Abstract
Theobroma cacao L. is cultivated in Mexico, primarily in Tabasco, a southern state where the climatic conditions are suitable for producing cacao, the source of chocolate. However, these conditions are also suitable for cacao pathogens such as Phytophthora spp., the causative agents of black pod rot, a disease that is difficult to eradicate once established. In this study, we collected cacao pods exhibiting the symptoms and signs of black pod rot from different locations in Tabasco. Several recovered isolates exhibited the typical colony morphology of Phytophthora, including a white mycelium, stellate with chrysanthemum shape and appressed appearance with slightly torulose hyphae. Both sporangia and chlamydospores were absent in culture media, but were observed after induction in a sterile soil solution. Sporangia were papillated, and the sporangial shapes were globose, ellipsoid, and obturbinate, while the sporangiospores were umbrella shaped and simple sympodial. Chlamydospores were spherical, terminal, and subterminal. The concatenated analysis of the internal transcribed spacer region (ITS1-5.8S-ITS2 = ITS), the cytochrome c oxidase subunit II (COXII), the translation elongation factor 1α (TEF1) and β-tubulin (BT) placed strain PtCa-14 among several P. tropicalis strains, confirming the identity of this species. The pathogenicity tests on pear and peach fruit and cacao pods showed the ability of this oomycete to induce rot. Taken together, these results indicated the presence of P. tropicalis in the Mexican state of Tabasco and demonstrated its ability to cause black pod rot.
Résumé
Theobroma cacao L. est cultivé au Mexique, principalement dans l’État de Tabasco qui est situé dans le sud où les conditions climatiques sont propices à la culture du cacao, la matière première du chocolat. Cependant, ces conditions conviennent également aux agents pathogènes qui s’attaquent au cacao, tels que Phytophthora spp., les agents causaux de la pourriture brune des cabosses, une maladie difficile à éradiquer une fois qu’elle est implantée. Dans le cadre de cette étude, nous avons collecté des cabosses présentant des symptômes et des signes de pourriture brune, provenant de différents endroits du Tabasco. Plusieurs des isolats récupérés affichaient la morphologie typique des colonies de Phytophthora, y compris du mycélium blanc, étoilé en forme de chrysanthème, apprimé avec des hyphes légèrement toruleux. Sur le milieu de culture, il n’y avait pas de sporanges ni de chlamydospores, mais on a pu les observer après induction dans une solution de terreau stérile. Les sporanges étaient papillés et de forme globuleuse, ellipsoïde et non turbinée, tandis que les sporangiospores, à sympode simple, avaient la forme d’une ombrelle. Les chlamydospores étaient sphériques, terminales et subterminales. L’analyse concaténée de la région de l’espaceur transcrit interne (ITS1-5.8S-ITS2 = ITS), de la sous-unité II du cytochrome c oxydase (COXII), du facteur d’élongation de la transcription 1α (TEF1) et de la β-tubuline (BT) a placé la souche PtCa-14 parmi plusieurs souches de P. tropicalis, confirmant l’identité de cette espèce. Les résultats des tests de pathogénicité effectués sur des poires et des pêches ainsi que sur des cabosses de cacao ont démontré la capacité de cet oomycète à causer la pourriture. Dans leur ensemble, ces résultats ont confirmé la présence de P. tropicalis dans l’État mexicain de Tabasco et démontré sa capacité à provoquer la pourriture brune des cabosses.
Introduction
The south of Mexico has a tropical climate that promotes the growth and production of cacao trees (Theobroma cacao L.). The cacao tree requires 3–5 years to bear its first fruit (pods), while the pods require 5–6 months to develop and mature. The cacao pods are the source of chocolate, a global industry valued at US 80 USD billion per year (Ploetz Citation2016). Despite its economic importance, cacao is primarily produced in low-input and low-output systems (http://www.worldcocoafoundation.org/).In Mexico, the primary area of cacao production is the state of Tabasco (64.8%), and globally, Mexico is the 14th largest producer with a production of 28,399 tons per year (http://www.fao.org/faostat/en/#data). Mexican cacao is exported to several countries, Switzerland being the main client ($973,368 in 2018) (SIAP Citation2019).
Constraints on cacao production include a number of phytopathogenic microorganisms, such as the fungi Moniliophthora roreri (Cif.) H.C. Evans, Stalpers, Samson & Benny (the cause of frosty pod or moniliasis), Moniliophthora perniciosa (Stahel) Aime & Phillips-Mora (the cause of witches’ broom), and Ceratocystis cacaofunesta Engelbr. & T.C.Harr. (the cause of ‘mal de machete’, triggering cacao wilt), as well as the oomycetes Phytophthora spp., which cause black pod rot (Phillips and Cerda Citation2011). A number of Phytophthora spp. have been shown to cause black pod rot, such as P. palmivora Butler, P. megakarya Brasier & M.J. Griffin, P. capsici Leonian, P. citrophthora (R.E. Sm. & E.H. Sm.) Leonian, P. arecae (L.C.Coleman) Pethybridge, P. megasperma Drechsler, and P. tropicalis Ola’h. The presence of each species is related to the continent where the disease is present (Rocha Citation1965; Evans Citation1971; Griffin et al. Citation1981; Smith Citation1981; Evans and Prior Citation1987; Erwin and Ribeiro Citation1996; Drenth and Guest Citation2004; Phillips and Wilkinson Citation2007). Typically, the identification and characterization of Phytophthora spp. has been based on morphological features (Waterhouse Citation1963), including the type of mycelium, the shape, size, and development of sporangia, the production of sexual reproductive structures, and the formation of chlamydospores (Pintos et al. Citation2004). However, due to intraspecific morphological variation, the exact identification of species is often difficult, even for specialists. For this reason, molecular techniques that analyse genetic sequences have been identified which aid in the identification and delimitation of species (Álvarez et al. Citation2016).
There are few reports regarding diseases of cacao in Mexico, and those that are available have been provided by the Mexican government (https://www.gob.mx/agricultura). These reports have established that M. roreri and P. palmivora are present in Mexico. However, there is no information about other Phytophthora species related to this disease. In this study, cacao pods were collected from Tabasco and used to isolate Phytophthora spp. Morphological characters and multigene phylogenetic analysis were used in species identification, and pathogenicity tests were conducted.
Materials and methods
Isolation and growth conditions
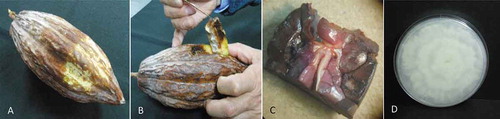
Cacao pods with black rot were collected from Comalcalco, Paraíso, Cárdenas, and Cunduacán in Tabasco, Mexico, in June 2014 and March 2016. At each location, a site was chosen and divided into three sections. From each section three cacao trees were selected to gather one infected pod per tree, resulting in nine pods being obtained per site for a total of 36 pods collected in each of 2014 and 2016. The pods were surface-disinfected with 3% sodium hypochlorite for 5 min and then washed 3 times with sterile water. To isolate fungi and oomycetes, a sterile scalpel was used to remove 3 cm of the surface of the pod from the edge of active lesions. Then, a 0.5 cm section of internal tissue was collected and placed on potato dextrose agar (PDA; 39 g/L; MCD Lab, State of Mexico) (). Ten internal tissue sections per pod were collected and incubated at 28°C for 5–7 days, and the isolates were purified by successive mass transfer on PDA.
Morphological observations
Many isolates were obtained with morphologies typical of Phytophthora, Fusarium, Colletotrichum, Moniliophthora, Rhizopus, Pestalotia, and Lasiodiplodia. For this study, Phytophthora-like isolates were selected for comparison and characterization. One of the isolates (named PtCa-14) was selected for further analysis. The isolate was grown in different culture media for the assessment of colony morphology features. The growth media used and their components (per 1 L, 15 g agar) were as follows: V8 medium (200 mL of V8 juice and 3 g calcium carbonate); corn medium (47 g of corn flour); chocolate agar (15 g of commercial chocolate with a 70% cocoa content); PDA; and Sabouraud Dextrose Agar (SDA; MCD Lab, State of Mexico). Three times mycelial plugs (5 mm diameter) were taken from the margins of growing cultures on PDA, inoculated in the centre of Petri dishes containing the different media and incubated at 28 or 35°C for 5 days. To induce the production of sporangia, 5 mm plugs (10–15 pieces) from the oomycete culture were collected after 5 days of incubation on V8 medium. The plugs were transferred to a Petri dish containing a sterile soil solution and incubated at 28°C with constant light. The development of sporangia was assessed every 24 h for 5 days. The morphology of the isolate was examined microscopically, and images were captured with an AxiozCam HRc camera using ZEN (blue edition) software. The colony morphology, structure, and growth rate were documented using three replicates, while the sporangial features and chlamydospore size were determined with 50 replicates. The morphological characteristics were measured at 200×, 400×, and 1000× magnification.
Pathogenicity studies
Healthy pears and peaches were surface-sterilized with 3% sodium hypochlorite for 5 min and washed 3 times with sterile distilled water. Using a needle, each pear and peach was wounded, and a 5 mm agar plug containing mycelium from a 5-day-old PtCa-14 culture grown on PDA was placed in the wound. For the control, an agar plug without microbial growth was placed in the wound. The pears and peaches were incubated in a plastic box at 28°C. Wound development was observed every day for 5 days for pears and 3 days for peaches. At the end of the incubation period, the surface of the infected area was removed, and small pieces (5 mm) were taken from the internal tissue and placed on PDA medium and incubated for 5 days at 28°C to isolate the pathogen. For each treatment, three biological replicates were prepared. Pathogenicity tests were also performed using cacao pods in the field (Mohamed-Azni et al. Citation2017) using pods that were approximately 120 days old. The pods, which remained attached to the tree, were surface disinfected following the same procedure described for the pears and peaches. Then, each pod were wrapped in a polyethylene bag containing a damp paper towel to maintain humidity and to create a closed system to prevent the dissemination of the pathogen. Each control pod was wounded with a needle, and a 5 mm agar plug, without any microbial growth, was placed in the wound. The same procedure was performed for the pathogenicity test except that the plug contained oomycete growth. The experiment was performed using five replicates with three independent experiments (first assay in April 2017, second and third assays in October 2019). The pods were monitored every 24 h for 5 d, and the phytopathogen was isolated to confirm Koch’s postulates.
Molecular identification
DNA isolation was performed according to Allers and Lichten (Citation2000), substituting liquid nitrogen for dry ice in the mechanical lysis step. The pathogen mycelia used for the isolation were collected by gentle scraping with a sterile scalpel from a 5-day-old culture grown on the PDA. The isolate DNA was amplified and sequenced using the following DNA markers: (a) the internal spaced sequence (ITS1-5.8S-ITS2 = ITS) with the primers ITS1/ITS4, resulting in a fragment of 754–834 bp (White et al. Citation1990); (b) the mitochondrial cytochrome c oxidase subunit II (COXII) gene with the primers FM35/FMPyy-10b, which amplify a fragment of 1000 bp (Martin and Tooley Citation2003); (c) the translation elongation factor-1a (TEF1) gene with the primers EF1AF/EF1AR, which amplify a fragment of 971 bp; and (d) b-tubulin (BT) with the primers Btub-F1/Btub-R2, which amplify a fragment of 1228 bp (Kroon et al. Citation2004). The PCR fragments were sequenced at Macrogen Inc., Seoul, Korea, (https://dna.macrogen.com/) using the same primers used for amplification. The forward and reverse sequences were edited and assembled with ChromasPro (Technelysium Pty Ltd., South Brisbane, QLD, Australia). Next the consensus sequences were compared against the Phytophthora database, which contains sequences from more than 1200 strains (http://www.phytophthoradb.org/welcome.php?a=intro). After the comparison, sequences were selected to perform a multiple alignment for each individual marker, similar to that described in our previous work on the bacterium Burkh0olderia (Estrada-de Los Santos et al. Citation2013). The alignments were carried out with Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) and then concatenated using Mesquite version 3.51 (http://www.mesquiteproject.org). Phylogenetic trees were constructed with the maximum likelihood method using the programme PhyML 3.0 under the model GTR+I+G (Guindon et al. Citation2009). The phylogenetic tree was visualized using the programme MEGA 7.0 (Kumar et al. Citation2016).
Results
Isolation and morphological observations
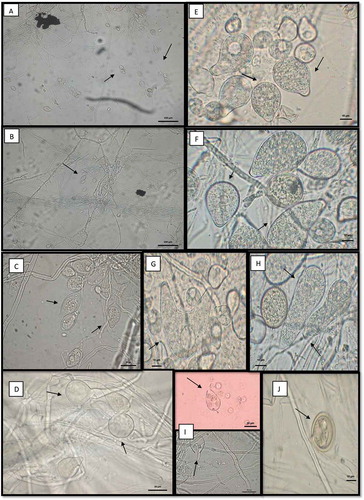
The cacao pods with signs and symptoms of infection yielded many colonies with typical growth of Phytophthora. The colonies presented a white mycelium, stellate with chrysanthemum shape, appressed and exhibited slightly torulose hyphae corresponding to the oomycete Phytophthora (). The isolate PtCa-14 grew faster on PDA than on the other media used, with colonies growing to 9.3 mm in diameter on PDA compared to 4–6 mm on the other media when it was incubated for 5 days at 28°C. The colony morphology of the isolate was similar on all media except for the one containing corn flour (). Hyphae were hyaline, and no sporangia and chlamydospores were observed until after the isolate was induced to form these structures in sterile soil solution. Both the sporangia and chlamydospores were abundant and exhibited features that were typical of P. tropicalis (, ). The asexual phase consisted of sporangia papillate, caducous with long often curved pedicels, which presented ovoid, obovoid, pyriform, ellipsoidal, or distorted shapes (36.761 ± 4.45 µm length × 23.097 ± 2.92 µm width), with tapered bases; originated on simple sympodial or umbellate sporangiophores. Hyphal swelling was absent. Chlamydospores were globose or subglobose (24.09 ± 3.37 µm diam), terminal or intercalary. Cacao pods with signs and symptoms of infection were collected for the first time in 2014 and then again in 2016. We have visited the area of study every year since 2016 and have observed the same indications of infection, suggesting the permanent presence of this disease at this location. The strain was preserved at CM-CNRG (Colección de Microorganismos, Centro Nacional de Recursos Genéticos, Jalisco, México) at INIFAP (Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, Veracruz, México) with the culture collection number CM-CNRG 705.
Table 1. Morphological features of Phytophthora tropicalis, Phytophthora palmivora and Phytophthora capsici.
Fig. 2 (Colour online) Colony morphology of Phytophthora tropicalis PtCa-14 in different media. (a) PDA; (b) SDA; (c) corn flour; (d) chocolate; and (e) V8. Stellate to petallate colony pattern after the phytopathogen was grown for 5 d at 28°C. No sexual structures were observed
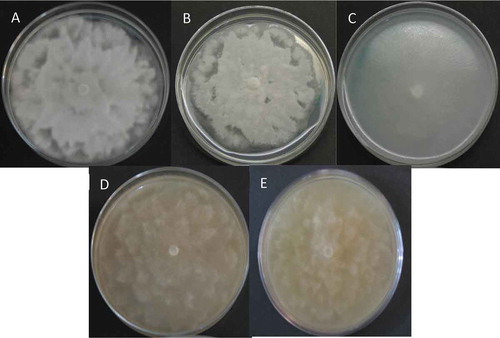
Fig. 3 (Colour online) Phytophthora tropicalis PtCa-14 observed by transmission electronic microscopy. (a) Sporangia showing sympodial with long pedicel and umbellate sporangiophores; (b) attachment of sporangiosphores is in close and simple sympodial arrangement; (c) sporangium typically papillate, ellipsoid; (d) chlamidosporas with base bulbous. Polymorphism sporangia; (e) sporangia limoniforme; (f) ellipsoid sporangia; (g) bi-papillate/tulbinate; (h) elongate; (i) release of zoospores with water; and (j) chlamydospore. Magnification: a, b, 20X; c, d, g, 40X; e, f, h, i, j, 100X
Molecular identification
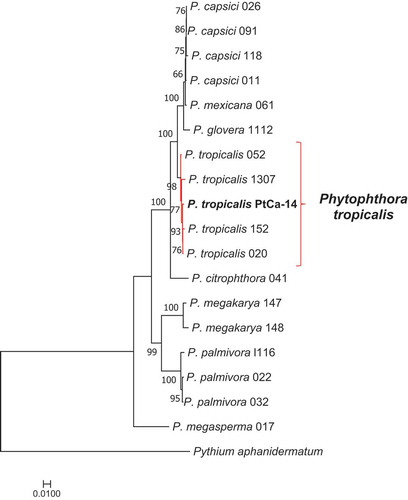
The comparison of the molecular markers in the Phytophthora database showed that all sequences from strain PtCa-14 had high similarity to P. tropicalis; ITS (MN563068) was 99.74% similar, COXII (MN796124) was 100% similar, TEF-1a (MN796125) was 99.66% similar, and tubulin beta chain (MN796126) was 99.78% similar. Moreover, the phylogenetic analysis using the four concatenated markers, showed that the strain PtCa-14 grouped with several P. tropicalis strains (), therefore identifying it as this species.
Fig. 4 (Colour online) Phylogenetic analysis of Phytophthora species, comparing the concatenated molecular markers ITS/coxII/TEF-1a/beta tubulin with maximum likelihood using the model GTR+I+G. The bar represents the differences among the sequences. The sequences were gathered from the Phytophthora database (http://www.phytophthoradb.org/)
Pathogenicity tests
Pears and peaches inoculated with strain PtCa-14 showed disease symptoms after 24 h. The lesions were necrotic with dark and shiny spots. White mycelium was observed after 5 days for pears and 3 days for peaches (). No lesions were observed in control pears and peaches. In addition, P. tropicalis PtCa-14 was successfully re-isolated from the pear and peach lesions and identified by morphological characteristics to confirm Koch postulates. In cacao pods, the symptoms were observed after 24 h. The lesions rapidly propagated, developing a mycelial efflorescence at day 4 and fully covering the pods by day 7 (). Control cacao pods did not exhibit any lesions. The oomycete was recovered from the cacao lesions, fulfiling Koch’s postulates.
Fig. 5 (Colour online) Pathogenicity assays of Phytophthora tropicalis PtCa-14 using pear fruits and cacao pods. (a-c) pear fruits, 5 d after inoculation with strain PtCa-14; (d) uninoculated pear fruit; (e) peach fruit, 3 d after inoculation with train PtCa-14; (f) uninoculated peach fruit; (g-i) cacao pods, 7 d after inoculation with strain PtCa-14; (h) uninoculated cacao pod

Discussion
In Mexico, cacao production is threatened by black pod rot and moniliasis. Through a multidisciplinary project, the Mexican government is currently supporting the study of strategies to improve the production of cacao (SAGARPA-CONACYT-2017-2-291417). As part of the development of biocontrol agents for this project, our research group isolated cacao phytopathogens to survey the microorganisms responsible for causing T. cacao damage in the states of Tabasco and Chiapas. Consequently, P. tropicalis was isolated from cacao pods affected by black pod rot in Tabasco. Given the specificity and wide distribution of P. palmivora as a cacao phytopathogen, the isolated strains were initially thought to belong to this species, but the morphological features did not match those of P. palmivora. Phytophthora palmivora is characterized by sporangia with short pedicels and sympodial sporangiophores (Mchau and Coffey Citation1994). The form of the chlamydospores and hyphal swellings are also useful for the identification of P. palmivora (Brasier and Griffin Citation1979). The morphological features of strain PtCa-14 included the production of a caducous long pedicel, elongated-narrow sporangia with a tapering base, and the frequent production of chlamydospores. This is also different from P. capsici, as it presents an asexual phase with the production of caducous, short, medium, and long pedicel sporangia which are subglobose to ellipsoid, and the absence of chlamydospores (Leonian Citation1922). According to the morphological analyses, the strain PtCa-14 belongs to the species P. tropicalis. This oomycete was recently described and includes isolates that were originally classified as P. capsici (Brasier and Griffin Citation1979) and P. palmivora (Aragaki and Uchida Citation2001). Although P. tropicalis and P. capsici are similar in morphological features, a molecular comparison performed in a previous study showed that they are different species (Zhang et al. Citation2004; Donahoo and Lamour Citation2008). In this study, the use of four molecular markers (ITS/coxII/TEF-1a/tubulin beta chain) greatly aided in correctly identifying the phytopathogen as P. tropicalis.
The phytopathogenic spectrum of P. tropicalis is wide. It has been recovered from a number of woody perennial and herbaceous hosts, including Piper nigrum, Macadamia integrifolia, Cuphea ignea, and Theobroma cacao (Aragaki and Uchida Citation2001), and it has also been identified as the causal agent of crown and root rot in Prunus armeniaca and Albizia julibrissin (Cacciola et al. Citation2006; Pane et al. Citation2008; Luongo et al. Citation2016). Furthermore, P. tropicalis has recently been described as affecting 23 plant families and it is often recovered from plant nurseries and economically important trees (Uchida and Kadooka Citation2013). In our analysis, the pathogenicity in P. tropicalis was confirmed in multiple biological models, namely, pears, peaches, and ultimately with cacao pods. Peaches rapidly displayed the symptoms and signs of black pod rot, showing that it is a good model to work with.
The distribution of P. tropicalis in Mexico is poorly understood, and although Donahoo and Lamour (Citation2008) re-classified some P. capsici strains as P. tropicalis, it is unknown where the strains were isolated. Ortega-Acosta et al. (Citation2017) observed P. tropicalis causing stem and root rot on Sesame indicum in the state of Guerrero, Mexico, identifying a new host for this pathogen. The authors also suggested that the genetic variation, pathogenicity, and virulence of this genus may be important in the adaptation to new hosts, which can make its control difficult and promote its dissemination to novel places and hosts. Therefore, it is important to identify Phytophthora species associated with different hosts and geographic locations correctly, using both morphological and molecular characters (Mchau and Coffey Citation1995; Aragaki and Uchida Citation2001; Luongo et al. Citation2016). This study revealed for the first time the presence of P. tropicalis in Tabasco, Mexico, as a causal agent of black pod of cacao. The proper identification of the causal agent of this disease may be useful to design effective strategies for its management. Currently, we are continuing to characterize Phytophthora isolates from different locations and cacao genotypes in Chiapas, Mexico, to assess the distribution and genetic diversity of P. tropicalis and determine whether other Phytophthora species are involved in black pod disease of cacao.
Acknowledgements
We thank Arturo Moreno Aviles and Isaac Luna Romero for technical support. Belén Chávez-Ramírez and Nadia Denisse Rodríguez-Velázquez were recipients of a fellowship from the Consejo Nacional de Ciencia y Tecnología. María Soledad Vásquez-Murrieta, Minerva Aurora Hernández-Gallegos, José Rodolfo Velazquez-Martínez, Carlos Hugo Avendaño-Arrazate, Paulina Estrada-de los Santos gratefully acknowledge the support of SNI. María Soledad Vásquez-Murrieta and Paulina Estrada-de los Santos also thank EDI and the Comisión de Operación y Fomento de Actividades Académicas del IPN for their support.
Additional information
Funding
References
- Allers T, Lichten M. 2000. A method for preparing genomic DNA that restrains branch migration of Holliday junctions. Nucleic Acids Res. 28:e6. doi:https://doi.org/10.1093/nar/28.2.e6.
- Álvarez RB, Carrillo FJA, García ERS, Allende MR, Santos CME. 2016. Caracterización de Phytophthora nicotianae causante de tizón de vinca en áreas urbanas y viveros de ornamentales en Culiacán, Mexico. Revista Mexicana de Fitopatología. 34:242–257.
- Aragaki M, Uchida JY. 2001. Morphological distinctions between Phytophthora capsici and Phytopthora tropicalis sp. nov. Mycologia. 93(1):137–145. doi:https://doi.org/10.1080/00275514.2001.12061285.
- Brasier CM, Griffin MJ. 1979. Taxonomy of Phytophthora palmivora on cocoa. Trans Br Mycol Soc. 72(1):111–143. doi:https://doi.org/10.1016/S0007-1536(79)80015-7.
- Cacciola SO, Spica D, Cooke DEL, Raudino F, Magnano Di San Lio G. 2006. Wilt and collapse of Cuphea ignea caused by Phytophthora tropicalis in Italy. Plant Dis. 90(5):680–680. doi:https://doi.org/10.1094/PD-90-0680A.
- Donahoo RS, Lamour KH. 2008. Interspecific hybridization and apomixes between Phytophthora capsici and Phytophthora tropicalis. Mycologia. 100(6):911–920. doi:https://doi.org/10.3852/08-028.
- Drenth A, Guest DI, editors. 2004. Diversity and management of Phytophthora in Southeast Asia. Australian Government, Australian Centre for International Agricultural Research. Monograph 114.
- Erwin DC, Ribeiro OK. 1996. Phytophthora diseases worldwide. St. Paul (MN): The American Phytopathological Society Press; p. 592.
- Estrada-de Los Santos P, Vinuesa P, Martínez-Aguilar L, Hirsch AM, Caballero-Mellado J. 2013. Phylogenetic analysis of Burkholderia species by multilocus sequence analysis. Curr Microbiol. 67:51–60. doi:https://doi.org/10.1007/s00284-013-0330-9.
- Evans HC. 1971. Transmission of Phytophthora pod rot of cocoa by invertebrates. Nature. 232(5309):346–347. doi:https://doi.org/10.1038/232346a0.
- Evans HC, Prior C. 1987. Cocoa pod diseases: causal agents and control. Outlook Agr. 16(1):35–41. doi:https://doi.org/10.1177/003072708701600106.
- Griffin MJ, Idowu AC, Maddison AC, Taylor B, Ward MR. 1981. Sources of infection. In: Gregory PH, Maddison AC, editors. Epidemiology of Phytophthora on cocoa in Nigeria. Kew (UK): Commonwealth Mycological Institute paper 25; p. 75–95.
- Guindon S, Delsuc F, Dufayard JF, Gascuel O. 2009. Estimating maximum likelihood phylogenies with PhyML. In: Posada D, editor. Bioinformatics for DNA sequence analysis. Hatfield, Hertfordshire: Springer Protocols; p. 113–137.
- Kroon LP, Bakker FT, van den Bosch GB, Bonants PJ, Flier WG. 2004. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet Biol. 41(8):766–782. doi:https://doi.org/10.1016/j.fgb.2004.03.007.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874. doi:https://doi.org/10.1093/molbev/msw054.
- Leonian LW. 1922. Stem and fruit blight of pepper caused by P. capsici species nov. Phytopathol. 12:401–408.
- Luongo L, Vitale S, Galli M, Haegi A, Wagner S, Werres S, Belisario A. 2016. Morphological and molecular identification of Phytophthora tropicalis as causal agent of crown and root rot on Albizia julibrissin. J Phytopathol. 164(11/12):959–966. doi:https://doi.org/10.1111/jph.12516.
- Martin FN, Tooley PW. 2003. Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia. 95(2):269–284. doi:https://doi.org/10.1080/15572536.2004.11833112.
- Mchau GR, Coffey MD. 1994. Isozyme diversity in Phytophthora palmivora: evidence for a southeast Asian centre of origin. Mycol Res. 98(9):1035–1043. doi:https://doi.org/10.1016/S0953-7562(09)80430-9.
- Mchau GR, Coffey MD. 1995. Evidence for the existence of two subpopulations in Phytophthora capsici and a redescription of the species. Mycol Res. 99(1):89–102. doi:https://doi.org/10.1016/S0953-7562(09)80321-3.
- Mohamed-Azni IN, Sundram S, Ramachandran V, Seman IA. 2017. An in vitro investigation of Malaysian Phytophthora palmivora isolates and pathogenicity study on oil palm. J Phytopathol. 165(11–12):800–812. doi:https://doi.org/10.1111/jph.12620.
- Ortega-Acosta SÁ, Hernández-Morales J, Ochoa-Martínez DL, Ayala-Escobar V. 2017. First report of Phytophthora tropicalis causing stem and root rot on sesame (Sesamum indicum) in Mexico. Plant Dis. 101(1):258–258. doi:https://doi.org/10.1094/PDIS-04-16-0482-PDN.
- Pane A, Cacciola SO, Chimento A, Allatta C, Scibetta S, Magnano Di San Lio G. 2008. First report of Phytophthora spp. as pathogens of Pandorea jasminoides in Italy. Plant Dis. 92(2):313. doi:https://doi.org/10.1094/PDIS-92-2-0313B.
- Phillips MW, Cerda BR. 2011. Catálogo, enfermedades del cacao en Centroamérica. Colombia CATIE No. 93.
- Phillips MW, Wilkinson MJ. 2007. Frosty pod of cacao: a disease with a limited geographic range but unlimited potential of damage. Phytopathology. 97(12):1644–1647. doi:https://doi.org/10.1094/PHYTO-97-12-1644.
- Pintos VC, Mansilla VJ, Aguín CO. 2004. Phytophthora ramorum nuevo patógeno en España sobre Camellia japonica y Viburnum tinus. Boletín de Sanidad Vegetal Plagas. 30:97–111.
- Ploetz R. 2016. The impact of diseases on cacao production: a global overview. In: Bailey BA, Meinhardt LW, editors. Cacao diseases: a history of old enemies and new encounters. Switzerland: Springer International Publishing; p. 33–59.
- Rocha HM. 1965. Cacao varieties resistant to Phytophthora palmivora (Butl.): a literature review. Cacao Costa Rica. 10(1):1–9.
- [SIAP] Servicio de Información Agroalimentaria y Pesquera. 2019. Atlas Agroalimentario 2019. Mexico. https://nube.siap.gob.mx/gobmx_publicaciones_siap/pag/2019/Atlas-Agroalimentario–2019.
- Smith ESC. 1981. An integrated control scheme for cocoa pests and diseases in Papua New Guinea. Trop Pest Manag. 27(3):351–359. doi:https://doi.org/10.1080/09670878109413804.
- Uchida J, Aragaki M. 1985. Occurrence of chlamydospores in Phytophthora capsica. Mycologia. 77(5):832–835. doi:https://doi.org/10.1080/00275514.1985.12025170.
- Uchida J, Kadooka CY. 2013. Distribution and biology of Phytophthora tropicalis. In: Lamour K, editor. Phytophthora: a global perspective. Wallingford (UK): CABI Plant Protection Series No. 2; p. 178–186.
- Waterhouse G. 1963. Key to the species of Phytophthora de Bary, Grace M. Waterhouse. Mycological Papers No. 92. Commonwealth Mycological Institute, Kew, 23, sd, 6.
- White TJ, Bruns TD, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press; p. 315–322.
- Zhang Z, Zhang JY, Zheng XB, Yang YW, Ko WH. 2004. Molecular distinction between Phytophthora capsici and P. tropicalis based on ITS sequences of ribosomal DNA. J Phytopathol. 152:358–364. doi:https://doi.org/10.1111/j.1439-0434.2004.00856.x.