Abstract
Cannabis (Cannabis sativa L., marijuana) plants grown under greenhouse or controlled environments with symptoms of leaf yellowing, leaf necrosis and defoliation were observed during 2018–2019. Additional symptoms included crown rot and internal browning or blackening of the pith tissues. Stock (mother) plants as well as plants in the vegetative and flowering stages of 15 cannabis strains (genotypes) were affected. In addition, damping-off symptoms were observed on rooted cuttings in propagation rooms. Isolations from diseased tissues yielded predominantly Fusarium proliferatum, with some F. oxysporum also recovered. Phylogenetic analysis of sequences from the translation elongation factor 1α (TEF-1 α) region of 29 isolates of F. proliferatum from eight licenced production facilities in three provinces in Canada (British Columbia, Ontario and New Brunswick), and one cannabis production site in northern California, grouped isolates from cannabis with a large clade of isolates from a wide range of other hosts in different geographic regions. Pathogenicity studies confirmed the ability of F. proliferatum to cause symptoms of wilting, leaf and pith necrosis, and plant death on cuttings, rooted plants and stock plants. Inoculated tomato and cucumber plants developed similar symptoms. Stem colonization was more extensive by F. proliferatum compared to F. oxysporum on cannabis cuttings. Both grew optimally at 25°C on agar media although F. oxysporum grew faster than F. proliferatum at all temperatures tested. The occurrence of F. proliferatum on cannabis plants has not been previously reported, adding to recent reports of F. oxysporum and F. solani that cause similar symptoms on cannabis plants.
Résumé
En 2018-2019, on a observé des plants de cannabis (Cannabis sativa L., marijuana) cultivés en serre ou dans des environnements contrôlés affichant des symptômes de jaunissement et de nécrose des feuilles ainsi que de défoliation. D’autres symptômes incluaient la pourriture du collet et le brunissement interne ou le noircissement de la moelle. Des plants mères, de même que des plants au stade végétatif et de la floraison de 15 souches (génotypes) de cannabis étaient touchés. De plus, on a observé, dans les salles de propagation, des symptômes de fonte des semis chez les boutures racinées. Des isolements de tissus infectés ont révélé la présence de Fusarium proliferatum, principalement, ainsi que, dans une moindre mesure, de F. oxysporum. L’analyse phylogénétique des séquences de la région du facteur d’élongation de la traduction 1α (TEF-1 α) de 29 isolats de F. proliferatum provenant de 8 installations de production autorisées de 3 provinces canadiennes (Colombie-Britannique, Ontario et Nouveau-Brunswick) et d’une installation du nord de la Californie a regroupé les isolats de cannabis dans un grand clade d’isolats provenant d’une vaste gamme d’autres hôtes de différentes régions géographiques. Des études de pathogénicité ont confirmé la capacité de F. proliferatum de provoquer les symptômes du flétrissement, de la nécrose des feuilles et de la moelle ainsi que de causer la mort chez les boutures, les plants racinés et les plants mères. Des plants de tomate et de concombre inoculés ont développé des symptômes similaires. Chez les boutures de cannabis, la colonization des tiges par F. proliferatum était plus extensive que celle résultant de F. oxysporum. Les deux ont crû de façon optimale à 25°C sur de la gélose dextrosée à la pomme de terre, bien que F. oxysporum se soit développé plus rapidement que F. proliferatum, et ce, à toutes les températures testées. L’occurrence de F. proliferatum sur les plants de cannabis n’avait pas été rapportée jusqu’à maintenant, ce qui s’ajoute aux récents rapports traitant de F. oxysporum et de F. solani qui causent des symptômes analogues sur les plants de cannabis.
Introduction
Cultivation of cannabis (Cannabis sativa L., marijuana) occurs mainly in greenhouses and controlled environment facilities throughout Canada, although an increasing number of field sites are also being approved for production, in accordance with Health Canada requirements (Health Canada Citation2019). With the increasing cultivation area of cannabis plants, the incidence of previously unreported pathogens is growing. Identification of the pathogens that affect cannabis plants and recognition of the associated symptoms is necessary in order for cannabis producers to be able to identify and manage the increased prevalence of these diseases. Currently, a number of pathogens infecting cannabis plants have been described in Canada. These include pathogens infecting the inflorescences, causing bud rots, such as Botrytis cinerea, Penicillium species, and several Fusarium species (F. oxysporum, F. solani, F. sporotrichioides) (Punja et al. Citation2019; Punja Citation2020b, Citation2020d). Pathogens which infect the foliage include the powdery mildew pathogens (Punja Citation2018, Citation2020c). Finally, the previously described root and crown-infecting pathogens include Fusarium oxysporum, F. solani, several Pythium species (Punja and Rodriguez Citation2018; Punja et al. Citation2020; Punja Citation2020a), and Cylindrocarpon (Punja Citation2020e).
In this study, the occurrence of F. proliferatum causing root and crown rot, and pith necrosis, of cannabis plants is described for the first time. The pathogen was found to be present in seven licenced production facilities in three provinces in Canada, and in one cannabis production site in northern California. The pathogen was observed to be more aggressive in pathogenicity tests compared to the recently described report of F. oxysporum (Punja Citation2020a), causing root and crown rot, and caused disease at all stages of cannabis production, from propagation to vegetative growth to flowering, as well as infecting the inflorescences. The progression of the pathogen into the pith tissues following inoculation is demonstrated through light and scanning electron microscopy.
Materials and methods
Sampling of plants
Cannabis plants showing symptoms of leaf yellowing, stunted growth, and necrosis of leaves and browning of roots, were included in the study. The plants were sampled at various stages of development, ranging from early propagation (1–2 weeks old) to vegetative growth (3–4 weeks of age) to onset of full flowering period (6–12 weeks of age) (Punja Citation2020a). They were grown in greenhouses or in controlled environment rooms in Health Canada approved licenced facilities. Five were located in British Columbia, one in Ontario, and one in New Brunswick. In addition, one production site in northern California was included. The propagation medium used was either coco fibre (coco coir) or rockwool blocks and plants were grown with the appropriate nutrient regimes and lighting conditions as required for commercial hydroponic production (Small Citation2017). Microbial isolations were conducted during July 2018–November 2019 and were made from over 200 symptomatic plants belonging to 15 cannabis strains (genotypes). In addition, stock (mother) plants grown in 10 L containers (6–10 months of age) were included for sampling (). The main stem of six diseased stock plants (8–10 months of age) was cut down and partitioned into 30-cm long increments, beginning at the crown and proceeding alongside branches to the top of the plant (>200 cm). From each increment, a 5-cm long stem piece was dissected from the mid-point and surface-sterilized as described below.
Table 1. Isolates of Fusarium proliferatum originating from cannabis (Cannabis sativa L., marijuana) plants included in this study
Fig. 1 Symptoms of infection caused by Fusaium proliferatum on stock (mother plants). (a) Healthy plants approximately 6 months of age. (b) Advanced infection causing yellowing and necrosis of leaves, and wilting symptoms. (c) Total collapse and defoliation of a severely diseased plant. (d) Symptoms of crown decay in the plant shown in (b) with black streaks. (e) Cross-section through a stem of a stock plant showing early internal black discoloration in the pith tissues. (f) More advanced internal stem discoloration and decay of the pith and xylem tissues. (g) Pith decay resulting from infection by F. proliferatum at a distance of 100 cm from the crown. Stem on the far right is from a healthy plant. (h) More advanced pith decay showing black streaks in the pith and shredding of the tissues. (i) Colonies of F. proliferatum recovered from diseased pith tissues shown in (g)

Fig. 2 Recovery of Fusarium proliferatum from diseased stem tissues and growth characteristics on potato dextrose agar. (a) Surface view (left) and bottom view (right) of isolation plates showing 100% recovery of F. proliferatum from diseased pith tissues after 7 days. (b) A one-month-old isolation plate showing fluffy white aerial mycelium. (c, d) Six week-old colonies with white aerial mycelial strands on the colony surface (c) and intense purple pigmentation produced on the underside of the colonies (d). (e) Comparison of the growth of six isolates of F. proliferatum on PDA after 10 days. (f-h) Scanning electron micrographs of mycelium and spore production by F. proliferatum in culture. (f) Microconidia produced in false heads. (g, h) Microconidia produced in long chains
Isolation from tissues
Small pieces, ca. 0.5 cm in length of root segments or 0.2–0.4 cm2 for cuttings, stem pieces and bud tissues, were surface-disinfested by dipping them in a 10% bleach solution (0.625% NaOCl) for 1 min, followed by 30 s in 70% EtOH. The tissues were rinsed in sterile water and blotted on paper towels. For larger diameter stem and pith tissues, the sterilization protocol was increased to 2 min in NaOCl and 30 s in EtOH. Tissue pieces were plated onto potato dextrose agar (PDA, Sigma Chemicals, St. Louis, MO) amended with 130 mg L−1 of streptomycin sulphate (PDA+S). At least 20 pieces were plated from each sample, four in each Petri dish. Dishes were incubated under ambient laboratory conditions (temperature range of 21–24°C with 10–12 hr day−1 fluorescent lighting) for 5–10 days. Emerging colonies were transferred to fresh PDA+S dishes for subsequent identification to genus level using morphological criteria, including colony colour and size and microscopic examination of spores. Up to 150 isolates putatively identified as Fusarium spp. by morphological features as described by Leslie and Summerell (Citation2006) were obtained from the different tissue types, among which both F. oxysporum and F. proliferatum were then identified using the PCR method described below. From these, 29 representative isolates of F. proliferatum were selected for phylogenetic analysis to represent different tissue sources and origins (). Colonies were transferred to water agar and hyphal tip transfers were made onto PDA+S prior to molecular analysis.
Molecular identification
Species-level identification of Fusarium isolates was conducted using PCR with primers for the translation elongation factor 1α (TEF-1 α) region: EF- 1 (5ʹATG GGT AAG GAG GAC AAG AC 3ʹ) and EF-2 (5ʹ GGA GGT ACC AGT GAT CAT GTT 3ʹ) (O’Donnell et al. Citation1998). Cultures of representative isolates from different tissue sources () were grown on potato dextrose agar at room temperature for 7 days and DNA was extracted from harvested mycelium scraped from the colony surface using the QIAGEN DNeasy Plant Mini Kit. Aliquots of 1 uL containing 5–20 ng DNA was used for PCR in a 25 uL reaction volume consisting of 2.5 uL 10X buffer (containing 15 mM MgCl2), 0.5 uL 10 mM dNTP, 0.25 uL Taq DNA Polymerase (QIAGEN), 0.25 uL 10 mM forward and reverse primers, as well as 20.25 uL DNAse- and RNAse-free water (Invitrogen). All PCR amplifications were performed in a MyCycler thermocycler (BIORAD) with the following program: 3 min at 94°C; 30 s at 94°C, 30 s at 60°C, 3 min at 72°C (35 cycles); and 7 min at 72°C. PCR products were separated on 1% agarose gels and bands of the expected size (ca. 700 bp) were purified with QIAquick Gel Extraction Kit and sent to Eurofins Genomics (Eurofins MWG Operon LLC 2016, Louisville, KY) for sequencing. The resulting sequences were compared to the corresponding EF-1 α sequences from the National Centre for Biotechnology Information (NCBI) GenBank database. Multiple sequence alignment of the respective isolates was done using the CLUSTAL W program (http://www.genome.jp/tools/clustalw). The sequences of F. proliferatum were subsequently included in a phylogenetic analysis using the neighbour-joining (NJ) method and a bootstrap consensus tree was inferred from 1000 replicates as described previously (Punja and Rodriguez Citation2018).
Effect of temperature on radial growth
To assess the effects of temperature on colony growth, 9-cm diameter Petri dishes containing 25 mL of PDA were inoculated with a 5 mm2 mycelial plug taken from one-week old cultures of F. proliferatum originating from pith tissues (isolate BC-5) and F. oxysporum (isolate BC-4). Replicates of five Petri dishes were placed in temperature controlled incubators maintained at 5, 10, 15, 20, 25, 30 and 35°C. The actual observed temperatures were within ± 1°C. The diameters of all colonies were measured after 6 days of incubation from two perpendicular measurements of each colony and averaged. The experiment was conducted twice and the data averaged.
Pathogenicity studies
Test-tube inoculations
A cotton plug was placed in the bottom of 18 × 2.5-cm glass test-tubes and moistened with sterile distilled water. A mycelial plug (8-mm diameter) from 7-day old PDA cultures of either F. proliferatum or F. oxysporum was placed on top of the cotton plug, mycelial side facing up. The pathogenicity of an isolate of F. proliferatum obtained from diseased pith tissues (isolate BC-5, ) was compared to isolate BC-4 of F. oxysporum from damped-off cuttings (Punja Citation2020a). A stem cutting (approx. 15 cm in height) was then inserted into the test-tube to ensure contact was made between the base of the cutting and the mycelial plug. Control tubes received a PDA plug. The test-tubes were sealed with caps and placed upright in a test-tube rack for 7–10 days under ambient conditions. After this time, the extent of mycelial development on the stem cutting was measured with a ruler. Five cannabis strains were assessed: ‘OG Kush’, ‘Hash Plant’, ‘Copenhagen Kush’, ‘White Rhino’ and ‘Island Honey’. There were four replicates of each strain and isolate and the experiment was conducted twice. The data from both experiments were averaged and variation was expressed as standard errors.
Stem cutting inoculations
For inoculation of stem cuttings, the base of stem segments (15-cm height) from stock plants of strains ‘Hash Plant’ and ‘White Rhino’ were dipped into a spore suspension of F. proliferatum for 5 min and inserted into pre-moistened 4 cm2 rockwool cubes (Grodan). The isolate was previously grown on PDA plates for 7–10 days under ambient laboratory conditions. The plates were flooded with 10 mL of sterile distilled water, the colony surface was rubbed with a glass rod, and the resulting spore suspension was poured through two layers of cheesecloth. Water was added as required to achieve a concentration of 1 × 106 spores mL−1 as quantified in a haemocytometer. All rockwool cubes containing inoculated cuttings were placed inside a plastic tray, a 1-cm layer of water was added, and covered with a plastic dome to maintain high humidity and placed in a Conviron incubator set at 24°C with a 24-hr photoperiod for two weeks. Controls consisted of noninoculated cuttings. For each experimental trial, there were five replicate cuttings used. Re-isolations were made from diseased tissues following the surface-sterilization procedure described previously and plating tissues onto PDA+S. The experiment was conducted three times.
Rooted plant inoculation
Cuttings of strain ‘Hash Plant’ or ‘Critical Kali Mist’ were inserted into Jiffy-7® peat pellets (http://www.jiffypot.com/) or rockwool cubes that had been pre-soaked in Current Culture H2O® hydroponic nutrient solution (https://www.cch2o.com/) to promote rooting (Punja and Rodriguez Citation2018). After two weeks, the rooted cuttings were transferred to a coco coir:perlite (3:1) potting medium in 10 cm2 pots. An isolate of F. proliferatum originating from pith tissues (BC-5) was grown in potato dextrose broth shake cultures (100 mL) for 7–10 days at 150 rpm and the mycelial mat from two flasks was blended with 200 mL of water for 20 s. To determine the inoculum level, dilutions of the mycelial + spore suspension were made in sterile distilled water (up to 10−3) and 50 uL was spread onto the surface of PDA+S plates and incubated at 21–24°C for 5 days, at which time colony-forming units (CFU mL−1) were quantified visually. Plants were inoculated with 20 mL of the mycelial + spore suspension which was poured around the base of each plant. A scalpel was then inserted into the soil at multiple points around the roots to create wounds. The pots were placed in a plastic tray containing a 1-cm layer of water, and covered with a plastic dome to maintain high humidity and placed in a Conviron incubator set at 24°C with a 24-hr photoperiod. Symptoms of yellowing or stunting of the plants were recorded after one and two weeks. There were four replicate plants for each strain. Re-isolations were made from diseased tissues following the surface-sterilization procedure described previously and plating tissues onto PDA+S. The experiment was conducted twice.
Stock plant inoculations
Inoculations were made on 4 month old stock plants of strain ‘Space Queen’ grown in the coco-perlite mix by pouring 150 mL of a mycelial + spore suspension prepared as described above around the base of the plant. A scalpel was then inserted into the soil at multiple points around the roots to create wounds. The plants were placed in a Conviron incubator set at 24°C with a 24-hr photoperiod. Symptoms of yellowing, necrosis or stunting of the plants were recorded after one and two weeks. Re-isolations were made from diseased crown tissues following the surface-sterilization procedure described previously and plating tissues onto PDA+S. The experiment was conducted twice.
Inoculation of tomato and cucumber plants
To determine whether F. proliferatum from cannabis plants could infect tomato and cucumber plants, the following experiments were conducted. Seeds of tomato ‘Moneymaker’ (West Coast Seeds) were planted in the coco:perlite mix and plants grown for two weeks under 16-hr day−1 supplemental lighting at 23–26°C. The plants (replicated six times) were then uprooted, roots washed under running tap water, and the bottom 4 cm was trimmed off with a pair of scissors. The plants were immersed for 10 min in a mycelial + spore suspension prepared as described above. Control plants (six replicates) had the roots trimmed and were dipped in PDB broth. All plants were re-potted and grown for an additional two weeks, at which time symptoms of disease were recorded. Cucumber seeds ‘Tasty Green’ (West Coast Seeds) were grown for three weeks under the same conditions as the tomato plants and were uprooted and had the roots trimmed and dipped in inoculum for 10 min and re-potted. Symptoms of disease were recorded after one week. The experiments were conducted twice.
Light and scanning electron microscopy
Fungal samples
Colonies of F. proliferatum growing on PDA+S for two weeks were used to examine spore morphology. Small pieces of agar (3 mm2) with mycelium were adhered to an SEM stub using a graphite-water colloidal mixture (G303 Colloidal Graphite, Agar Scientific, UK) and Tissue-Tek (O.C.T. Compound, Sakura Finetek, NL). The sample was submerged in a nitrogen slush for 10–20 s to rapidly freeze it. After freezing, the sample was placed in the preparation chamber of a Quorum PP3010 T cryosystem attached to a FEI Helios NanoLab 650 scanning electron microscope (4D Labs., Simon Fraser University). The frozen sample was sublimed for 5 min at −80°C, after which a thin layer of platinum (10 nm thickness) was sputter-coated onto the sample for 30 s at a current of 10 mA. The sample was moved into the SEM chamber and the electron beam was set to a current of 50 pA at 3 kV. Images were captured at a working distance of 4 mm, at a scanning resolution of 3072 × 2207 collected over 128 low-dose scanning passes with drift correction.
Pith tissue samples
Stem cuttings were taken from stock plants of strains ‘Hash Plant’ and ‘Island Honey’. The basal portion of the cutting was sliced into 5-mm long sections which were each placed under a dissecting microscope and photographed to show the internal structure and extent of development of the pith tissues. The same stems were used to obtain similar slices which were examined under the scanning electron microscope as described above to show the cellular composition of the pith cells and surrounding tissues. Stem sections were also stained with a 1% solution of safranin red for 30 min to show the lignin deposition within the xylem tissues.
Inoculated cuttings
Stem cuttings were inoculated with F. proliferatum by dipping them in a spore suspension as described above and incubated at 24°C. Pieces of stem segments (5 mm in length) that were showing early and advanced stages of external colonization by mycelium and with light brown external tissue discoloration (4–8 days after inoculation) were excised and examined under the dissecting and scanning electron microscopes as described above. Controls consisted of noninoculated stem cuttings incubated under similar conditions. Observations were made of the integrity of the pith tissues and of parenchyma cells comprising the pith.
Results
Pathogen distribution in stock plants
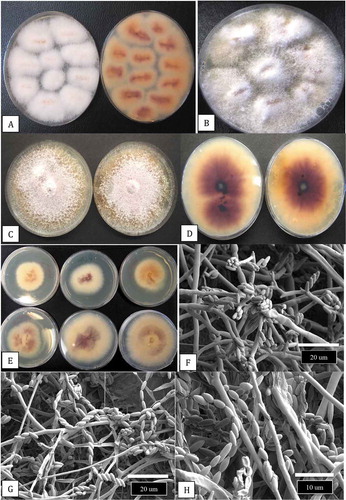
Stock (mother) plants sampled in this study had reached a height of 2–3 m and attained a basal stem diameter of 4–5 cm after 6–10 months of growth (). Symptoms of disease that affected some of the plants (5–10% of the total) included necrosis of leaves and defoliation (). At advanced stages of disease development, necrosis of leaves and stem death led to plant mortality (). At the crown region of many diseased plants, the internal tissues were discoloured (). A black rot was visible internally around the pith tissues when the plants were cut down (). The internal discoloration was present in the stem at distances of 100–150 cm from the crown (). At advanced stages of disease development, the pith and surrounding tissues were shredded (). From surface-sterilized crown and stem tissues of diseased plants, colonies of Fusarium proliferatum (identified as described below) were recovered at a frequency of 90–100% from the crown region (). The colonies produced fluffy aerial mycelium on PDA and developed an intense purple pigment when viewed from the underside (). A range of colony pigmentation and growth rates were observed among isolates originating from different sample sources () which were all identified as F. proliferatum. Under the scanning electron microscope, microconidia were observed to be produced in large numbers on PDA cultures, both in false heads at the ends of phialides as well as in long chains ().
Development of disease symptoms
Plants showing symptoms from which F. proliferatum was isolated included rooted vegetative cuttings growing in coco blocks displaying extensive necrosis of leaves and plant collapse (). Symptoms on flowering plants included yellowing of leaves, browning of the tips and margins of leaves, and wilting (). When the stems of these symptomatic plants were cut open, extensive decay of the pith and surrounding tissues was observed, which extended to distances of 30–50 cm from the crown region (). Asymptomatic cuttings taken from 100–150 cm distance away from the crown of stock plants and inserted into PDA showed presence of F. proliferatum at a frequency of around 1% after 7 days ().
Fig. 3 Development of symptoms caused by Fusarium proliferatum on cannabis plants in the vegetative and flowering stages. (a) Foliar necrosis and collapse of rooted cuttings. (b) Marginal necrosis of leaves. (c, d) Extensive yellowing and necrosis of foliage of flowering plants of strains ‘Hash Plant’ and ‘White Rhino’. (e, f) Internal stem necrosis of the pith and xylem tissues of flowering plants shown in (c) and (d). (g) Recovery of F. proliferatum from cuttings taken 100 cm from the crown of diseased plants and inserted into PDA slants. Mycelial growth was visible after 5 days

Molecular identification and phylogenetic analysis
Isolations made from around 200 symptomatic plants representing 15 cannabis strains (genotypes) resulted in over 300 isolates of Fusarium spp. (identified by morphological features as described by Leslie and Summerell Citation2006) being recovered, of which 150 were subcultured and retained. These isolates were examined for colony characteristics, pigmentation and spore production using the morphological features shown in to attempt to distinguish F. proliferatum from F. oxysporum which was also recovered from these tissues. Subsequently, PCR of the TEF-1 α region was conducted to yield a band size of approximately 700 bp () in all isolates. Sequence analysis showed that among the 150 isolates, 105 were F. proliferatum and 45 were F. oxysporum. A subset of 29 isolates of F. proliferatum () were included in the phylogenetic analysis. They included isolates recovered from 15 cannabis strains (genotypes) from eight licenced production facilities in three provinces in Canada, and from one cannabis production site in northern California. The tissues from which the isolates were obtained included diseased crowns and stem and pith tissues of stock plants and flowering plants, damped-off cuttings, brown roots on flowering plants, and infected flower buds (). A few isolates were also recovered from air sampling conducted by exposing Petri dishes in the growing environment for 60 min as described by Punja (Citation2020a). The phylogenetic analysis () grouped isolates of F. proliferatum from cannabis within a large clade of isolates originating from a wide range of other hosts in different geographical locations worldwide. There was limited genetic variability among isolates representing the species based on EF-1 sequences. Isolates of F. oxysporum and F. solani previously isolated from cannabis (Punja and Rodriguez Citation2018) were clearly separated from F. proliferatum ().
Fig. 4 PCR band of size ca. 700 bp obtained for 20 isolates of Fusarium proliferatum from cannabis plants (BC-5, BC-6, BC-8, BC-9, BC-10, BC-11, BC-12, BC-13, BC-14, BC-15, BC-17, BC-18, BC-21, BC-22, BC-24, BJ-1, NB-2, ON-1, CA-1, CA-2) using the EF-1α primer set. Lane C is the water control. Molecular weight standard is shown (L)
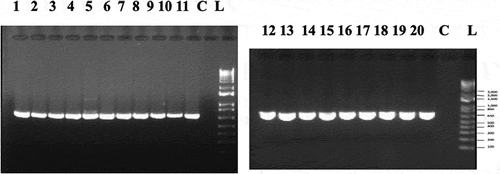
Fig. 5 Phylogenetic analysis of 23 isolates of Fusarium proliferatum originating from cannabis plants (see ) using EF-1α sequences compared to isolates from other hosts (GenBank numbers are shown). Isolates were obtained from a range of tissue sources and from different licenced facilities in BC, ON, and NB, and one site in CA. A bootstrap consensus tree was inferred from 1000 replicates to represent the distance using the neighbour-joining (NJ) method. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates were collapsed. The outgroup was Sclerotinia sclerotiorum.
Effect of temperature on radial growth
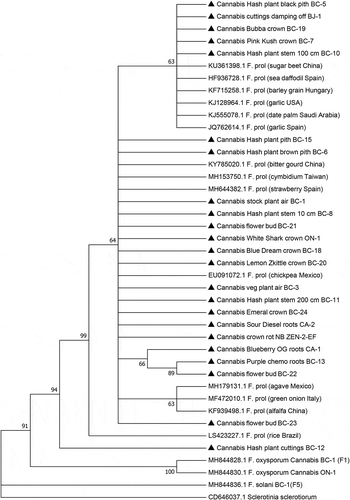
A comparison of the extent of colony growth of an isolate each of F. proliferatum and F. oxysporum on PDA at seven temperatures is shown in . The optimal temperature for radial growth of both species was 25°C, and growth was reduced at 5–10°C but still occurred at 35°C. At all temperatures tested, F. oxysporum grew faster than F. proliferatum and colony diameters after 6 days at 25°C were 72 mm and 50 mm, respectively (). When viewed from the underside, colonies grown at all temperatures showed a pink-purple pigmentation, which was most pronounced at 20, 25 and 30°C ().
Fig. 6 (a) Comparison of radial growth of an isolate of Fusarium proliferatum (BC-5) with F. oxysporum (BC-4) at seven temperatures after 6 days. Means from five replicate dishes ± standard error are shown. The experiment was conducted twice. (b) Comparison of growth of F. proliferatum after 6 days at 10–35°C, at 5°C increments. The underside of the colonies are shown to reveal the extent of pigmentation produced
Pathogenicity studies
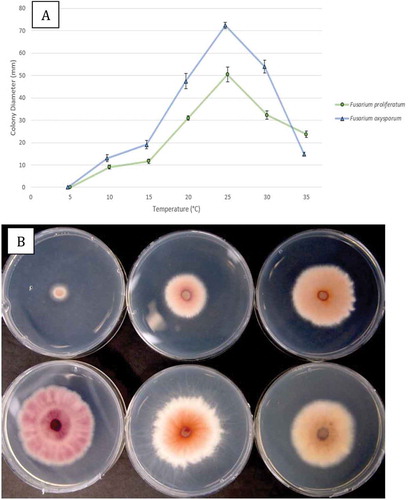
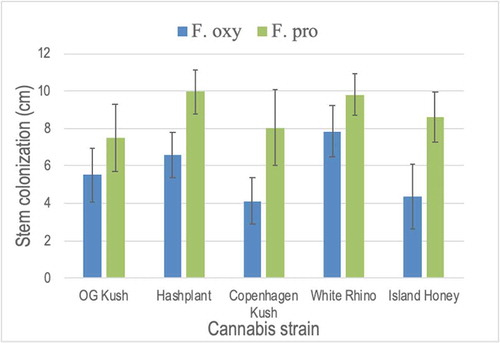
In the test-tube inoculation method using cuttings, mycelial growth of F. proliferatum was extensive after 7 days on ‘Hash Plant’ (), and infected cuttings removed from the test-tube showed necrosis and wilting of the leaves (). Inoculation of cuttings of ‘White Rhino’ grown in rockwool cubes showed similar extensive growth of mycelium on the stems, resulting in wilting of the plant (). In a coco:vermiculite growing medium, inoculation of cuttings of ‘Hash Plant’ with an inoculum level of 1 × 106 CFU mL−1 showed mycelial growth developing on the stem and initial wilting was observed after 5 days (), with more extensive mycelial development and yellowing and wilting of the plants occurring within 8 days (). Noninoculated control plants showed no symptoms (). On rooted inoculated cannabis plants of strain ‘Critical Kali Mist’, symptoms of leaf necrosis and wilting of plants were observed within 7–10 days (). On inoculated tomato and cucumber plants, disease symptoms were severe and included total plant collapse and necrosis of leaves within one week on cucumber plants and within two weeks for tomato (). On larger cannabis stock plants of strain ‘Space Queen’, symptoms of foliar necrosis developed on lower leaves within 5 days (), and entire branches showed necrosis and wilting after two weeks (). The crown of affected plants was discoloured and mycelial growth was evident in the pith tissues further up the stem (). Isolations made from diseased plants yielded colonies of F. proliferatum. Using the test-tube inoculation method to compare the pathogenicity of F. proliferatum and F. oxysporum on five cannabis strains, the results showed that stem colonization was more extensive by F. proliferatum in ‘Hash Plant’, ‘Copenhagen Kush’ and ‘Island Honey’ and similar to F. oxysporum in ‘OG Kush’ and ‘White Rhino’ (). All cannabis strains were considered to be susceptible to both Fusarium species, with F. proliferatum appearing to be more pathogenic on some strains.
Fig. 7 Pathogenicity testing of Fusarium proliferatum using three different methods. (a) Stem cuttings of ‘Hash Plant’ were inoculated in a test-tube with a mycelial plug. Mycelial growth on the stem is shown after 7 days of incubation. (b) Cuttings removed from the test-tubes to show mycelial colonization of the stem and wilting of the cuttings. (c) Inoculation of cuttings of ‘Hash Plant’ by dipping in a spore suspension for 5 min and inserting into a rockwool block. Stem colonization and wilting can be seen after 7 days. (d, e) Inoculation of rooted cuttings of ‘Hash Plant’ grown in a coco coir: perlite potting mixture with a mycelial+spore suspension. (d) Wilting symptoms after 7 days. (e) Stem colonization and plant death after two weeks. (f) Non-inoculated control plant shows no symptoms after two weeks. (g) Symptoms on ‘Critical Kali Mist’ plants two weeks after inoculation with a mycelial + spore suspension. Extensive wilting and foliar necrosis can be seen on inoculated plant (right). (h) Symptoms on a tomato plant inoculated with F. proliferatum (right) compared to the non-inoculated control plant (left) after two weeks. (i) Symptoms on a cucumber plant inoculated with F. proliferatum (right) compared to the non-inoculated control plant (left) after two weeks. (j) Symptoms of foliar necrosis developing on the lower part of a stock plant inoculated with a mycelial + spore suspension of F. proliferatum after one week. (k) The same plant after two weeks showing stem die-back and wilting. (l) Decay of the crown tissues and mycelial growth inside the stem of the diseased plant shown in (k)

Fig. 8 Pathogenicity testing and response of five cannabis strains to inoculation with Fusarium oxysporum and F. proliferatum in a test-tube assay. The extent of stem colonization was measured after 7 and 10 days. Data shown are for 10 days and are means of four replicates ± standard errors. The experiment was conducted twice. Strains tested in each test-tube were: ‘OG Kush’, ‘Hash Plant’, ‘Copenhagen Kush’, ‘White Rhino’ and ‘Island Honey’. While there were no differences among strains in response to the two Fusarium species, colonization by F. proliferatum was significantly greater on ‘Hash Plant’, ‘Copenhagen Kush’, and ‘Island Honey’ when compared to F. oxysporum.
Light and scanning electron microscopy of pith tissues
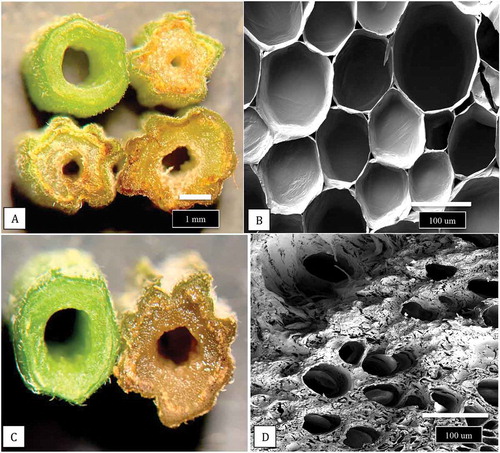
Sections made through the stems of cuttings of cannabis plants, when examined under both dissecting and scanning electron microscopes, revealed the developmental progression of the pith tissues (). The cuttings showed a progression from larger diameter pith areas with densely packed pith cells () to smaller diameter pith areas with loosely packed cells (), to pith regions in which large hollow centres developed (). The development of xylem vessel elements and tracheids can be seen in . Scanning electron microscopy of a healthy stem cutting taken from a stock plant showed the hollow pith surrounded by a ring of xylem tissues (). Healthy stem sections that were stained with 1% safranin red showed the uptake of the red dye in the ring of xylem cells (). Scanning electron microscopy of a section made through a young cannabis stem cutting showed the organization of the outermost epidermal cell layer, with a dense covering of hair-like trichomes, the inner ring of xylem cells, and the innermost central parenchyma pith cells. The cutting was inoculated with a spore suspension and the mycelium progressed into the pith within 5 days after inoculation. In contrast to non-inoculated stem cuttings, in which the parenchyma cells surrounding the pith region were normal and the tissues were green (), inoculated stem tissues showed disruption of these tissues and intense browning developed (). When cells were examined by scanning electron microscopy, they had collapsed and there was a build-up of a dense extracellular matrix that could be a combination of cellular contents and polysaccharides () compared to healthy cells ().
Fig. 9 The extent of development of the central pith tissues in cannabis stem cuttings. The cuttings were obtained from stock plants at the same stage of growth and sequential stem pieces were taken (from top to bottom) and sectioned by hand and 3 mm thick slices were placed on the observation stage of a dissecting microscope and photographed at the same magnification. (a, b) Early stage of pith development with densely packed central parenchyma cells. (c, d) Loosely packed parenchyma cells in the pith region. (e, f) Development of hollow central pith where parenchyma cells are absent. (g, h) Complete development of central pith in stem cutting of cannabis strain ‘Hash Plant’. Scale bars shown in a and b apply to all figures
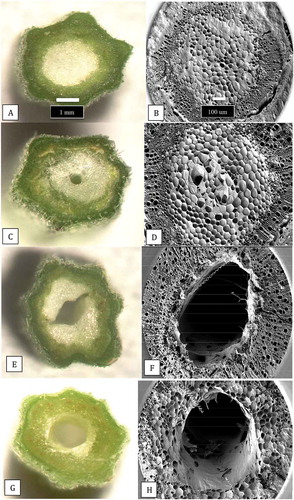
Fig. 10 (a) Scanning electron micrograph of a healthy stem cutting taken from a stock plant showing the hollow pith surrounded by a ring of xylem tissues. A portion of the remaining pith cells (arrow) that has not disintegrated can be seen. (b) Healthy stem sections that were stained with 1% safranin red to show the uptake of the red dye in the ring of xylem cells (arrow). (c) Scanning electron micrograph of a section made through a young cannabis stem cutting to show the organization of the outermost epidermal cell layer with a dense covering of hair-like trichomes, the inner ring of xylem cells, and the innermost central parenchyma pith cells. The cutting was inoculated with a spore suspension of Fusarium proliferatum and the mycelium has progressed into the pith (white arrow). The advancement of the mycelial front is shown by the blue arrows. Photo was taken 5 days after inoculation
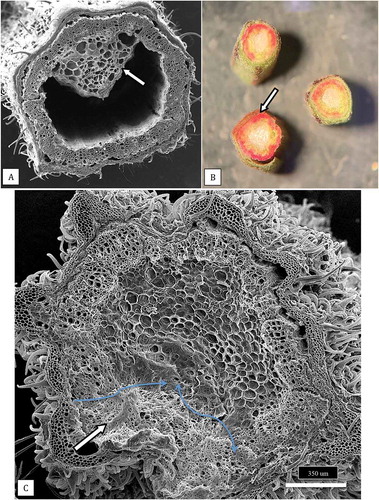
Fig. 11 Comparison of hand sections made through a non-inoculated stem cutting with those infected by F. proliferatum. (a) Healthy cutting (top left) compared with three different stages of necrosis and tissue disruption caused by pathogen invasion of inoculated stem cuttings. (b) Healthy parenchyma cells viewed under the scanning electron microscope. (c) Close-up of a healthy cutting (left) with a diseased cutting (right). (d) Parenchyma cells in a diseased cutting that are disrupted and show accumulation of a dense matrix that could be a combination of cellular contents and polysaccharides
Discussion
The isolation of F. proliferatum (Matsushima) Nirenberg (Gibberella intermedia Kuhlman) from a range of symptomatic tissues of cannabis plants, as described in this study, adds to the previously described reports of F. oxysporum and F. solani causing root and crown rot (Punja and Rodriguez Citation2018; Punja Citation2020a), as well as F. brachygibbossum causing crown infection on field-grown plants (Punja et al. Citation2018). The overlapping symptoms that develop following infection by these four pathogens requires that isolation and pathogenicity studies be conducted to confirm which species are present. In this study, F. oxysporum was frequently recovered in conjunction with F. proliferatum. The pathogenicity of isolates of F. oxysporum has been confirmed in a previous study (Punja Citation2020a). While F. proliferatum produces more white aerial mycelium, forms spores in chains and in false heads, develops a more intense purple pigment on the underside of colonies on PDA, and grows more slowly than F. oxysporum, PCR of the EF-1 region was required to conclusively confirm the identity of the isolates. Additional molecular approaches used to distinguish between these two Fusarium species have included the use of calmodulin gene sequences (Mule et al. Citation2004; Chang et al. Citation2015), the internal transcribed spacer region of rDNA (Quazi Citation2013), and the intergeneric spacer region of rDNA (Palmero et al. Citation2012). The optimal temperature for growth of F. proliferatum was 25°C, confirming results from an earlier study (Marin et al. Citation1995). The growth of F. proliferatum was slower than that of F. oxysporum but it was more pathogenic on cannabis stem cuttings.
The epidemiology of F. proliferatum is quite similar to that of F. oxysporum (Punja Citation2020a), and the pathogen was recovered from cuttings, vegetative and flowering plants, and stock plants, and was aerially dispersed and also recovered from infected inflorescences. The primary differences in symptomology between the two pathogens were that F. proliferatum caused a distinct browning/blackening within the pith tissues of affected plants and foliar necrosis was more noticeable compared to yellowing of leaves caused by F. oxysporum (Punja Citation2020a). The pith tissues of plants are prone to infection by a range of pathogens affecting a number of different hosts. Visible browning of the pith tissues similar to that observed on cannabis plants in this study has been reported in several other diseases e.g. black shank of tobacco (Phytophthora nicotianae) (Gallup et al. Citation2006), brown stem rot of soybeans (Phialophora gregata) (Hadi Citation2016), fusarium stalk rot of corn (Fusarium moniliforme) (Faske and Kirkpatrick Citation2015), and pith necrosis of tomato (Pseudomonas corrugata) (Scarlett et al. Citation2015) and Xanthomonas perforans (Aiello et al. Citation2013). The pith tissues consist of loosely organized parenchyma cells that store and transport nutrients (Fujimoto et al. Citation2018). The morphological features of parenchyma cells and pith development in cannabis stem tissues, as shown by light and scanning electron microscopy in this study, have not been previously described. During the development of a central pith in plants such as sorghum, corn and rice, death of the cells in the stem pith parenchyma occurs in a manner similar to that of programmed cell death (PCD) (Beers Citation1997; Fujimoto et al. Citation2018). Activation of autolytic cell wall degrading enzymes resulted in the development of a central cavity (Beers Citation1997; Fujimoto et al. Citation2018). In sorghum, this cell death resulted in increased susceptibility to the anthracnose pathogen, Colletotrichum graminicola (Katsanos and Pappelis Citation1966). It is not clear whether pathogen spores and/or pathogen metabolites can spread within the pith tissues of cannabis plants but recovery of the pathogen at distances of 100–150 cm from the crown suggest that long-range spread internally of F. proliferatum is occurring in diseased plants, similar to that reported for F. oxysporum (Punja Citation2020a). The rapidity of symptom development on inoculated cannabis plants and the development of necrosis and wilting symptoms also suggest that pathogen metabolites are a part of the disease syndrome. SEM images of the pith tissues from inoculated cuttings showed collapse of parenchyma cells and cell death within 5 days, and was accompanied by an accumulation of a dense matrix in diseased pith tissues, likely due to an accumulation of polysaccharides and the result of host cell wall destruction. Mycelial growth was observed ramifying from the epidermal cells into the pith tissues.
Fusarium proliferatum is a member of the Gibberella fujikuroi species complex, which is comprised at least of 15 reproductively isolated biological species (mating populations), and infects a broad range of plant species and produces a range of mycotoxins (Leslie et al. Citation2004; Niehaus et al. Citation2016). Some of the more economically important host species affected by F. proliferatum are asparagus (Elmer Citation1990, Citation1995; Borrego-Benjumea et al. Citation2014), alfalfa (Cong et al. Citation2016), banana (Jimenez et al. Citation1993), citrus fruits (Hyun et al. Citation2000), corn (Logrieco et al. Citation1995; Munkvold Citation2003), garlic (Dugan et al. Citation2003; Palmero et al. Citation2012; Galvez et al. Citation2017), mango (Zhan et al. Citation2010), onion (Stankovic et al. Citation2007; Carrieri et al. Citation2013), orchids (Benyon et al. Citation1996), peach palm (Jarek et al. Citation2018), pistachio (Crespo et al. Citation2019), rice (Desjardins et al. Citation2000; Kim et al. Citation2012; Quazi et al. Citation2013; Choi et al. Citation2017, Citation2018), safflower (Kim et al. Citation2016), sesame (Torabi et al. Citation2014), soybean (Chang et al. Citation2015), sorghum (Leslie Citation2003), strawberry (Borrero et al. Citation2019), and wheat (Jurado et al. Citation2006; Conner et al. Citation2009). Symptoms on these hosts included damping-off, wilting, yellowing of leaves, soft rot, root and crown rot, vascular discoloration, as well as blackening of the crown and roots of diseased plants as seen on palm and strawberry plants (Jarek et al. Citation2018; Borrero et al. Citation2019). All of these symptoms were observed on diseased cannabis plants affected by F. proliferatum in this study.
The mycotoxins reported to be produced by F. proliferatum include the polyketide-derived fumonisins, with fumonisin B1 (FB1) being the most prevalent (Rheeder et al. Citation2002; Jurado et al. Citation2010; Stepien et al. Citation2011; Choi et al. Citation2017; Galvez et al. Citation2017). In addition, the following mycotoxins – beauvericin, enniatin, fusaric acid, fusarin, fusaproliferin, and moniliformin – are also produced by F. proliferatum (Marasas et al. Citation1986; Ritieni et al. Citation1995; Moretti et al. Citation1996; Bacon et al. Citation1996; Desjardins et al. Citation2000; Logrieco et al. Citation2002; Leslie et al. Citation2004; Desjardins Citation2006; Proctor et al. Citation2010; Niehaus et al. Citation2016; Galvez et al. Citation2017). It is not known if any of these metabolites are produced in diseased cannabis tissues but the infection of inflorescences by this pathogen, as reported in this study and in a recent study (Punja Citation2020b), may be of concern. In addition, the occurrence of F. sporotrichioides on diseased inflorescences (Punja Citation2020b) could also raise concern over the potential for mycotoxin production. This species produces a range of trichothecene mycotoxins, including T-2 toxin, neosolaniol, nivalenol, and HT-2 toxin (Kokkonen et al. Citation2012). In wheat plants, Guo et al. (Citation2016) demonstrated that seed-borne infection by F. proliferatum resulted in internal systemic spread of the pathogen within the stem tissues, which led to seed kernel infection and mycotoxin accumulation (fuminosin and beauvericin). The potential for systemic movement of Fusarium species through the pith and xylem tissues of cannabis plants to result in infection of the inflorescence needs to be investigated. This could potentially occur given that pathogen recovery was confirmed in this study at distances as far as 100–150 cm from the crown region. Previous studies also showed that F. oxysporum demonstrated endophytic colonization of stems (Punja et al. Citation2019) and was transmitted through cuttings taken from symptomless cannabis plants (Punja Citation2020a). In corn, inoculation studies conducted with a GFP-marked strain of Fusarium verticilliodes showed that the pathogen, when applied at the seedling stage, resulted in infection through the radicle which progressed upward through the stem tissues to the ear to infect kernels (Gai et al. Citation2018). The extent to which Fusarium spp. are transmitted as seed-borne pathogens in cannabis plants is not known but a similar infection cycle that involves spread though the pith could potentially give rise to pathogen-contaminated seed.
In British Columbia, F. proliferatum has been previously isolated from garlic bulbs and pine seedlings (Joshi and Jeffries Citation2018). In other regions of Canada, it also causes crown and root rot of asparagus (Vujanovic et al. Citation2006; Borrego-Benjumea et al. Citation2014) and soybeans (Chang et al. Citation2015) and stalk rot of corn (Reid and Zhu Citation2004) and has been detected in wheat seed (Clear and Patrick Citation1990). Fusarium proliferatum has been reported to be more pathogenic that F. oxysporum on soybeans (Chang et al. Citation2015), an observation confirmed in this study on inoculated cannabis cuttings. The pathogen is also capable of extensive colonization of organic matter in soil (Gaige et al. Citation2019). Fusarium oxysporum and F. proliferatum are frequently isolated from the same diseased plants, e.g. in asparagus (Borrego-Benjumea et al. Citation2014), soybeans (Chang et al. Citation2015), and peach palm (Jarek et al. Citation2018), similar to what was observed in this study. Dual inoculations with both species were not conducted given the severe symptoms produced by each individual species. The ability to produce an array of metabolically active compounds could be important in the infection potential of F. proliferatum on other hosts (Niehaus et al. Citation2016). Both cucumber and tomato plants inoculated in this study developed rapid necrosis of the foliage, followed by plant death, which may suggest a role for extracellular compounds in symptom development. Fusaric acid, for example, has been shown to be associated with symptom development in plants affected by F. proliferatum (Palmero et al. Citation2012).
The approaches to management of F. proliferatum should be similar to those suggested for F. oxysporum (Punja Citation2020a), given that both pathogens can occur concurrently in diseased cannabis tissues despite the different symptomologies. However, specific studies to address the management of F. proliferatum have not yet been conducted given the pathogen has not been previously described from cannabis. This represents the first report of F. proliferatum causing root and crown rot, and pith necrosis, on cannabis plants. The detection of this pathogen in eight licenced production facilities in three provinces in Canada (British Columbia, Ontario and New Brunswick), and one cannabis production site in northern California, suggests the pathogen may have been present for some time and been spread through diseased planting stock or through other means and remained undetected. The recovery of the pathogen from 15 cannabis strains suggests that identification of genetic resistance may prove to be challenging unless a broader spectrum of genotypes, which include land races, are evaluated (Punja et al. Citation2017).
Acknowledgements
Technical assistance provided by Samantha Lung, Emily Betz, Cameron Scott, Darren Sutton, Hayley Reekie, and Sarah Chen during various aspects of this project, is gratefully acknowledged. This work made use of the 4D LABS scanning electron microscopy facility supported by the Canada Foundation for Innovation (CFI), British Columbia Knowledge Development Fund (BCKDF), Western Economic Diversification Canada (WD), and Simon Fraser University (SFU)
Additional information
Funding
References
- Aiello D, Scuderi G, Vitale A, Firrao G, Polizzi G, Cirvilleri G. 2013. A pith necrosis caused by Xanthomonas perforans on tomato plants. Eur J Plant Pathol. 137(1):29–41. doi:10.1007/s10658-013-0214-7.
- Bacon CW, Porter JK, Norred WP, Leslie JF. 1996. Production of fusaric acid by Fusarium species. Appl Environ Microbiol. 62:4039–4043. doi:10.1128/AEM.62.11.4039-4043.1996.
- Beers EP. 1997. Programmed cell death during plant growth and development. Cell Death Differ. 4:649–661. doi:10.1038/sj.cdd.4400297.
- Benyon F, Summerell BA, Burgess LW. 1996. Association of Fusarium species with root rot of Cymbidium orchids. Australas Plant Path. 25:226–228. doi:10.1071/AP96041.
- Borrego-Benjumea A, Basallote-Ureba MJ, Melero-Vara JM, Abbasi PA. 2014. Characterization of Fusarium isolates from asparagus fields in southwestern Ontario and influence of soil organic amendments on Fusarium crown and root rot. Phytopathology. 104(4):403–415. doi:10.1094/PHYTO-08-13-0231-R.
- Borrero C, Capote N, Gallardo MA, Aviles M. 2019. First report of vascular wilt caused by Fusarium proliferatum on strawberry in Spain. Plant Dis. 103(3):581. doi:10.1094/PDIS-09-18-1544-PDN.
- Carrieri R, Raimo F, Pentangelo A, Lahoz E. 2013. Fusarium proliferatum and Fusarium tricinctum as causal agents of pink rot of onion bulbs and the effect of soil solarization combined with compost amendment in controlling their infections in field. Crop Prot. 43:31–37. doi:10.1016/j.cropro.2012.09.013.
- Chang KF, Hwang SF, Conner RL, Ahmed HU, Zhou Q, Turnbull GD, Strelkov SE, McLaren DL, Gossen BD. 2015. First report of Fusarium proliferatum causing root rot in soybean (Glycine max L.) in Canada. Crop Prot. 67:52–58. doi:10.1016/j.cropro.2014.09.020.
- Choi H-W, Hong SK, Lee YK, Kim WG, Chun S. 2018. Taxonomy of Fusarium fujikuroi species complex associated with bakanae on rice in Korea. Australas Plant Pathol. 47(1):23–34. doi:10.1007/s13313-017-0536-6.
- Choi JH, Lee S, Nah JY, Kim HK, Paek JS, Lee S, Ham H, Hong SK, Yun SH, Lee T. 2017. Species composition of and fumonisin production by the Fusarium fujikuroi species complex isolated from Korean cereals. Intern. J Food Microbiol. 267:62–69. doi:10.1016/j.ijfoodmicro.2017.12.006.
- Clear RM, Patrick SK. 1990. Fusarium species isolated from wheat samples containing tomb-stone (scab) kernels from Ontario, Manitoba, and Saskatchewan. Can J Plant Sci. 70:1057–1069. doi:10.4141/cjps90-128.
- Cong LL, Sun Y, Kang JM, Li MN, Long RC, Zhang TJ, Yang QC. 2016. First report of root rot disease caused by Fusarium proliferatum on alfalfa in China. Plant Dis. 100(12):25–26. doi:10.1094/PDIS-08-14-0790-SR.
- Conner RL, Hwang SF, Stevens RR. 2009. Fusarium proliferatum: a new causal agent of black point in wheat. Can J Plant Pathol. 18:419–423. doi:10.1080/07060669609500598.
- Crespo M, Lawrence DP, Nouri MT, Doll DA, Trouillas FP. 2019. Characterization of Fusarium and Neocosmospora species associated with crown rot and stem canker of pistachio rootstocks in California. Plant Dis. 103(8):1931–1939. doi:10.1094/PDIS-11-18-2012-RE.
- Desjardins AE. 2006. Fusarium mycotoxins: chemistry, genetics, and biology. St. Paul (MN): APS Press.
- Desjardins AE, Manandhar HK, Plattner RD, Manandhar GG, Poling SM, Maragos CM. 2000. Fusarium species from Nepalese rice and production of mycotoxins and gibberellic acid by selected species. Appl Environ Microbiol. 66:1020–1025. doi:10.1128/AEM.66.3.1020-1025.2000.
- Dugan FM, Hellier BC, Lupien SL. 2003. First report of Fusarium proliferatum causing rot of garlic bulbs in North America. Plant Pathol. 52:426. doi:10.1046/j.1365-3059.2003.00852.x.
- Elmer WH. 1990. Fusarium proliferatum as a causal agent in Fusarium crown and root rot of asparagus. Plant Dis. 74:938. doi:10.1094/PD-74-0938E.
- Elmer WH. 1995. A single mating population of Gibberella fujikuroi (Fusarium proliferatum) predominates in asparagus fields in Connecticut, Massachusetts, and Michigan. Mycologia. 87:68–71. doi:10.1080/00275514.1995.12026504.
- Faske T, Kirkpatrick T 2015. Corn diseases and nematodes. Chapter 7. In Arkansas corn production handbook. University of Arkansas Research and Extension. MP437. Accessed 2020 Feb 24. https://www.uaex.edu/publications/pdf/mp437/chapter7corn.pdf.
- Fujimoto M, Sazuka T, Oda Y, Kawahigashi H, Wu J, Takanashi H, Ohnishi T, Yoneda J-I, Ishimori M, Kajiya-Kanegae H, et al. 2018. Transcriptional switch for programmed cell death in pith parenchyma of sorghum cells. Proc Natl Acad Sci USA. 115(37):E8783–E8792. doi:10.1073/pnas.1807501115
- Gai X, Dong H, Wang S, Liu B, Zhang Z, Li X, Gao Z. 2018. Infection cycle of maize stalk rot and ear rot caused by Fusarium verticillioides. PLoS ONE. 13(7):e0201588. doi:10.1371/journal.pone.0201588.
- Gaige AR, Giraldo M, Todd T, Stack JP. 2019. Growth and colonization of organic matter in soil by Fusarium proliferatum. Can J Plant Pathol. 41:242–250. doi:10.1080/07060661.2018.1522374.
- Gallup CA, Sullivan MJ, Shew HD. 2006. Black shank of tobacco. American Phytopathological Society. The Plant Health Instructor. doi:10.1094/PHI-I-2006-0717-01
- Galvez L, Urbaniak M, Waskiewicz A, Srepien L, Palmero D. 2017. Fusarium proliferatum – causal agent of garlic bulb rot in Spain: genetic variability and mycotoxin production. Food Microbiol. 67:41–48. doi:10.1016/j.fm.2017.05.006.
- Guo Z, Pfohl K, Karlovsky P, Dehne H-W, Altincicek B. 2016. Fuminosin B1 and beauvericin accumulation in wheat kernels after seed-borne infection with Fusarium proliferatum. Agricul Food Sci. 25:138–145. doi:10.23986/afsci.55539.
- Hadi B 2016. Brown stem rot of soybeans. Northern Plains Integrated Pest Management Guide. Accessed 2020 Feb 14. https://wiki.bugwood.org/NPIPM:Brown_stem_rot_of_soybean.
- Health Canada. 2019. Accessed 2019 Sept 16. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/industry-licensees-applicants/licensed-cultivators-processors-sellers.html.
- Hyun JW, Lee SC, Kim DH, Ko SW, Kim KS. 2000. Fusarium fruit rot of citrus in Jeju Island. Mycobiology. 28:158–162. doi:10.1080/12298093.2000.12015743.
- Jarek TM, Dos Santos AF, Tessmann DJ, Vieira ESN. 2018. Inoculation methods and aggressiveness of five Fusarium species against peach palm. Ciencia Rural. 48:04 e20170462. doi:10.1590/0103-8478cr20170462.
- Jimenez M, Logrieco A, Bottalico A. 1993. Occurrence and pathogenicity of Fusarium species in banana fruits. J. Phytopathol. 137(3):214–220. doi:10.1111/j.1439-0434.1993.tb01341.x.
- Joshi V, Jeffries M. 2018. Diseases/symptoms diagnosed on commercial crop samples submitted to the British Columbia Ministry of Agriculture (BCAGRI) Plant Health Laboratory in 2017. Can Plant Dis Survey. 98:6–16.
- Jurado M, Marín P, Callejas C, Moretti A, Vázquez C, González-Jaén MT. 2010. Genetic variability and fumonisin production by Fusarium proliferatum. Food Microbiol. 27:50–57. doi:10.1016/j.fm.2009.08.001.
- Jurado M, Vázquez C, Callejas C, González Jaén MT. 2006. Occurrence and variability of mycotoxigenic Fusarium species associated to wheat and maize in the southwest of Spain. Mycotoxin Res. 22:87–91. doi:10.1007/BF02956769.
- Katsanos RA, Pappelis AJ. 1966. Relationship of cell death patterns and spread of Colletotrichum graminicola in sorghum stalk tissue. Phytopathology. 56:468–469.
- Kim J-H, Kang M-R, Kim H-K, Lee S-H, Lee T, Yun S-H. 2012. Population structure of the Gibberella fujikuroi species complex associated with rice and corn in Korea. Plant Pathol. J. 28(4):357–363. doi:10.5423/PPJ.OA.09.2012.0134.
- Kim SG, Ko H-C, Hur O-S, Luitel BP, Rhee J-H, Yoon M-S, Baek H-J, Ryu K-Y, Sung JS. 2016. First report of Fusarium wilt caused by Fusarium proliferatum on safflower. Res Plant Dis. 22(2):111–115. doi:10.5423/RPD.2016.22.2.111.
- Kokkonen M, Jestoi M, Laitila A. 2012. Mycotoxin production of Fusarium langsethiae and Fusarium sporotrichioides on cereal-based substrates. Mycotoxin Res. 28:25–35. doi:10.1007/s12550-011-0113-8.
- Leslie JF. 2003. Sorghum and millet diseases. Ames (IA): Iowa State Press.
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Ames (IA): Blackwell.
- Leslie JF, Zeller KA, Logrieco A, Mulè G, Moretti A, Ritieni A. 2004. Species diversity of and toxin production by Gibberella fujikuroi species complex strains isolated from native prairie grasses in Kansas. Appl Environ Microbiol. 70:2254–2262. doi:10.1128/AEM.70.4.2254-2262.2004.
- Logrieco A, Moretti A, Ritieni A, Bottalico A, Corda P. 1995. Occurrence and toxigenicity of Fusarium proliferatum from preharvest maize ear rot, and associated mycotoxins, in Italy. Plant Dis. 79:727–731. doi:10.1094/PD-79-0727.
- Logrieco A, Mule G, Moretti A, Bottalico A. 2002. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur J Plant Pathol. 108:597–609. doi:10.1023/A:1020679029993.
- Marasas WFO, Thiel PG, Rabie CJ, Nelson PE, Toussoun TA. 1986. Moniliformin production in Fusarium section Liseola. Mycologia. 78:242–247. doi:10.1080/00275514.1986.12025235.
- Marin S, Sanchis V, Magan N. 1995. Water activity, temperature, and pH effects on growth of Fusarium moniliforme and Fusarium proliferatum isolates from maize. Can J Microbiol. 41:1063–1070. doi:10.1139/m95-149.
- Moretti A, Logrieco A, Bottalico A, Ritieni A, Fogliano V, Randazzo G. 1996. Diversity in beauvericin and fusaproliferin production by different populations of Gibberella fujikuroi (Fusarium section Liseola). Sydowia. 48:44–56.
- Mule G, Susca A, Stea G, Moretti A. 2004. Specific detection of the toxigenic species Fusarium proliferatum and F. oxysporum from asparagus plants using primers based on calmodulin gene sequences. FEWS Microbiol Lett. 230:235–240. doi:10.1016/S0378-1097(03)00926-1.
- Munkvold GP. 2003. Epidemiology of mycotoxin producing fungi. Netherlands: Springer.
- Niehaus E-M, Munsterkotter M, Proctor RH, Brown DW, Sharon A, Idan Y, Oren-Young L, Sieber CM, Novák O, Pěnčík A, et al. 2016. Comparative “Omics” of the Fusarium fujikuroi species complex highlights differences in genetic potential and metabolite synthesis. Genome Bio Evol. 8(11):3574–3599. doi:10.1093/gbe/evw259
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci USA. 95:2044–2049. doi:10.1073/pnas.95.5.2044.
- Palmero D, De Cara M, Nosir W, Galvez L, Cruz A, Woodward S, Gonzalez-Jaen MT, Tello JC. 2012. Fusarium proliferatum isolated from garlic in Spain: identification, toxigenic potential and pathogenicity on related Allium species. Phytopathol Mediter. 51:207–218.
- Proctor RH, Desjardins AE, Moretti A. 2010. Biological and chemical complexity of Fusarium proliferatum. In: Strange RN, Gullino ML, editors. The role of plant pathology in food safety and food security. Plant pathology in the 21st century. Netherlands: Springer Science; p. 97–111.
- Punja ZK. 2018. Flower and foliage-infecting pathogens of marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40:514–527. doi:10.1080/07060661.2018.1535467.
- Punja ZK.2020a. Epidemiology of Fusarium oxysporum causing root and crown rot of cannabis (Cannabis sativa L., marijuana) plants in commercial greenhouse production. Can J. Plant Pathol. 42(3). (in press)
- Punja ZK.2020b. The diverse mycoflora present on dried cannabis (Cannabis sativa L.) inflorescences in commercial production. Can J Plant Pathol. 42(3). (in press)
- Punja ZK. 2020c. First report of the hops powdery mildew pathogen, Podosphaeria macularis, on naturally infected marijuana (Cannabis sativa L.) plants in the field. Phytopathology (Abstr). (in press).
- Punja ZK. 2020d. Cannabis and hemp biology and pathology: an overview of the crop and emerging pathogens. Phytopathology (Abstr.). (in press).
- Punja ZK. 2020e. Brown root rot and crown rot of cannabis (Cannabis sativa L., marijuana) plants caused by Fusarium (Cylindrocarpon) lichenicola. Phytopathology (Abstr.). (in press).
- Punja ZK, Collyer D, Scott C, Lung S, Holmes J, Sutton D. 2019. Pathogens and molds affecting production and quality of Cannabis sativa L. Frontiers Plant Sci. (on-line). doi:10.3389/fpls.2019.01120.
- Punja ZK, Rodriguez G. 2018. Fusarium and Pythium species infecting roots of hydroponically grown marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40(4):498–513. doi:10.1080/07060661.2018.1535466.
- Punja ZK, Rodriguez G, Chen S. 2017. Assessing genetic diversity in Cannabis sativa using molecular approaches. In: Chandra S, Lata L, ElSohly MA, editors. Cannabis sativa L. Botany and Biotechnology. Berlin: Springer-Verlag; p. 395–418.
- Punja ZK, Scott C, Chen S. 2018. Root and crown rot pathogens causing wilt symptoms on field-grown marijuana (Cannabis sativa L.) plants. Can J Plant Pathol. 40(4):528–541. doi:10.1080/07060661.2018.1535470.
- Punja ZK, Scott C, Lung S, Roberts A. 2020. The Pythium species complex associated with crown and root rot on cannabis (Cannabis sativa L., marijuana) plants grown under commercial greenhouse conditions. Phytopathology. (Abstr.). (in press).
- Quazi SAJ, Meon S, Jaafar H, Ahmad ZABM. 2013. Characterization of Fusarium proliferatum through species specific primers and its virulence on rice seeds. Intern J Agric Biol. 15(4):649–656.
- Reid LM, Zhu X 2004. Common diseases of silage corn in Canada. In Chapt. 6: Corn Pests. Advanced Silage Corn Management 2004. Accessed 2020 Feb 17. Farwmwest.com. https://farmwest.com/node/966
- Rheeder JP, Marasas WF, Vismer HF. 2002. Production of fumonisin analogs by Fusarium species. Appl Environ Microb. 68:2101–2105. doi:10.1128/AEM.68.5.2101-2105.2002.
- Ritieni A, Fogliano V, Randazzo G, Scarallo A, Logrieco A, Moretti A, Bottalico A, Mannina L. 1995. Isolation and characterization of fusaproliferin, a new toxic metabolite from Fusarium proliferatum. Nat Toxins. 3:17–20. doi:10.1002/nt.2620030105.
- Scarlett K, Tesoriero L, Daniel R, Maffi D, Faoro F, Guest DI. 2015. Airborne inoculum of Fusarium oxysporum f. sp. cucumerinum. Eur J Plant Pathol. 141:779–787. doi:10.1007/s10658-014-0578-3.
- Small E. 2017. Cannabis. In: A Complete Guide. Boca Raton FL: CRC Press; 567 p.
- Stankovic S, Levic J, Petrovic T, Logrieco A, Moretti A. 2007. Pathogenicity and mycotoxin production by Fusarium proliferatum isolated from onion and garlic in Serbia. Eur J Plant Pathol. 118:165–172. doi:10.1007/s10658-007-9126-8.
- Stępień Ł, Koczyk G, Waśkiewicz A. 2011. Genetic and phenotypic variation of Fusarium proliferatum isolates from different host species. J Appl Genet. 52:487. doi:10.1007/s13353-011-0059-8.
- Torabi M, Ghorbany M, Salari M, Mirzaee MR. 2014. First report of sesame wilt disease caused by Fusarium proliferatum in Iran. J Plant Pathol. 96(S4):114.
- Vujanovic V, Hamel C, Yergeau E, St-Arnaud M. 2006. Biodiversity and biogeography of Fusarium species from northeastern North American asparagus fields based on microbiological and molecular approaches. Microb Ecol. 51:242–255. doi:10.1007/s00248-005-0046-x.
- Zhan R-L, Yang S-J, Ho -H-H, Liu F, Zhao Y-L, Chang J-M, He Y-B. 2010. Mango malformation.disease in south China caused by Fusarium proliferatum. J. Phytopathol. 158:721–725. doi:10.1111/j.1439-0434.2010.01688.x.
