effect of elicitors on plant defence-related enzyme activity. A 4 × 4 factorial design was used, with four treatments (ASM, MOS, silicate clay, and water), four experimental periods (6, 8, 10, and 12 DAI), and four replications. The daily average temperature was 25.4°C. Fifteen-day-old rice seedlings were inoculated with 1500 eggs and J2 of M. graminicola and treated by foliar spraying.
At 6, 8, 10, and 12 DAI, the roots were collected, washed, dried with paper towels, weighed, wrapped in aluminium foil, and stored at −18°C until use. Samples were ground in liquid nitrogen and 4 mL of a solution containing 1% (w/w) polyvinylpyrrolidone and 0.1 mM EDTA buffer in 50 mM potassium phosphate buffer (pH 7.0) was added. The mixture was homogenized and centrifuged at 15 000 rpm and 4°C for 30 min. The supernatant was stored at −18°C until analysis and used as enzyme extract for protein quantification and determination of enzyme activity.
Total proteins were determined by the Bradford method (Citation1976). Briefly, 50 µL of enzyme extract was added to 2.5 mL of Bradford reagent and homogenized for 5 min. Absorbance was read at 595 nm, and protein concentration was quantified against a standard curve of bovine serum albumin. Results are expressed as mg mL−1.
Phenylalanine ammonia-lyase (PAL, EC 4.3.1.5), catalase (CAT, EC. 1.11.1.6), peroxidase (POX, EC 1.11.1.7), and β-1,3-glucanase (GLU, EC 3.2.1.6) activities were determined spectrophotometrically (UV 5200S, Global Trade Technology, Brazil), in duplicate.
PAL activity was determined according to Mori et al. (Citation2001). Enzyme extract (100 µL) was mixed with 400 µL of 0.5 M Tris-EDTA buffer (pH 8.0) and 500 µL of phenylalanine solution. A blank was prepared by using distilled water instead of phenylalanine. The mixture was incubated at 40°C for 1 h, placed in an ice bath, and mixed with 60 µL of HCl buffer. Spectrophotometric readings were performed at 290 nm. PAL activity was determined against a standard curve of trans-cinnamic acid. Results are expressed as mg trans-cinnamic acid h−1 mg−1 protein (Umesha Citation2006).
CAT activity was determined according to Tománková et al. (Citation2006). Enzyme extract (100 µL) was added to 500 µL of substrate at 10s intervals. The mixture was incubated in a water bath at 37°C for 4 min, and the reaction was quenched using 500 µL of molybdate solution. A blank was prepared by adding 100 µL of enzyme extract, 500 µL of molybdate, and 500 µL of substrate directly to a cuvette in the spectrophotometer. Spectrophotometric analysis was carried out at 405 nm. CAT activity was estimated as the difference in H2O2 decomposition rate (extinction coefficient rate) between sample and control. Results are expressed as µmol min−1 mg−1 protein.
POX activity was determined by measuring the conversion of guaiacol to tetraguaiacol in the presence of hydrogen peroxide. Enzyme extract (100 µL) was mixed with 2.9 mL of substrate (7.25 µL of guaiacol and 8.874 µL of hydrogen peroxide diluted in 50 mM potassium phosphate buffer pH 7.0) and incubated at 30°C. Absorbance was read at 470 nm, and results were expressed as change in absorbance at 470 nm min−1 mg−1 protein (Lusso & Pascholati Citation1999).
GLU activity was determined by colourimetric quantification of reducing sugars released from laminarin (Vogelsang & Barz Citation1993). Briefly, 150 µL of enzyme extract was added to 150 µL of 2 mg mL−1 laminarin. A blank was prepared without laminarin. Samples were incubated in a water bath at 40°C for 60 min. After this period, 150 µL of laminarin was added to the control. Aliquots of 50 µL were mixed with 1.5 mL of 0.5% 4-hydroxybenzoic acid hydrazide in 0.5 M NaOH (Lever Citation1972), incubated at 100°C for 10 min, and cooled in an ice bath. Absorbance was read at 410 nm, and the concentration of released sugars was determined against a standard curve of glucose (0–1.3 mg mL−1). Results are expressed as mg glucose h−1 mg−1 protein.
Statistical analysis
Nematode reproduction, penetration, and enzyme activity data were transformed to to meet normality assumptions. Transformed data were subjected to analysis of variance (ANOVA) followed by the Scott–Knott test for comparisons between elicitors and the Student–Newman–Keuls test for other results. The level of significance was set at P < 0.05. Analyses were performed using Sisvar version 5.6. (Ferreira Citation2011).
Results
Nematode reproduction
Nematode population density was mainly reduced by MOS (90.7%), silicate clay (85.9%), and organic fertilizer (70.3%) (). Biological compost, ASM, and sulphur fertilizer reduced population density by 52.0, 50.7, and 48.7%, respectively. Copper fertilizer and manganese phosphite had the lowest efficiencies; population density in plants treated with these products did not differ from that of the control (). MOS and silicate clay resulted in the greatest reduction in M. graminicola reproduction factor compared with the control, 86.8 and 75.5%, respectively ().
Table 2. Shoot dry weight, root fresh weight, nematode per gram of root, and nematode reproduction factor of rice (Oryza sativa cv. IRGA 424) plants inoculated with 1500 eggs and second-stage juveniles of Meloidogyne graminicola and treated every two weeks with resistance elicitors at 60 days after inoculation
Treatments had no effect on vegetative parameters, except for shoot dry weight during the first experiment for elicitor selection (). Shoot dry weight was highest in the control, not varying between treatments.
MOS and silicate clay were chosen for further experiments, as they promoted the best results. ASM was also selected because it is officially registered in MAPA and for having presented reduction of nematodes per gram of root in the repetition of the experiment ().
A second experiment was conducted in 2017 to confirm the effectiveness of the selected products. ASM, MOS, and silicate clay reduced the number of nematodes per gram of root by 72.6%, 65.5%, and 70.1% at 30 DAI. At 60 DAI, MOS led to the highest reduction in population density (80.2%). ASM (59.6%) and silicate clay (33.0%) treatments did not differ from the control (). The reproduction factor decreased by 76.2% with ASM, by 60.3% with MOS, and by 72.0% with silicate clay at 30 DAI and by 64.2, 49.3, and 40.8% at 60 DAI, respectively ().
Table 3. Shoot dry weight, root fresh weight, nematode per gram of root, and nematode reproduction factor of rice (Oryza sativa cv. IRGA 424) plants inoculated with 1500 eggs and second-stage juveniles of Meloidogyne graminicola and treated every two weeks with resistance elicitors at 30 and 60 days after inoculation
Although no differences in vegetative variables were observed between 30 and 60 DAI (), it was clear that ASM affected plant development. The aerial parts of plants treated with ASM were yellowish, and shoot dry and root fresh weight decreased by 37.87 and 45.87% in relation to the control, respectively ().
Nematode penetration
At 6 DAI, all treatments decreased J2 penetration in rice roots compared with the control, with mean reductions of 72.5% for ASM, 55.5% for MOS, and 58.3% for silicate clay (). There were no differences in J2 penetration between control and treatments at 12 or 18 DAI, but a lower number of J3 was found in plants treated with ASM, MOS, and silicate clay (in descending order) compared with the control, possibly as a result of the lower J2 penetration at 6 DAI (). At 18 DAI, no differences in J2 numbers were observed between treatments, and J3 numbers followed the same trend observed at 12 DAI. The number of J4, however, was lower in ASM-treated plants than in the control (). At 24 DAI, there was an increase in J3 in all treatments, not differing from the control, but a lower number of J4 in plants treated with elicitors, confirming that elicitors delayed nematode development ().
Fig. 1 Number of J2, J3, J4, and females of Meloidogyne graminicola in rice (Oryza sativa cv. IRGA 424) plants treated every 2 weeks with resistance elicitors at 6 (a), 12 (b), 18 (c), 24 (d), and 30 (e) days after inoculation. (f) Total number of nematodes at 30 days after inoculation. Means followed by the same letter do not differ significantly at P < 0.05 by the Student–Newman–Keuls test
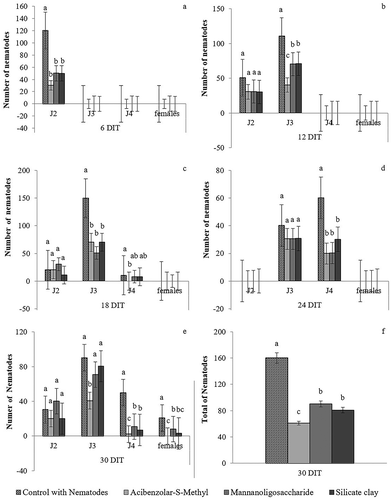
At 30 DAI, M. graminicola completed its life cycle. No differences were observed in J2 count between treatments. Plants treated with ASM had the lowest numbers of J3, J4, and females, showing the efficiency of the elicitor in delaying the development of parasites in rice. Plants treated with MOS or silicate clay had higher numbers of J4 and females at 30 DAI (). ASM, MOS, and silicate clay reduced nematode penetration by 57.5, 42.5, and 45.0%, respectively ().
Enzyme activity
There was a significant interaction of treatment and experimental period on enzyme activity, showing that enzyme activity varied over time ().
Table 4. ANOVA for enzyme activity in roots of rice (Oryza sativa cv. IRGA424) plants treated with resistance elicitors and inoculated with 1500 eggs and second-stage juveniles of Meloidogyne graminicola at 6, 8, 10, and 12 days after inoculation
Peak PAL activity was observed at 6 DAI in control plants and at 10 DAI in ASM-treated plants. PAL activity in MOS- and silicate-treated plants was highest at 6 and 10 DAI, not varying between the two periods. A significant difference between treatments was observed at 8 DAI, when PAL activity was highest in MOS, and at 10 DAI, when all treatments had higher PAL activity than the control ().
Fig. 2 Enzyme activity in roots of rice (Oryza sativa cv. IRGA424) plants treated with resistance elicitors and inoculated with 1500 eggs and second-stage juveniles of Meloidogyne graminicola at 6, 8, 10 and 12 days after inoculation (DAI). Within each period, means followed by the same lowercase letters do not differ at p < 0.05 by the Student–Newman–Keuls test. Within each treatment, means followed by the same uppercase letters do not differ at P < 0.05 by the Student–Newman–Keuls test
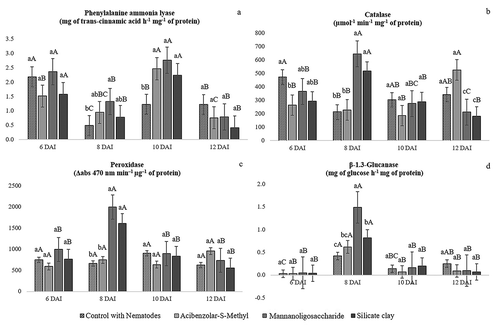
CAT activity in control plants varied little over time, decreasing slightly at 8 DAI but increasing thereafter. In ASM-treated plants, CAT activity remained low during most of the study period, with a peak at 12 DAI. For plants treated with MOS or silicate clay, enzyme activity peaked at 8 DAI (). An increase in CAT activity promoted by elicitor treatment was observed at 8 DAI, when plants treated with MOS and silicate clay had higher CAT activity than control and ASM-treated plants.
POX activity remained constant throughout the experimental period in control and ASM-treated plants. On the other hand, plants treated with MOS or silicate clay had higher POX activity at 8 DAI than plants treated with ASM and the control ().
For all treatments, including the control, GLU activity peaked at 8 DAI. At this time point, GLU activity was higher in plants treated with MOS than in plants treated with other elicitors ().
Discussion
The susceptibility of rice to M. graminicola is widely reported (Kyndt et al. Citation2014; Zhan et al. Citation2018). This nematode is a serious threat to crop health, especially because commercial rice cultivars are not resistant to plant-parasitic nematodes (Cabasan et al. Citation2018). Sedentary nematodes alter plant cell biology by injecting substances from oesophageal glands, impairing the host’s defence response and ensuring long-term feeding (Gheysen & Mitchum Citation2011). Meloidogyne graminicola infection reduces the expression of plant defence genes, such as OsPAL1, pathogenesis-related genes (OsPR10, OsPR1a, and OsPR1b), jasmonic acid carboxyl methyltransferase gene (OsJMT1), and ethylene and salicylic acid biosynthesis pathway gene (OsICS1) (Kyndt et al. Citation2011). According to Kyndt et al. (Citation2011), nematodes affect the hormonal homoeostasis of plants and modulate systemic signalling by suppressing local and systemic defence mechanisms.
In this study, plants treated with ASM, MOS, and silicate clay were more resistant to J2 penetration and nematode development. Elicitors were likely able to induce a state of alert, i.e., a primed state. Priming is a process by which plants prepare to respond more quickly and effectively to pathogens. Protective mechanisms, such as SAR or ISR, are activated, which trigger various physiological, transcriptional, metabolic, and epigenetic changes (Mauch-Mani et al. Citation2017). Under these conditions, the production of phytoalexins, glucosinolates, phenolic compounds, hormones, and pathogenesis-related proteins is increased. This state can be induced by pathogens, beneficial microorganisms, or chemical substances (Mauch-Mani et al. Citation2017).
Nematode penetration was detected only after 6 DAI, later than the expected for this pathosystem. This result probably occurred because the inoculum was composed mainly of eggs; egg development and hatching may take 5 to 6 days (Rao & Israel Citation1973). At 6 DAI, nematodes were detected mainly in control plants, whereas treated plants were more resistant to penetration. In some cases, plants may respond quickly to elicitor treatment. In a study of rice susceptibility to nematodes, plants treated with BABA showed reduced nematode penetration 50 h after application (Ji et al. Citation2015). Similar to the observed results in the current study, Puerari et al. (Citation2013) reported a reduction in Meloidogyne javanica (Treub) Chitwood penetration 7 days after maize was treated with ASM.
The hypothesis that elicitors induce a primed state in plants was confirmed by the increase in PAL expression. This enzyme plays a key role in the phenylpropanoid pathway, which involves the biosynthesis of polyphenols, flavonoids, and lignin precursors (Barros et al. Citation2016). Lignin is a component of the cell wall and vascular tissues. It confers mechanical resistance to cells, reducing nematode penetration (Gheysen & Jones Citation2006; Ji et al. Citation2015) and plant exposure to degradative enzymes released during the infection process (Gheysen & Jones Citation2006; Wuyts et al. Citation2007). Studies have shown that plants resistant to M. graminicola, as well as rice plants treated with elicitors, deposit and accumulate lignin in the endoderm of cells surrounding the nematode feeding site, affecting nematode nutrition (Ji et al. Citation2015; Galeng-Lawilao et al. Citation2018; Zhan et al. Citation2018). This process explains not only the reduction in J2 penetration in treated plants, but also the delay in nematode development observed in this study.
Other mechanisms may also have contributed for the greater resistance of plants to the nematode. There was an increase in CAT activity at 12 DAI in plants treated with ASM, and an increase in CAT and POX at 8 DAI in plants treated with MOS and silicate clay. These enzymes are related to resistance induction (Zhan et al. Citation2018; Puerari et al. Citation2019) and plant protection via promotion of hydrogen peroxide detoxification (Passardi et al. Citation2005). Hydrogen peroxide acts as a signalling molecule in local and systemic responses. These signals trigger the expression of defence genes and strengthen the cell wall by stimulating the formation of crosslinks between structural proteins (proline- or hydroxyproline-rich glycoproteins) and polysaccharides (Passardi et al. Citation2005; Fürstenberg-Hägg et al. Citation2013; Zhan et al. Citation2018). Moreover, hydrogen peroxide has a direct toxic effect on pathogens, inhibiting their development (Passardi et al. Citation2005; Ji et al. Citation2015; Huang et al. Citation2015).
POX accumulation is related to the release of reactive oxygen species by the plant. The enzyme reduces oxidative stress caused by nematode infection (Melillo et al. Citation2006; Nguyen et al. Citation2011). Previous studies have shown that hydrogen peroxide-mediated defence responses are more expressive in plants treated with resistance inducers (Anita & Samiyappan Citation2012; Mostafanezhad et al. Citation2014; Zhan et al. Citation2018).
GLU activity was high at 8 DAI in plants treated with MOS or silicate clay. This enzyme is a pathogenesis-related protein, and its expression is induced as a response to biotic and abiotic stresses via the signalling pathways of ethylene, salicylic acid, and jasmonic acid (Bernard et al. Citation2017). GLU is commonly expressed in nematode-infected plants, as it acts in the lysis of glycoprotein and carbohydrate complexes accumulated in the nematode cuticle (Sundararaj & Kathiresan Citation2012). In plants treated with resistance inducers, however, the increase in GLU is associated with the accumulation of phytoalexins, which act directly on a variety of pathogens (Ahuja et al. Citation2012).
The results confirmed our initial hypothesis that elicitors can induce resistance in rice, indicating their potential in integrated nematode management. Control strategies that reduce initial penetration and limit nematode development and reproduction inside the root are particularly important in flooded cultivation systems. Primary M. graminicola infection usually occurs when the soil is not completely flooded; however, juveniles hatched from eggs deposited under flooding may not succeed in infecting roots (Cabasan et al. Citation2012). On the other hand, it is common for females to lay eggs inside the root, increasing the difficulty of controlling nematodes with products that are applied to the soil.
ASM and silicate clay increased PAL activity at 10 and 8 DAI, respectively. ASM also activated CAT at 12 DAI, which suggests that ASM may have contributed to cell wall lignification and reduced oxidative stress. ASM is an analogue of salicylic acid, which characteristically promotes SAR activation through the expression of pathogenesis-related proteins (Molinari & Baser Citation2010). Despite affording good results in M. graminicola control, ASM was less efficient than the other treatments.
Silicate clay, in addition to enhancing PAL activity at 8 and 10 DAI, increased CAT, POX and GLU expression at 8 DAI. Silicate likely contributed to cell resistance, hampering the accesses of the pathogen to cellular contents, as suggested in previous studies (Mattei et al. Citation2017; Zhan et al. Citation2018). POX can promote callose deposition, accumulation of hydrogen peroxide, and production of lignin and phenolic compounds in root galls (Araújo et al. Citation2016; Nascimento et al. Citation2016).
Of all the elicitors investigated, MOS promoted the highest increase in enzyme activities; all enzymes were activated in at least one evaluation period. This is the first study to report the resistance-inducing effects of MOS in rice, but similar effects were observed in lettuce, eggplant, and cucumber against M. javanica (Karajeh Citation2013; Mokbel & Alharbi Citation2014; Toninato et al. Citation2019).
We highlight that, although elicitors may be effective in controlling M. graminicola, they may affect vegetative development, as observed in ASM-treated plants. Reduction in plant growth can be attributed to the high-energy expenditure associated with activation of resistance mechanisms (Walters & Heil Citation2007). Field experiments are needed to assess the impact of resistance elicitors on crop productivity and investigate whether the presence of beneficial microorganisms and adequate plant nutrition can minimize side effects.
This study underscores the importance of investigating not only known products, such as ASM and silicate clay, but also novel elicitors, such as MOS, as potential agents to control M. graminicola in rice in a sustainable, integrated management. Further research is needed to better understand the metabolic pathways and genes activated by MOS in plants.
References
- Ahuja I, Kissen R, Bones AM. 2012. Phytoalexin in defense against pathogens. Trends Plant Sci. 17:73–90. doi:10.1016/j.tplants.2011.11.002.
- Anita B, Samiyappan R. 2012. Induction of systemic resistance in rice by Pseudomonas fluorescens against rice root knot nematode Meloidogyne graminicola. J Biopesticides. 5:53–59.
- Araújo L, Paschoalino RS, Rodrigues FA. 2016. Microscopic aspects of silicon-mediated rice resistance to leaf scald. Phytopathology. 106:132–141. doi:10.1094/PHYTO-04-15-0109-R.
- Barros J, Serrani-Yarce JC, Chen F, Baxter D, Venables BJ, Dixon RA. 2016. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat Plants. 2:1–8. doi:10.1038/nplants.2016.50.
- Bernard GC, Egnin M, Bonsi C. 2017. The impact of plant-parasitic nematodes on agriculture and methods of control. In: Shah MM, Mahamood M, editors. Nematology - concepts, diagnosis and control. Rijeka (Croatia): Intech Open; p. 121–150.
- Boneti JI, Ferraz S. 1981. Modificações do método de Hussey and Barker para extração de ovos de Meloidogyne exigua em raízes de cafeeiro [Modification of the Hussey and Barker method for extracting Meloidogyne exigua eggs from coffee roots]. Fitopatol Bras. 6:551–553. (Portuguese).
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 7:248–254. doi:10.1016/0003-2697(76)90527-3.
- Byrd DW, Kirpatrick T, Barker KR. 1983. An improved technique for clearing and staining plant tissue for detection of nematodes. J Nematol. 15:142–143.
- Cabasan MTN, Kumar A, DeWaele D. 2012. Comparison of migration, penetration, development and reproduction of Meloidogyne graminicola on susceptible and resistant rice genotypes. Nematology. 14:405–415. doi:10.1163/156854111X602613.
- Cabasan MTN, Kumar A, DeWaele D. 2018. Evaluation of resistance and tolerance of rice genotypes from crosses of Oryza glaberrima and O. sativa to the rice root-knot nematode, Meloidogyne graminicola. Trop Plant Pathol. 43:230–241. doi:10.1007/s40858-018-0210-8.
- Dutta TK, Ajoy K, Ganguly GHS. 2012. Global status of rice root-knot nematode, Meloidogyne graminicola. Afr J Microbiol Res. 6:6016–6021. doi:10.5897/AJMR12.707.
- Empresa Brasileira de Pesquisa Agropecuária-EMBRAPA. 2018. Sistema Brasileiro de Classificação de Solos. Revista e Ampliada. 5:356.
- Ferreira DF. 2011. Sisvar: a computer statistical analysis system. Ciência Agrotecnologia. 35:1039–1042. doi:10.1590/S1413-70542011000600001.
- Fürstenberg-Hägg J, Zagrobelny M, Soren B. 2013. Plant defense against insect herbivores. Int J Mol Sci. 14:10242–10297.
- Galeng-Lawilao J, Kumar A, Ma A, Cabasan TN, DeWaele D. 2018. Comparison of the penetration, development and reproduction of Meloidogyne graminicola, and analysis of lignin and total phenolic content in partially resistant and resistant recombinant inbred lines of Oryza sativa. Trop Plant Pathol. 1:1–12.
- Gheysen G, Jones J. 2006. Molecular aspects of plant-nematode interactions. In: RN P, Mones M, editors. Plant nematology. Wallingford: CABI; p. 234–254.
- Gheysen G, Mitchum MG. 2011. How nematodes manipulate plant development pathways for infection. Curr Opin Plant Biol. 14:415–421. doi:10.1016/j.pbi.2011.03.012.
- Huang WK, Ji HL, Gheysen G, Debode J, Kyndt T. 2015. Biochar-amended potting medium reduces the susceptibility of rice to root-knot nematode infections. BMC Plant Biol. 15:267. doi:10.1186/s12870-015-0654-7.
- Hussey RS, Barker KRA. 1973. Comparison of methods for colecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Rep. 57:1025–1028.
- Ji H, Kyndt T, He W, Vanholme B, Gheysen G. 2015. β-aminobutyric acid-induced resistance against root-knot nematodes in rice is based on increased basal defense. MPMI. 28:519–533. doi:10.1094/MPMI-09-14-0260-R.
- Karajeh MR. 2013. Efficacy of Saccharomyces cerevisiae on controlling the root-knot nematode (Meloidogyne javanica) infection and promoting cucumber growth and yield under laboratory and field conditions. Arch Phytopathol P Flanzenschutz. 46:2492–2500. doi:10.1080/03235408.2013.799819.
- Kumari C, Dutta TK, Banakar P, Rao U. 2016. Comparing the defence-related gene expression changes upon root-knot nematode attack in susceptible versus resistant cultivars of rice. Sci Rep. 6:1–17. doi:10.1038/srep22846.
- Kyndt T, Fernandez DA, Gheysen G. 2014. Plant-parasitic nematode infections in rice: molecular and cellular insights. Annu Review Phytopathol. 52:135–153. doi:10.1146/annurev-phyto-102313-050111.
- Kyndt T, Nahar K, Haegeman A, De Vleesschauwer D, Hofte M, Gheysen G. 2011. Comparing systemic defense-related gene expression changes upon migratory and sedentary nematode attack in rice. Plant Biology. 14:73–82. doi:10.1111/j.1438-8677.2011.00524.x.
- Kyndt T, Vieira P, Gheysen G, Almeida-Engler J. 2013. Nematode feeding sites: unique organs in plant roots. Planta. 238:807–818. doi:10.1007/s00425-013-1923-z.
- Le HTT, Padgham JL, Sikora RA. 2009. Biological control of the rice root-knot nematode Meloidogyne graminicola on rice, using endophytic and rhizosphere fungi. Int J Pest Manage. 55:31–36. doi:10.1080/09670870802450235.
- Lever M. 1972. A new reaction for colorimetric determination of carbohydrates. Anal Biochem. 47:273–279. doi:10.1016/0003-2697(72)90301-6.
- Lusso MFG, Pascholati SF. 1999. Activity and isoenzymatic pattern of soluble peroxidases in maize tissues after mechanical injury or fungal inoculation. Summa Phytopathol. 25:244–249.
- Mattei D, Dias-Arieira CR, Lopes APM, Miamoto A. 2017. Influence of Rocksil®, Silifort® and wollastonite on penetration and development of Meloidogyne javanica in Poaceae and Fabaceae. J Phytopathol. 165:91–97. doi:10.1111/jph.12540.
- Mauch-Mani B, Baccelli I, Luna E, Flors V. 2017. Defense priming: an adaptive part of induced resistance. Annu Rev Plant Biol. 68:485–512. doi:10.1146/annurev-arplant-042916-041132.
- Melillo MT, Leonetti P, Bongiovanni M, Castagnone-Sereno P, Bleve-Zacheo T. 2006. Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato-root-knot nematode interactions. New Phytol. 170:501–512. doi:10.1111/j.1469-8137.2006.01724.x.
- Mokbel AA, Alharbi AA. 2014. Suppressive effect of some microbial agents on root-knot nematode, Meloidogyne javanica infected eggplant. Aust J Crop Sci. 8:1428–1434.
- Molinari S, Baser N. 2010. Induction of resistance to root-knot nematodes by SAR elicitors in tomato. Crop Prot. 29:1354–1362. doi:10.1016/j.cropro.2010.07.012.
- Molinari S, Fanelli E, Leonetti P. 2014. Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol Plant Pathol. 152:55–64.
- Mori T, Sakutai M, Sakuta M. 2001. Effects of conditioned medium on activities of PAL, CHS, DAHP synthase (DS-Co and Ds-Mn) and anthocyanin production in suspension cultures of Fragaria ananassa. Plant Sci. 160:355–360. doi:10.1016/S0168-9452(00)00399-X.
- Mostafanezhad H, Sahebani N, Zarghani SN. 2014. Induction of resistance in tomato against root-knot nematode Meloidogyne javanica with salicylic acid. Crop Prot. 3:499–508.
- Nascimento KJT, Araujo LR, Sousa R, Schurt DAS, Washington L, Rodrigues FA. 2016. Silicon, acibenzolar-S-methyl and potassium phosphite in the control of brown spot in rice. Bragantia. 75:212–221. doi:10.1590/1678-4499.281.
- Nguyen DMC, Seo DJ, Park RD, Lee BR, Jung WJ. 2011. Changes in antioxidative enzyme activities in cucumber plants with regard to biological control of root-knot. J Korean Soc Appl Bi. 54:507–514.
- Passardi F, Cosio C, Penel C, Dunand C. 2005. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 24:255–265. doi:10.1007/s00299-005-0972-6.
- Prasad R, Shivay YS, Kumar D. 2017. Current status, challenges, and opportunities in rice production. In: Chauhan SB, Jabran K, Mahajan G, editors. Rice production worldwide. Cham (Switzerland): Springer International Publishing; p. 1–15.
- Puerari HH, Dias-Arieira CR, Dadazio TS, Mattei D, Silva TRB, Ribeiro RCF. 2013. Evaluation of acibenzolar-S-methyl for the control of Meloidogyne javanica and effects on the development of susceptible and resistant soybean. Trop Plant Pathol. 38:44–48. doi:10.1590/S1982-56762013000100006.
- Puerari HH, Miamoto A, Jardinett VA, KRF S-E, Dias-Arieira CR. 2019. Enzymatic activity induced by acibenzolar-S-methyl for control of Pratylenchus brachyurus in maize. J Plant Physiol Pathol. 7:1–9.
- Rao Y, Israel PS. 1973. Life history and bionomics of Meloidogyne graminicola the rice root-knot nematode. Indian Phytopathol. 16:333–336.
- Seenivasan N, David PMM, Vivekanandan P, Samiyappan R. 2012. Biological control of rice root-knot nematode, Meloidogyne graminicola through mixture of Pseudomonas fluorescens strains. Biocontrol Sci Techn. 22:611–632. doi:10.1080/09583157.2012.675052.
- Sundararaj P, Kathiresan T. 2012. Induction of β-1,3-glucanase and chitinase activities in resistant and susceptible sugarcane clones inoculated with Pratylenchus zeae. Int Nematol. 22:1–10.
- Tománková K, Lenka L, Marek PI, Pavel P, Ales L. 2006. Biochemical aspects of reactive oxygen species formation in the interaction between Lycopersicon spp. and Oidium neolycopersici. Physiol Mol Plant Path. 68:22–32. doi:10.1016/j.pmpp.2006.05.005.
- Toninato BO, Souza DHG, Pontalti PR, Lopes APM, Dias-Arieira CR. 2019. Meloidogyne javanica control in lettuce with fertilizers applied isolated or associated with biological product. Horticultura Brasileira. 37:271–276. doi:10.1590/s0102-053620190404.
- Umesha S. 2006. Phenylalanine ammonia lyase activity in tomato seedlings and its relationship to bacterial canker disease resistance. Phytoparasitica. 34:68–71. doi:10.1007/BF02981341.
- Vogelsang R, Barz W. 1993. Purification, characterization and differential hormonal regulation of a beta-1.3-glucanase and two chitinases from chickpea (Cicer arietinum L.). Planta. 189:60–69. doi:10.1007/BF00201344.
- Walters D, Heil M. 2007. Costs and trade-offs associated with induced resistance. Physiol Mol Plant Path. 71:3–7. doi:10.1016/j.pmpp.2007.09.008.
- Wuyts N, Lognay G, Verscheure M, Marlier M, DeWaele D, Swennen R. 2007. Potential physical and chemical barriers to infection by the burrowing nematode Radopholus similis in roots of susceptible and resistant banana (Musa spp.). Plant Pathol. 56:878–890. doi:10.1111/j.1365-3059.2007.01607.x.
- Zhan LP, Peng DL, Wang X, Kong LA, Peng H, Liu SM, Liu Y, Huang WK. 2018. Priming effect of root-applied silicon on the enhancement of induced resistance to the root-knot nematode Meloidogyne graminicola in rice. BMC Plant Biol. 18:50. doi:10.1186/s12870-018-1266-9.
- Zhu QH, Stephen S, Kazan K, Jin G, Fan L, Taylor J, Dennis ES, Helliwell CA, Wang MB. 2013. Characterization of the defense transcriptome responsive to Fusarium oxysporum-infection in Arabidopsis using RNA-seq. Gene. 512:259–266. doi:10.1016/j.gene.2012.10.036.
