Abstract
Gray blight, caused by Pestalotiopsis-like species, is one of the most serious diseases in tea [Camellia sinensis (L.) O. Kuntze] production. In this study, eight Pestalotiopsis isolates obtained from diseased tea leaves collected in Chongqing, China were identified as P. chamaeropis Maharachch, K.D. Hyde & Crous. Pestalotiopsis chamaeropis as a causal agent of tea gray blight disease was clearly distinguished from other species in the genus Pestalotiopsis by morphological characteristics and phylogenetic analysis based on a combination of the internal transcribed spacer (ITS), beta-tubulin (β-tubulin), and partial translation elongation factor 1-alpha (TEF) genes. Pathogenicity was confirmed by inoculation of a conidial suspension of P. chamaeropis on wounded tea leaves. Difenoconazole, a highly active and low toxicity fungicide, demonstrated significant antifungal effect towards P. chamaeropis, with an EC50 of 0.470 µg/mL.
Résumé
La moisissure grise, causée par les espèces du genre Pestalotiopsis, est une des maladies les plus graves du thé (Camellia sinensis [L.] O. Kuntze). Dans cette étude, huit isolats de Pestalotiopsis ont été obtenus de feuilles de thé infectées collectées dans la municipalité autonome de Chongqing, en Chine, et ont été identifiés en tant que P. chamaeropis Maharachch., K. D. Hyde & Crous. Pestalotiopsis chamaeropis, en tant qu’agent causal de la moisissure grise du thé, a été nettement différencié des autres espèces du genre Pestalotiopsis par ses caractéristiques morphologiques et l’analyse phylogénétique basée sur la région de l’espaceur transcrit interne (ITS), sur le gène de la bêta tubuline (β-tubuline) et sur une partie du gène codant pour le facteur de la traduction de l’élongation 1-alpha (TEF). La pathogénicité a été confirmée en inoculant des feuilles de thé présentant des lésions avec une suspension conidiale de P. chamaeropis. Le difénoconazole, un fongicide très actif et de faible toxicité, a démontré, à une CE50 de 0,470 µg/mL, un effet antifongique notable à l’égard de P. chamaeropis.
Introduction
Gray blight of tea [Camellia sinensis (L.) O. Kuntze] is an important disease in terms of economics as it causes significant production loss and quality decreases in many commercial tea plantations in India (Joshi et al. Citation2009), Japan (Horikawa Citation1986), and China (Liu et al. Citation2017; Chen et al. Citation2017, Citation2018a, Citation2018b). Therefore, an understanding of the pathogens that cause this disease is a priority. For a long time, gray blight disease was thought to be caused mainly by Pestalotiopsis theae (Sawata) Steyaert (Jeewon et al. Citation2003; Keith et al. Citation2006; Hu et al. Citation2007; Maharachchikumbura et al. Citation2011, Citation2013a, Citation2013b). More recently, a combination of the study of the morphological characteristics (i.e., length and width of conidia, median cell length, colour of median cells, and number and length of the apical appendages) and the molecular phylogenies derived from multi-gene analysis of the internal transcribed spacer (ITS), beta-tubulin (β-tubulin) and partial translation elongation factor 1-alpha (TEF) genes has proven to be an effective for species identification, resulting in greatly improved the understanding of Pestalotiopsis taxonomy on C. sinensis (Wei et al. Citation2007; Maharachchikumbura et al. Citation2014, Citation2016; Liu et al. Citation2017; Chen et al. Citation2018a). Based on this approach, several studies identifying Pestalotiopsis-like species as the causal agent of gray blight disease on C. sinensis have shown that there is considerable species diversity in the genus Pestalotiopsis (Chen et al. Citation2017, Citation2018a, Citation2018b; Wang et al. Citation2019a, Citation2019b).
Pestalotiopsis species are common phytopathogens and can cause many types of diseases on a wide range of host plants, including hazelnut, bayberry, longan, apple, blueberry, chestnut, rambutan, grape and guava (Sun and Cao Citation1990; Sangchote et al. Citation1998; Xu et al. Citation1999; Keith et al. Citation2006; Joshi et al. Citation2009; Keith and Zee Citation2010; Crous et al. Citation2011; Chen et al. Citation2011; Maharachchikumbura et al. Citation2012, Citation2013a, Citation2013b; Evidente et al. Citation2012; Ren et al. Citation2013). Chemical fungicides as a standard commercial practice, have been considered effective tools for the management of various plant diseases. Given its low mammalian toxicity and high efficiency, difenoconazole is often recommended for the management of foliar crop diseases including anthracnose caused by Colletotrichum spp. (Baggio et al. Citation2018). However, the use of this fungicide has not been evaluated for the management of gray blight disease of tea plants. In our study, the characteristics of a new Pestalotiopsis species from symptomatic tissues of tea plants in Chongqing city of China was described based on its morphological characters and multi-gene phylogenies based on ITS, β-tubulin and TEF genes. Furthermore, the effect of difenoconazole as a potential fungicide active ingredient against the new Pestalotiopsis species was evaluated.
Materials and methods
Isolates from diseased tea leaves
More than 20 naturally infected leaves were collected from commercial tea plantations located in Ersheng, in the Chongqing district of China (N29ʹ38, E106ʹ52), in 2017. The surfaces of symptomatic tea leaves were washed with running tap water. Leaf pieces (4 × 4 mm) were taken from the margins of symptomatic tissues, and surface-sterilized by dipping in 0.1% HgCl2 for 30 s, then in 75% ethanol for 30 s, and rinsed three times with sterile water. The surface-sterilized leaf pieces were transferred to potato dextrose agar (PDA) in Petri dishes and incubated in a growth chamber at 25 ± 2°C with 12 h light photoperiod. Two days after incubation, mycelia growing from the symptomatic tissues were transferred to fresh PDA by taking agar discs from the margins of colonies.
Morphological analysis
Pure cultures of the fungi were grown on PDA at 25 ± 2ºC and checked daily. Colony morphology was examined after incubation for 5–10 days and photographs of the upper and lower surfaces of all the colonies were taken on the sixth day. The shape, colour, cell number, and number of apical and basal appendages of conidia were determined using a U-TV0.5XC-3 microscope (Olympus, Japan). The size (length and width) of conidia and the length of apical and basal appendages were determined for 30 randomly selected conidia from a conidial suspension of each isolate that was prepared in sterile water.
DNA extraction, PCR amplification and sequencing
The total genomic DNA was extracted from fresh mycelium of each of eight isolates following a CTAB method described previously (Chen et al. Citation2018b). Fresh mycelium was scraped off the PDA surface after six days of growth. The partial gene sequences of the ITS, β-tubulin, and TEF genes were amplified with the universal primers ITS4/ITS5 (White et al. Citation1990), T1/Bt-2b (Glass and Donaldson Citation1995; O’Donnell and Cigelnik Citation1997) and EF1-728F/EF-2 (O’Donnell et al. Citation1998;Carbone and Kohn Citation1999) (Supplementary Table 1). The PCR conditions for each of ITS, β-tubulin and TEF were as follows: an initial denaturing for 3 min at 95°C, followed by 38 cycles of denaturation for 30 s at 95 °C, annealing for 30 s at 56°C and elongation for 1 min at 72°C, with a final extension step for 10 min at 72°C. The amplification products were resolved in 1% agarose gels, and purified using a Gel Extraction Kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s instructions, then cloned into a pGW-T Vector (Tiangen, Beijing). The positive transformants were sequenced at the Shanghai Sangon Biotech Company (Shanghai, China).
Phylogenetic analyses
New obtained gene sequences were deposited in GenBank and the sequences of homologous Pestalotiopsis species were retrieved from GenBank for comparison (). Neopestalotiopsis clavispora CBS 447.73 was used as the outgroup. The gene sequences of the isolates were aligned with the representative sequences in the NCBI database using Clustal W (Tamura et al. Citation2013). Phylogenetic trees were built and viewed in Molecular Evolutionary Genetic Analysis (MEGA) software v. 7. Phylogenetic analyses of the gene sequences consisted of Maximum Parsimony (MP) and Neighbour-Joining (NJ) methods based on both the individual and combined ITS, β-tubulin and TEF gene sequences. To determine the confidence values for the grouping within a tree, a bootstrap analysis was performed using 1,000 resamplings of the data (Saitou and Nei Citation1987; Tamura et al. Citation2013). The names and accession numbers were recorded on phylogenetic trees.
Table 1. Fungal species isolated from tea plants and the reference isolates used for phylogenetic analyses
Pathogenicity tests
Pathogenicity tests for each isolate were conducted on healthy tea leaves of the common tea cultivar cv. Fudingdabai, which were surfaced-disinfected using 75% ethanol. For each isolate, 10 leaves were inoculated by the wound method that was described previously (Chen et al. Citation2018a, Citation2018b). Eight microlitres of a conidial suspension (1 × 106 conidia/mL) was placed over each wound. Control tea leaves were inoculated with an equal volume of sterile water. All inoculated tea leaves were placed into Petri dishes with wet filter paper to maintain high humidity and then placed in a growth chamber at 25 ± 2°C until disease symptoms appeared. All fungal isolates included in the pathogenicity tests were re-isolated from the diseased tea leaves to confirm their identity by both molecular and morphological methods as described above. Experiments were repeated three times with similar results. The disease incidences (%) were calculated by counting the number of infected tea leaves over the total inoculated tea leaves. The diameter of the lesions developed on tea leaves was measured after six days of incubation.
Antifungal activity of difenoconazole
Difenoconazole (Syngenta, China) was evaluated for its antifungal activity based on its capacity to inhibit the mycelial growth of isolate LB61-3. The test fungicide was dissolved in water and used to amend PDA medium at concentrations of 0, 0.1, 1.0, 2.0, 4.0, and 6.0 µg difenoconazole/mL. A mycelial disk of 5-mm-diameter of fungal isolate LB61-3 taken from a 5-day-old culture was placed at the centre of the PDA medium. Mycelial disks on PDA without any test chemical served as controls. All the Petri dishes were incubated at 25 ± 2°C for 6 days. The percentage inhibition was calculated using a formula: percentage inhibition = [(d c-d t)/d c] ×100, where d c and d t are the mean colony diameters of the control and treatment sets, respectively. There were three replicates for each treatment. The effective concentration of the fungicide causing growth of inhibition of 50% (EC50) was determined by probit-log analysis (Wang et al. Citation2016).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 20.0. Data were analysed by one-way variance (ANOVA) and means were compared according to Turkey’s HSD (p ≤ 0.05).
Results
Gray blight disease was observed in the commercial tea plantations of Chongqing, China, in 2017. The disease generally began with small brown spots. As the disease developed, the spots got larger and the colour of necrotic lesions changed to light gray at the late stage of the disease (), ultimately leading to serious losses in tea production. These symptoms were similar to our previous study (Chen et al. Citation2018b).
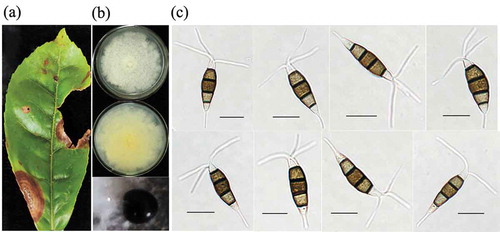
Fig. 1 The morphological characteristics of Pestalotiopsis-like species isolated from tea plants. (a) Symptoms caused by gray blight disease on tea plants; (b) Cultural traits of Pestalotiopsis isolates on potato dextrose agar (PDA) medium at 25 ± 2°C for six days; (c) Conidial morphology of Pestalotiopsis isolates on PDA medium after 8 days incubation (scale bar = 10 um)
Morphological characteristics of Pestalotiopsis species
Eight isolates (LB5-1, LB5-2, LB11-1, LB11-2, LB11-3, LB61-1, LB61-2, and LB61-3) were collected from symptomatic tea leaves. All the isolates reached 65–75 mm diam. after 7 days incubation at 25 ± 2°C on PDA. Colonies on PDA were white, with moderate aerial mycelium on the surface and light yellow colour on the reverse, with an undulating margin (). The conidiomata of all isolates forming on the surface were black and gathered closely, diffusing throughout the culture medium after 7–10 days incubation (). Conidia were fusoid, straight to slightly curved, five celled, 4-septate, with septa darker than the rest of the cells, 19.7–28.4 (mean ± SD = 25.1 ± 3.2) μm in length and 6.1–9.3 (7.1 ± 1.1) μm in width; three median cells were doliiform to sub-cylindrical, concolorous, brown, but the two upper median cells were slightly darker than the lower median cell, with the length ranging from 13.7 to 18.1 (15.5 ± 1.6) μm; basal cells were hyaline, obconic with a truncate base; basal appendages arising from the apical crest were single, straight or slightly curved, tubular, and 4.6–8.5 μm long; the apical appendages were 2 to 4 (mostly 3), with the length ranging from 10.1 to 18.9 μm ().
Phylogenetic analyses of the combined datasets
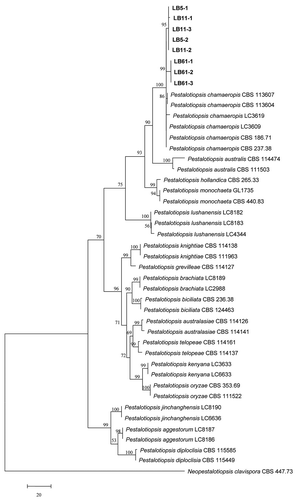
Phylogenetic analyses of 43 isolates in the genus Pestalotiopsis were performed using both MP and NJ methods on the basis of the single locus and concatenated datasets of the ITS, β-tubulin and TEF genes. Both methods produced similar results, thus only the MP tree is presented. As shown in , 43 isolates from C. sinensis and other host plants of Pestalotiopsis clustered in 16 clades, which represented 16 known species including P. chamaeropis, P. hollandica, P. australis, P. monochaeta, P. lushanensis, P. grevilleae, P. knightiae, P. brachiata, P. australasiae, P. kenyana, P. telopeae, P. jinchanghensis, P. aggestorum, P. biciliata, P. diploclisia and P. oryzae. The eight isolates (LB5-1, LB5-2, LB11-1, LB11-2, LB11-3, LB61-1, LB61-2, and LB61-3) in this study clustered with previously identified strains of P. chamaeropis with a high bootstrap support of 100%. BLAST searches indicated that the ITS, β-tubulin and TEF gene sequences of the isolates in this study shared 98–100% similarity with those from some other hosts published in GenBank in the same clade. Based on the molecular analysis and morphological characteristics, the eight isolates in this study were identified as Pestalotiopsis chamaeropis Maharachch, K.D. Hyde & Crous.
Fig. 2 Phylogenetic tree obtained using the MP method on the basis of combined ITS, β-tubulin and TEF gene sequence data from distinctive Pestalotiopsis species in this study (bold font) and published sequences. Neopestalotiopsis clavispora CBS 447.73 served as the outgroup. The numbers in the bootstrap test (1,000 replicates) are indicated above the branches and only values > 50% are shown
Pathogencity analysis
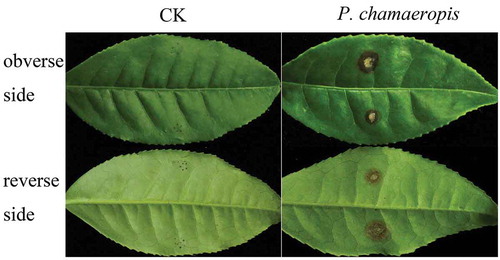
In pathogenicity tests of all the P. chamaeropis isolates, 40.0–76.7% of the inoculated tea leaves showed obvious disease symptoms (). Symptoms began as tiny brown or dark spots around the wound, two days after inoculation. These spots expanded to surrounding healthy tissues six days after inoculation (), with 3.0–6.5 mm diameter lesions (). Finally black acervuli were formed on the surface of the lesions. Although all of the P. chamaeropis isolates were pathogenic to tea leaves, they showed differences in virulence. Isolate LB61-3 was the most virulent producing typical lesions on infected leaves of 6.5 ± 0.3 mm diameter, while isolate LB5-1 was the least virulent with a lesion diameter of 3.0 ± 0.1 mm (). All the symptoms were identical to those observed in the field. The control tea leaves inoculated with sterile water did not show any symptoms throughout the duration of the experiments (). The fungi re-isolated from the lesions showed the same morphological characteristics and 100% sequence similarities with the ones used for the inoculations. The recovery frequency of P. chamaeropis from the inoculated lesions was 100%, while a recovery of 0% was recorded for the control treatment.
Table 2. Pathogenicity of the isolates of P. chamaeropis on the tea cv. Fudingdabai six days after inoculation
Effect of difenoconazole
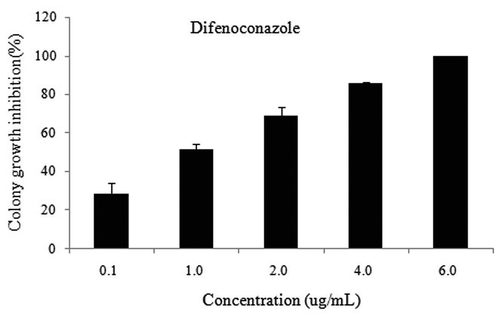
Difenoconazole exhibited antifungal activity against P. chamaeropis which was dose-dependent in a wide range of concentrations from 0.1 to 6.0 µg/mL (). The inhibition percent ranged from 28.8% to 100.0%. Difenoconazole was very effective, with a 28.8% inhibition even at the low concentration of 0.1 µg/mL, and when the concentration reached 6.0 µg/mL, growth of the fungus was completely inhibited. The EC50 of difenoconazole was 0.470 µg/mL.
Discussion
More than 20 Pestalotiopsis-like species have been thought to be associated with gray blight disease on C. sinensis. All the species belong to the three genera Pestalotiopsis, Neopestalotiopsis and Pseudopestalotiopsis, including P. longiseta, P. camelliae, P. furcate, N. clavispora, N. ellipsospora, P. menhaiensis, P. sichuanensis, P. kenyana, P. rhodomyrtus, P. chamaeropis, P. longiappendiculata, P. yanglingensis, P. trachicarpicola, P. lushanensis, P. jinchanghensis, Ps. camelliae-sinensis, Ps. ignota, Ps. cocos, Ps. indica, Ps. kubahensis, Ps. ampullacea, Ps. Chinensis, and Ps. theae (Takeda Citation2002; Joshi et al. Citation2009; Maharachchikumbura et al. Citation2014, Citation2016; Lateef et al. Citation2015; Liu et al. Citation2017; Chen et al. Citation2017, Citation2018a, Citation2018b; Wang et al. Citation2019a). Pseudopestalotiopsis and Neopestalotiopsis are two novel genera segregated from Pestalotiopsis, and in a previous study, five Pestalotiopsis-like species belonging to the three genera were found to be associated with tea gray blight disease in Chongqing city, China (Chen et al. Citation2017, Citation2018a, Citation2018b). In this study, eight P. chamaeropis isolates from diseased tea leaves were identified, and this is the first description of the characteristics of P. chamaeropis causing gray blight disease on C. sinensis in Chongqing city. The shape and size of the conidia of P. chamaeropis have some differences with the other four species, and the most important differences are with respect to the median cells and apical appendages of the conidia. Three median cells of the conidia of P. chamaeropis are concolourous, which can be easily distinguished from N. clavispora (Maharachchikumbura et al. Citation2014; Chen et al. Citation2018a, Citation2018b). The numbers and lengths of apical appendages of P. chamaeropis range from two to four (mostly three) and 10.1 to 18.9 μm, while P. camelliae has three to seven apical appendages, with the length ranging from 13.5 to 32.4 μm (Chen et al. Citation2017, Citation2018a, Citation2018b). Although P. chamaeropis has some distinct morphological differences with other species, a combination of morphological evaluation with an analysis of the ITS, β-tubulin and TEF gene regions provides the best resolution for distinguishing closely related species.
All the eight P. chamaeropis isolates used in this study were shown to be pathogenic to wounded tea plants; in contrast, symptoms were not usually observed on unwounded leaves (data not shown), suggesting that wounding may be necessary for symptom development. Similar results also have been confirmed in previous studies (Chen et al. Citation2018b; Wang et al. Citation2019a). Pestalotiopsis chamaeropis, initially isolated from the diseased leaves of dwarf palm (Chamaerops humilis), has also been identified as the pathogen-causing necrotic spots on mint-bush, Prostanthera rotundifolia, in Australia (Maharachchikumbura et al. Citation2014) and Camellia sp. in Jiangxi, China (Liu et al. Citation2017). The virulence of the eight P. chamaeropis isolates varied to some extent, which was consistent with the results that different Pestalotiopsis-like isolates within the same species showed variability in pathogenicity (Chen et al. Citation2018b; Wang et al. Citation2019a, Citation2019b). This indicates that pathogenicity may be isolate dependent rather than species dependent. Further research is needed to understand the basis for the differences in virulence among the isolates within the same species or species within the same genus. Isolate LB61-3, which showed stable and strong pathogenicity on tea plants, was used to test the antifungal activity of difenoconazole and the results showed that difenoconazole was effective against P. chamaeropis. Fungicides are the most important and commonly method used to control gray blight. To date, however, this fungicide has not been applied for the management of tea gray blight. Difenoconazole has been proven a useful fungicide for the management of chilli anthracnose caused by Colletotrichum acutatum (Gao et al. Citation2017), although it has a lower EC50 value (0.22 μg/mL) against this fungus than for inhibiting growth of P. chamaeropis. The efficacy of fungicides and the sensitivity of the pathogen to those fungicides are important knowledge for effective disease management in the field.
Supplementary_table_1.doc
Download MS Word (14 KB)Supplementary material
Supplemental data for this article can be accessed online here: https://doi.org/10.1080/07060661.2020.1816582.
Additional information
Funding
References
- Baggio JS, Wang NY, Peres NA, Amorim L. 2018. Baseline sensitivity of Colletotrichum acutatum isolates from Brazilian strawberry fields to azoxystrobin, difenoconazole, and thiophanate-methyl. Trop Plant Pathol. 43(6):533–542. doi:10.1007/s40858-018-0232-2.
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 91(3):553–556. https://www.jstor.org/stable/3761358.
- Chen CQ, Zhang B, Yang LN, Gao J. 2011. Identification and biological characteristics of round leaf spot on blueberry caused by Pestalotiopsis photiniae (in Chinese). J Northeast for Univ. 39:95–98.
- Chen YJ, Zeng L, Meng Q, Tong HR. 2018a. Occurrence of Pestalotiopsis lushanensis causing gray blight disease on Camellia sinensis in China. Plant Dis. 102(12):2654. doi:10.1094/PDIS-04-18-0640-PDN.
- Chen YJ, Zeng L, Shu N, Jiang MY, Wang H, Huang YJ, Tong HR. 2018b. Pestalotiopsis-like species causing gray blight disease on Camellia sinensis in China. Plant Dis. 102(1):98–106. doi:10.1094/PDIS-05-17-0642-RE.
- Chen YJ, Zeng L, Shu N, Wang H, Tong HR. 2017. First report of Pestalotiopsis camelliae causing gray blight disease on Camellia sinensis in China. Plant Dis. 101(6):1034. doi:10.1094/PDIS-01-17-0033-PDN.
- Crous PW, Summerell BA, Swart L, Denman S, Taylor JE, Bezuidenhout CM, Palm ME, Marincowitz S, Groenewald JZ. 2011. Fungal pathogens of Proteaceae. Persoonia. 27(1):20–45. doi:10.3767/003158511X606239.
- Evidente A, Zonno MC, Andolfi A, Troise C, Cimmino A, Vurro M. 2012. Phytotoxic α-pyrones produced by Pestalotiopsis guepinii, the causal agent of hazelnut twig blight. J Antibiot. 65(4):203–206. doi:10.1038/ja.2011.134.
- Gao YY, He LF, Li BX, Mu W, Lin J, Liu F. 2017. Sensitivity of Colletotrichum acutatum to six fungicides and reduction in incidence and severity of chili anthracnose using pyraclostrobin. Australasian Plant Pathol. 46(6):521–528. doi:10.1007/s13313-017-0518-8.
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Micro. 61(4):1323–1330. doi:10.1128/AEM.61.4.1323-1330.1995.
- Horikawa T. 1986. Yield loss of new tea shoots due to gray blight caused by Pestalotia longiseta Spegazzini. Bull Shizuoka Tea Exp Station (Japan). 12:1–8.
- Hu HL, Jeewon R, Zhou DQ, Zhou TX, Hyde KD. 2007. Phylogenetic diversity of endophytic Pestalotiopsis species in Pinusarmandii and Ribes spp.: evidence from rDNA and β- tubulin gene phylogenies. Fungal Divers. 24:1–22.
- Jeewon R, Liew ECY, Simpson JA, Hodgkiss IJ, Hyde KD. 2003. Phylogenetic significance of morphological characters in the taxonomy of Pestalotiopsis species. Mol Phylogenet Evol. 27(3):372–383. doi:10.1016/s1055-7903(03)00010-1.
- Joshi SD, Sanjay R, Baby UI, Mandal AKA. 2009. Molecular characterization of Pestalotiopsis spp. associated with tea (Camellia sinensis) in southern India using RAPD and ISSR markers. Indian J Biotechnol. 8:377–383. doi:10.1101/2020.03.17.994806.
- Keith LM, Velasquez ME, Zee FT. 2006. Identification and characterization of Pestalotiopsis spp. causing scab disease of guava, Psidium guajava in Hawaii. Plant Dis. 90(1):16–23. doi:10.1094/PD-90-0016.
- Keith LM, Zee FT. 2010. Guava disease in Hawaii and the characterization of Pestalotiopsis spp. affecting guava. Acta Hortic. 849(849):269–276. doi:10.17660/ActaHortic.2010.849.31.
- Lateef AA, Sepiah M, Bolhassan MH. 2015. Description of Pseudopestalotiopsis kubahensis sp. nov., a new species of microfungi from Kubah National Park, Sarawak, Malaysia. Curr Res Environ Appl Mycol. 5(4):376–381. http://www.creamjournal.org/pdf/Cream_5_4_8.pdf.
- Liu F, Hou L, Raza M, Cai L. 2017. Pestalotiopsis and allied genera from Camellia, with description of 11 new species from China. Sci Rep-UK. 7(1):866. doi:10.1038/s41598-017-00972-5.
- Maharachchikumbura SSN, Chukeatirote E, Guo LD, Crous PW, McKenzie EHC, Hyde KD. 2013a. Pestalotiopsis species associated with Camellia sinensis (tea). Mycotaxon. 123(1):47–61. doi:10.5248/123.47.
- Maharachchikumbura SSN, Guo LD, Cai L, Chukeatirote E, Wu WP, Sun X, Crous PW, Bhat DJ, McKenzie EHC, Bahkali AH, et al. 2012. A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers. 56(1):95–129. doi:10.1007/s13225-012-0198-1.
- Maharachchikumbura SSN, Guo LD, Chukeatirote E, Bahkali AH, Hyde KD. 2011. Pestalotiopsis—morphology, phylogeny, biochemistry and diversity. Fungal Divers. 50(1):167–187. doi:10.1007/s13225-011-0125-x.
- Maharachchikumbura SSN, Guo LD, Chukeatirote E, Hyde KD. 2013b. Improving the backbone tree for the genus Pestalotiopsis; addition of P. steyaertii and P. magna sp. nov. Mycol Prog. 13(3):617–624.
- Maharachchikumbura SSN, Guo LD, Liu ZY, Hyde KD. 2016. Pseudopestalotiopsis ignota and Ps. camelliae spp. nov. associated with gray blight disease of tea in China. Mycol Prog. 15(3):1–7. doi:10.1007/s11557-016-1162-3.
- Maharachchikumbura SSN, Hyde KD, Groenewald JZ, Xu J, Crous PW. 2014. Pestalotiopsis revisited. Stud Mycol. 79:121–186. doi:10.1016/j.simyco.2014.09.005.
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the FungusFusariumAre Nonorthologous. Mol Phylogenet Evol. 7(1):103–116. doi:10.1006/mpev.1996.0376.
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. P Natl Acad Sci USA. 95(5):2044–2049. doi:10.1073/pnas.95.5.2044.
- Ren HY, Li G, Qi XJ, Fang L, Wang HR, Wei JG, Zhong SB. 2013. Identification and characterization of Pestalotiopsis spp. causing twig blight disease of bayberry (Myrica rubra Sieb. & Zucc) in China. Eur J Plant Pathol. 137(3):451–461. doi:10.1007/s10658-013-0255-y.
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4(406–4255). doi:10.1093/oxfordjournals.molbev.a040454.
- Sangchote S, Farungsang U, Farungsang N. 1998. Pre- and postharvest infection of rambutan by pathogens and effect on postharvest treatments. In: Coates LM, Hofman PJ, Johnson GI, editors. Disease control and storage life extension in fruits. ACIAR Proceedings No. 81; May 22–23 1997; Chaing Mai, Thailand; p. 87–91.
- Sun HT, Cao RB. 1990. Identification of Pestalotiopsis parasitized on fruit crops (in Chinese). Acta Agr University Zhejiangensis. 16:179–185.
- Takeda Y. 2002. Genetic analysis of tea gray blight resistance in tea plants. Jpn Agric Res Q. 36(3):143–150. doi:10.6090/jarq.36.143.
- Tamura K, Stecher G, Peterson D, Lewis PO. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30(12):2725–2729. doi:10.1093/molbev/msw054.
- Wang SS, Mi XZ, Wu ZR, Zhang LX, Wei CL. 2019a. Characterization and pathogenicity of Pestalotiopsis-like species associated with gray blight disease on Camellia sinensis in Anhui province, China. Plant Dis. 103(11):2786–2797. doi:10.1094/PDIS-02-19-0412-RE.
- Wang YC, Qian WJ, Li NN, Hao XY, Wang L, Xiao B, Wang XC, Yang YJ. 2016. Metabolic changes of caffeine in tea plant (Camellia sinensis (L.) O. Kuntze) as defense response to Colletotrichum fructicola. J Agri Food Chem. 64(35):6685–6693. doi:10.1021/acs.jafc.6b02044.
- Wang YC, Xiong F, Lu QH, Hao XY, Zheng MX, Wang L, Li NN, Ding CQ, Wang XC, Yang YJ. 2019b. Diversity of Pestalotiopsis-like species causing gray blight disease of tea plants (Camellia sinensis) in China, including two novel Pestalotiopsis species, and analysis of their pathogenicity. Plant Dis. 103(10):2548–2558. doi:10.1094/PDIS-02-19-0264-RE.
- Wei JG, Xu T, Guo LD, Liu AR, Zhang Y, Pan XH. 2007. Endophytic Pestalotiopsis species associated with plants of Podocarpaceae, Theaceae and Taxaceae in southern China. Fungal Divers. 24:55–74.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and amplifications. San Diego: Academic Press; p. 315–322. doi:10.1016/b978-0-12-372180-8.50042-1
- Xu L, Kusakari S, Hosomi A, Toyoda H. 1999. Postharvest disease of grape caused by Pestalotiopsis species. Ann Phytopathol Soc Japan. 65(3):305–311. doi:10.3186/jjphytopath.65.305.